Abstract
It is becoming apparent that over the years cell infection by virus seems to have evolved into a multistep process in which many viruses employ distinct cell surface molecules for their attachment and cell entry. In this study the attachment and entry pathway of coxsackievirus A9 (CAV-9), a member of the Picornaviridae family, was investigated. It has been known that, although integrin αvβ3 is utilized as a receptor, its presence alone is insufficient for CAV-9 infection and that CAV-9 also requires a 70-kDa major histocompatibility complex class I (MHC-I)-associated protein (MAP-70) as a coreceptor molecule. We document by protein isolation and peptide sequencing that the 70-kDa protein is GRP78, a member of the heat shock protein 70 family of stress proteins. Furthermore we show by using fluorescence resonance energy transfer (FRET) that GRP78 is also expressed on the cell surface and associates with MHC-I molecules. In addition CAV-9 infection of permissive cells requires GRP78 and also MHC-I molecules, which are essential for virus internalization. The identification of GRP78 as a coreceptor for CAV-9 and the revelation of GRP78 and MHC-I associations have provided new insights into the life cycle of CAV-9, which utilizes integrin αvβ3 and GRP78 as receptor molecules whereas MHC-I molecules serve as the internalization pathway of this virus to mammalian cells.
It has been clear for many years that viruses which propagate within vertebrate hosts have had to adapt to survive the hostile environment imposed by the host immunity by utilizing more than one cell surface molecule for their attachment and cell entry (24). Identification of virus receptors and characterization of their interaction with the virus are major goals in virology.
In this study we focused on receptor interactions of coxsackievirus A9 (CAV-9), a nonenveloped RNA virus which causes flaccid paralysis and chronic dilated cardiomyopathy (10) and which is implicated in autoimmune episodes that lead to insulin-dependent diabetes mellitus (IDDM) (22, 23). Substantial knowledge of the receptors utilized by CAV-9 will allow some mechanisms of host recognition by the virus to be understood and consequently open ways for therapeutic intervention. It has been known that integrin αvβ3 is a receptor for CAV-9 (21, 32, 33). However its presence alone is insufficient for CAV-9 infection, thus leading us to believe that other cell surface molecules may be required for efficient CAV-9 infection (33).
Our previous studies have identified a 70-kDa major histocompatibility complex class I (MHC-I)-associated protein (MAP-70) as another receptor molecule for CAV-9 (31). Here we present evidence that this 70-kDa protein is GRP78. This glucose-regulated 78-kDa protein is a member of the heat shock protein 70 (HSP70) family. GRP78 acts as a molecular chaperon and is involved in the folding and translocation of nascent peptide chains including the folding and assembly of MHC-I molecules (8, 9). Even though mostly intracellular, HSPs have been found to be expressed on the surfaces of cells and to function as antigen-presenting structures carrying viral peptides (5), minor histocompatibility (1), and model antigens for CD8 T cells (4).
In this study, we further studied the association of MHC-I with GRP78 and the significance of this association in the CAV-9 infectious cycle. Using fluorescence resonance energy transfer (FRET) studies we discovered that GRP78 associates with MHC-I molecules on the cell surface and that MHC-I molecules play an essential role in the virus internalization process.
MATERIALS AND METHODS
Cell lines.
The green monkey kidney cell line (GMK) was maintained in minimal essential medium containing 1% nonessential amino acids, 10% heat-inactivated fetal bovine serum, and 100 μg of gentamicin/liter. B-lymphoma cell lines Daudi and Daudi-MHC+ (transfected with the β2-microglobulin gene) (20) were maintained in RPMI medium with Glutamax (Gibco) supplemented with 100 μg of antibiotics (penicillin and streptomycin)/ml, 0.1% (wt/vol) sodium pyruvate, and 10% (wt/vol) fetal calf serum at 37°C in a 7% humidified atmosphere.
Antibodies.
Integrin αvβ3-specific monoclonal antibody (MAb) MCA757G, which recognizes a domain between the αv and β3 molecules including the RGD motif recognition sequence, and β2-microglobulin-specific MAb MCA1115 were obtained from Serotec. HLA-A-, -B-, and -C-specific MAb W6/32, which recognizes a monomorphic epitope complexed by the heavy chain and β2-microglobulin of MHC-I, was obtained from the American Type Culture Collection. The MAB1976 integrin αvβ3-specific MAb, which recognizes the vitronectin receptor complex of integrin αvβ3, was obtained from Chemicon. GRP78-specific goat polyclonal serum was obtained from RDI. CAV-9-neutralizing rabbit polyclonal serum was obtained from the Public Health Laboratories. Fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse IgG and FITC-conjugated swine anti-rabbit Ig were obtained from Dako. The transferrin-specific M073401 MAb was also obtained from Dako. Antibodies were conjugated to either Cy3 or Cy5 using the Cy3 and Cy5 FluoroLink labeling kits from Amersham Pharmacia.
Immunoprecipitation protocols.
