Abstract
1 The β-adrenoceptor is one of a number of G protein-coupled receptors which have been proposed to contain seven transmembrane α-helices. The function of this receptor appears to be regulated by phosphorylation by a specific enzyme, the β-adrenoceptor kinase. Synthetic peptides which comprise each of the proposed intra- and extracellular domains of the β2-adrenoceptor have been tested as potential substrates and inhibitors of the β-adrenoceptor kinase.
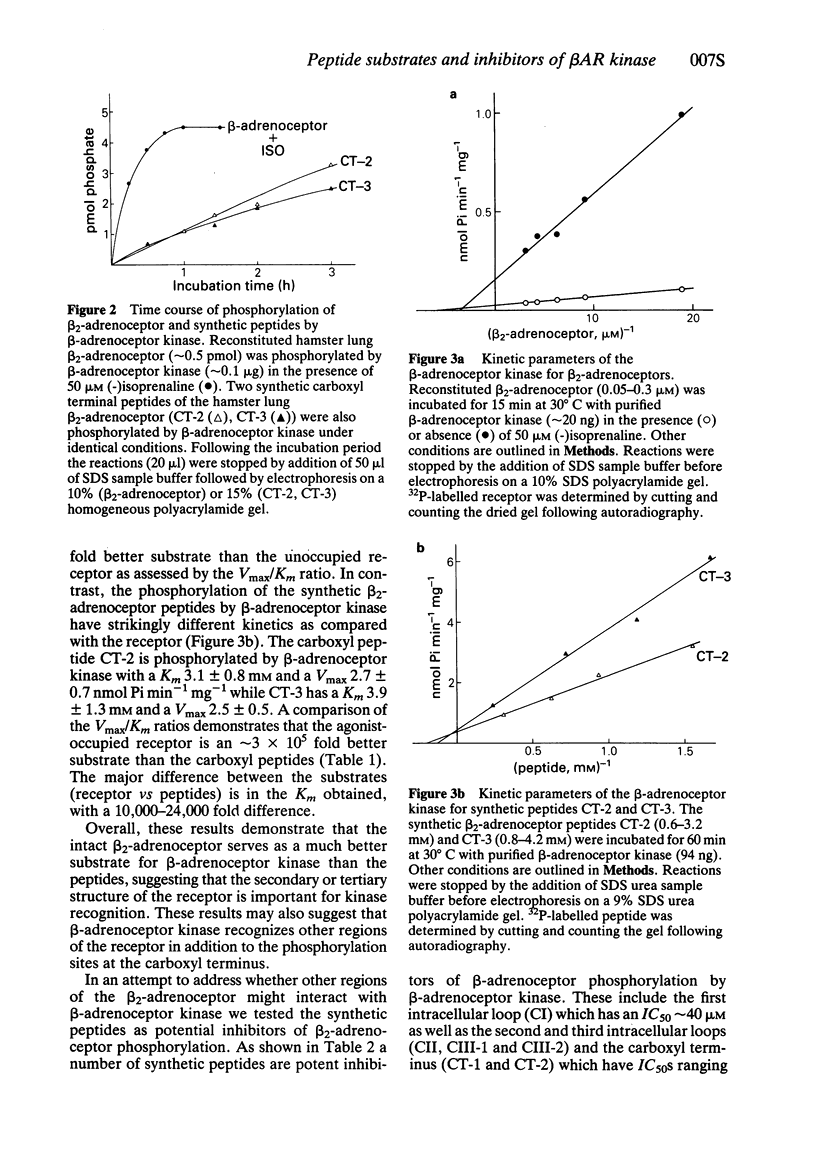
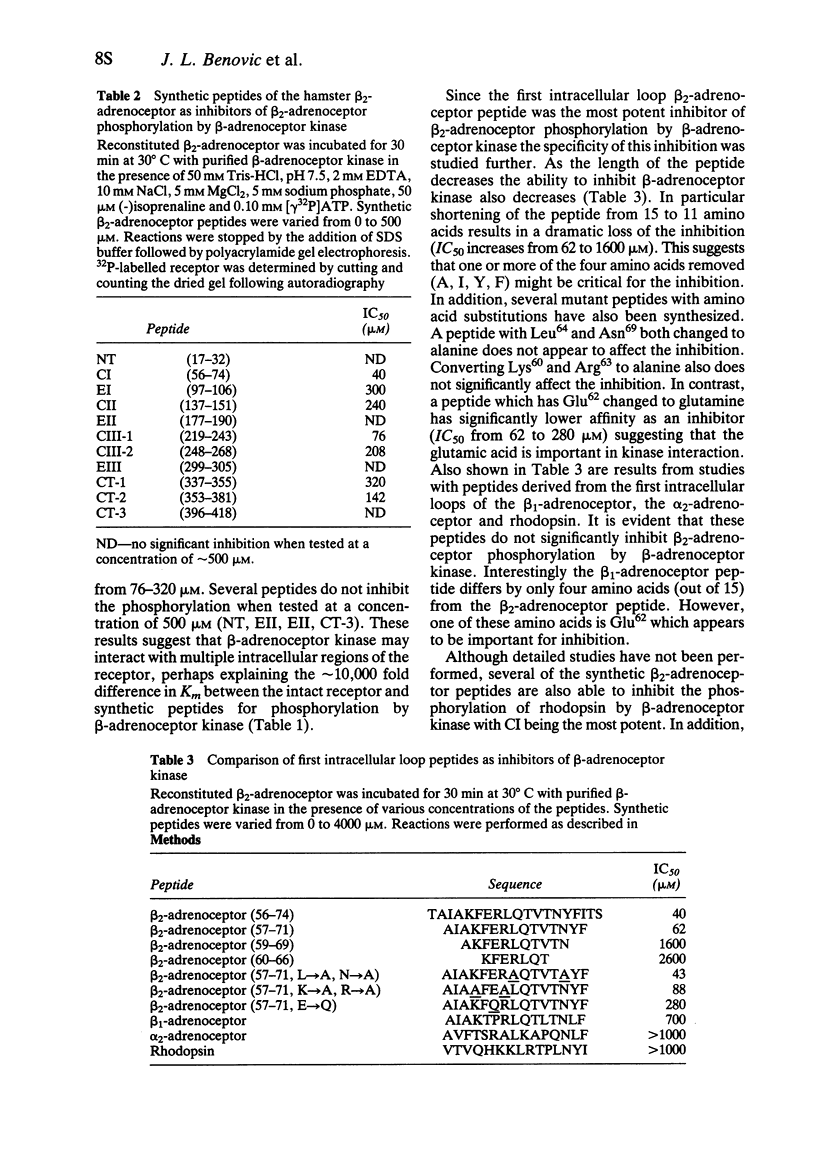
2 Two peptides which encompass the middle and terminal portions of the carboxyl tail of the receptor served as substrates by β-adrenoceptor kinase. The kinetics of the phosphorylation reaction, however, suggest that these peptides are 106-fold poorer substrates than the agonist occupied receptor.
3 A number of synthetic peptides also served as inhibitors of β2-adrenoceptor phosphorylation by β-adrenoceptor kinase. In particular, a peptide which comprised the first intracellular loop of the β2-adrenoceptor (amino acids 56-74) inhibited most effectively with an IC50 of 40 μM.
4 These results suggest that multiple intracellular regions of the β-receptor may serve as potential sites of interaction with β-adrenoceptor kinase. Moreover, these regions may serve as potential targets for the development of specific inhibitors of β-adrenoceptor kinase which could be used to block homologous desensitization.
Keywords: Receptors, desensitization, phosphorylation, kinases, inhibitors
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benovic J. L., Bouvier M., Caron M. G., Lefkowitz R. J. Regulation of adenylyl cyclase-coupled beta-adrenergic receptors. Annu Rev Cell Biol. 1988;4:405–428. doi: 10.1146/annurev.cb.04.110188.002201. [DOI] [PubMed] [Google Scholar]
- Benovic J. L., Mayor F., Jr, Staniszewski C., Lefkowitz R. J., Caron M. G. Purification and characterization of the beta-adrenergic receptor kinase. J Biol Chem. 1987 Jul 5;262(19):9026–9032. [PubMed] [Google Scholar]
- Benovic J. L., Pike L. J., Cerione R. A., Staniszewski C., Yoshimasa T., Codina J., Caron M. G., Lefkowitz R. J. Phosphorylation of the mammalian beta-adrenergic receptor by cyclic AMP-dependent protein kinase. Regulation of the rate of receptor phosphorylation and dephosphorylation by agonist occupancy and effects on coupling of the receptor to the stimulatory guanine nucleotide regulatory protein. J Biol Chem. 1985 Jun 10;260(11):7094–7101. [PubMed] [Google Scholar]
- Benovic J. L., Regan J. W., Matsui H., Mayor F., Jr, Cotecchia S., Leeb-Lundberg L. M., Caron M. G., Lefkowitz R. J. Agonist-dependent phosphorylation of the alpha 2-adrenergic receptor by the beta-adrenergic receptor kinase. J Biol Chem. 1987 Dec 25;262(36):17251–17253. [PubMed] [Google Scholar]
- Benovic J. L., Shorr R. G., Caron M. G., Lefkowitz R. J. The mammalian beta 2-adrenergic receptor: purification and characterization. Biochemistry. 1984 Sep 25;23(20):4510–4518. doi: 10.1021/bi00315a002. [DOI] [PubMed] [Google Scholar]
- Benovic J. L., Stone W. C., Caron M. G., Lefkowitz R. J. Inhibition of the beta-adrenergic receptor kinase by polyanions. J Biol Chem. 1989 Apr 25;264(12):6707–6710. [PubMed] [Google Scholar]
- Benovic J. L., Strasser R. H., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M., Collins S., O'Dowd B. F., Campbell P. T., de Blasi A., Kobilka B. K., MacGregor C., Irons G. P., Caron M. G., Lefkowitz R. J. Two distinct pathways for cAMP-mediated down-regulation of the beta 2-adrenergic receptor. Phosphorylation of the receptor and regulation of its mRNA level. J Biol Chem. 1989 Oct 5;264(28):16786–16792. [PubMed] [Google Scholar]
- Bouvier M., Leeb-Lundberg L. M., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of adrenergic receptor function by phosphorylation. II. Effects of agonist occupancy on phosphorylation of alpha 1- and beta 2-adrenergic receptors by protein kinase C and the cyclic AMP-dependent protein kinase. J Biol Chem. 1987 Mar 5;262(7):3106–3113. [PubMed] [Google Scholar]
- Cerione R. A., Strulovici B., Benovic J. L., Lefkowitz R. J., Caron M. G. Pure beta-adrenergic receptor: the single polypeptide confers catecholamine responsiveness to adenylate cyclase. Nature. 1983 Dec 8;306(5943):562–566. doi: 10.1038/306562a0. [DOI] [PubMed] [Google Scholar]
- Cook P. F., Neville M. E., Jr, Vrana K. E., Hartl F. T., Roskoski R., Jr Adenosine cyclic 3',5'-monophosphate dependent protein kinase: kinetic mechanism for the bovine skeletal muscle catalytic subunit. Biochemistry. 1982 Nov 9;21(23):5794–5799. doi: 10.1021/bi00266a011. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Bouvier M., Benovic J. L., Caron M. G., Lefkowitz R. J. The multiple membrane spanning topography of the beta 2-adrenergic receptor. Localization of the sites of binding, glycosylation, and regulatory phosphorylation by limited proteolysis. J Biol Chem. 1987 Oct 15;262(29):14282–14288. [PubMed] [Google Scholar]
- Dohlman H. G., Caron M. G., Lefkowitz R. J. A family of receptors coupled to guanine nucleotide regulatory proteins. Biochemistry. 1987 May 19;26(10):2657–2664. doi: 10.1021/bi00384a001. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Matsui H., Kobilka T. S., Yang-Feng T. L., Francke U., Caron M. G., Lefkowitz R. J., Regan J. W. Cloning, sequencing, and expression of the gene coding for the human platelet alpha 2-adrenergic receptor. Science. 1987 Oct 30;238(4827):650–656. doi: 10.1126/science.2823383. [DOI] [PubMed] [Google Scholar]
- Kuenzel E. A., Krebs E. G. A synthetic peptide substrate specific for casein kinase II. Proc Natl Acad Sci U S A. 1985 Feb;82(3):737–741. doi: 10.1073/pnas.82.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwatra M. M., Benovic J. L., Caron M. G., Lefkowitz R. J., Hosey M. M. Phosphorylation of chick heart muscarinic cholinergic receptors by the beta-adrenergic receptor kinase. Biochemistry. 1989 May 30;28(11):4543–4547. doi: 10.1021/bi00437a005. [DOI] [PubMed] [Google Scholar]
- Kwatra M. M., Hosey M. M. Phosphorylation of the cardiac muscarinic receptor in intact chick heart and its regulation by a muscarinic agonist. J Biol Chem. 1986 Sep 25;261(27):12429–12432. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Cotecchia S., DeBlasi A., Caron M. G., Lefkowitz R. J. Regulation of adrenergic receptor function by phosphorylation. I. Agonist-promoted desensitization and phosphorylation of alpha 1-adrenergic receptors coupled to inositol phospholipid metabolism in DDT1 MF-2 smooth muscle cells. J Biol Chem. 1987 Mar 5;262(7):3098–3105. [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Cotecchia S., Lomasney J. W., DeBernardis J. F., Lefkowitz R. J., Caron M. G. Phorbol esters promote alpha 1-adrenergic receptor phosphorylation and receptor uncoupling from inositol phospholipid metabolism. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5651–5655. doi: 10.1073/pnas.82.17.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse M. J., Lefkowitz R. J., Caron M. G., Benovic J. L. Inhibition of beta-adrenergic receptor kinase prevents rapid homologous desensitization of beta 2-adrenergic receptors. Proc Natl Acad Sci U S A. 1989 May;86(9):3011–3015. doi: 10.1073/pnas.86.9.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dowd B. F., Lefkowitz R. J., Caron M. G. Structure of the adrenergic and related receptors. Annu Rev Neurosci. 1989;12:67–83. doi: 10.1146/annurev.ne.12.030189.000435. [DOI] [PubMed] [Google Scholar]
- Palczewski K., McDowell J. H., Hargrave P. A. Rhodopsin kinase: substrate specificity and factors that influence activity. Biochemistry. 1988 Apr 5;27(7):2306–2313. doi: 10.1021/bi00407a010. [DOI] [PubMed] [Google Scholar]
- Shichi H., Somers R. L. Light-dependent phosphorylation of rhodopsin. Purification and properties of rhodopsin kinase. J Biol Chem. 1978 Oct 10;253(19):7040–7046. [PubMed] [Google Scholar]
- Sibley D. R., Strasser R. H., Caron M. G., Lefkowitz R. J. Homologous desensitization of adenylate cyclase is associated with phosphorylation of the beta-adrenergic receptor. J Biol Chem. 1985 Apr 10;260(7):3883–3886. [PubMed] [Google Scholar]
- Stiles G. L., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptors: biochemical mechanisms of physiological regulation. Physiol Rev. 1984 Apr;64(2):661–743. doi: 10.1152/physrev.1984.64.2.661. [DOI] [PubMed] [Google Scholar]
- Strasser R. H., Sibley D. R., Lefkowitz R. J. A novel catecholamine-activated adenosine cyclic 3',5'-phosphate independent pathway for beta-adrenergic receptor phosphorylation in wild-type and mutant S49 lymphoma cells: mechanism of homologous desensitization of adenylate cyclase. Biochemistry. 1986 Mar 25;25(6):1371–1377. doi: 10.1021/bi00354a027. [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Wilden U., Hall S. W., Kühn H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilden U., Kühn H. Light-dependent phosphorylation of rhodopsin: number of phosphorylation sites. Biochemistry. 1982 Jun 8;21(12):3014–3022. doi: 10.1021/bi00541a032. [DOI] [PubMed] [Google Scholar]