GMK cells were surface labeled with N-hydroxy-succinamide-biotin and lysed in lysis buffer (31). The lysate was precleared with normal rabbit serum or normal mouse serum followed by the addition of 10% protein A-Sepharose slurry (Pharmacia Biotech, Uppsala, Sweden) to remove nonspecific binding material. Virus-receptor complexes were immunoprecipitated by the addition of 1.5 × 106 PFU of virus, which was incubated for 1 h at room temperature, followed by the addition of 2 μg of anti-CAV-9 serum for 1 h at 4°C. The resulting immune complexes were isolated with 10% protein A-Sepharose slurry. Immune complexes were eluted from protein A-Sepharose beads with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (125 mM Tris-HCl, 4% SDS, 20% glycerol, 1.4 M β-mercaptoethanol, 0.1% bromophenol blue). Eluates were electrophoresed in 4 to 20% gradient polyacrylamide gels (Ready Gel; Bio-Rad) or subjected to two-dimensional nonequilibrium pH gradient electrophoresis. Biotin-labeled proteins were transferred to a nitrocellulose membrane, and for the cell surface-labeled lysates the gel was Western blotted with streptavidin-horseradish peroxidase (HRP) conjugate as described below.
Immunoblotting.
Immunoprecipitates were separated by SDS-PAGE and transferred onto a nitrocellulose filter (Schleicher & Schuell) or Immobilon P membranes (Millipore). After transfer, the membrane was immersed for 1 h in blocking solution (5% low-fat dried milk dissolved in 0.1% phosphate-buffered saline [PBS]-Tween) and washed with 0.1% PBS-Tween (two rinses, a 15-min wash, and two 10-min washes). The membrane was then incubated with HRP-conjugated GRP78-specific goat polyclonal serum. The optimum antibody concentration was determined by dot blot assay (data not shown). After extensive washing with 0.1% PBS-Tween, the antigen was visualized using the ECL procedure (Amersham) according to the manufacturer’s instructions.
Cell labeling for FRET.
GMK cells on microchamber culture slides (Lab-tek; Gibco) were labeled with 100 μl of a mixture of a donor-conjugated antibody (Cy3) and an acceptor-conjugated antibody (Cy5). The cells were rinsed twice in PBS-0.02% bovine serum albumin prior to fixation with 4% formaldehyde for 15 min. The cells were fixed in order to prevent potential reorganization of the proteins during the course of the experiment.
Confocal imaging.
Cells were imaged on a Carl Zeiss, Inc., confocal microscope (with an Axiovert 100 fluorescence microscope) using a 1.4-numerical aperture 63× Zeiss objective. The images were analyzed using LSM, version 2.5, image analysis software (Carl Zeiss, Inc.). Cy3 and Cy5 were detected using the appropriate filter sets. When typical exposure times for image acquisition (less than 5 s) were used, no fluorescence was observed from a Cy3-labeled specimen using the Cy5 filters nor was Cy5 fluorescence detected using the Cy3 filter sets.
FRET measurements.
The FRET assay is a noninvasive imaging technique used to determine molecular proximity. Energy transfer can occur over 1- to 10-nm distances and effectively increases the resolution of light microscopy to the molecular level. It involves nonradiative transfer of energy from the excited state of a donor molecule to an appropriate acceptor (6, 7, 35). The rate of energy transfer is inversely proportional to the sixth power of the distance between donor and acceptor (15, 16). The efficiency of energy transfer (E) is defined with respect to r and R0, the characteristic Forster distance, by E = 1/[1 + (r/R0)6], where r is the radius of the distance between the fluorophores. In the present study, FRET was measured using a method previously described (2, 3, 15). Briefly, samples were labeled with donor- and acceptor-conjugated antibodies and energy transfer was detected as an increase in donor fluorescence (dequenching) after complete photobleaching of the acceptor molecule.
Cells labeled only with the GRP78-Cy5 probe were used in order to determine the minimum time required to bleach Cy5. Cy5 was bleached by continuous excitation with an arc lamp using a Cy5 filter set for 10 min. Under these conditions, Cy3 was not bleached.
FRET images were calculated from the increase in donor fluorescence after acceptor photobleaching by E (percent) × 100 = 10,000 × [(Cy3 postbleach) − Cy3 prebleach)/Cy3 postbleach]. The scaling factor of 10,000 was used in order to expand E to the scale of the 12-bit images.
Virus-blocking assays.
GMK cells were grown as a monolayer in six-well plates (Nunc). MAbs (2.5 to 10 μg) were added in 1 ml of serum-free medium and incubated at room temperature for 50 min before the addition of approximately 250 PFU of CAV-9 virus particles and further incubation at room temperature for 50 min. The monolayer was washed with culture medium and overlayed with 0.5% (wt/vol) carboxymethylcellulose in culture medium. The incubation was continued for 48 to 72 h in a 7% CO2 humidified incubator before plaque visualization with crystal violet. Control plates with isotype control IgG were similarly treated.
Virus binding assays by flow cytometry.
Cells were harvested by gentle agitation and washed with blocking buffer (PBS containing 0.02% [vol/vol] donor calf serum, 0.02% [wt/vol] NaN3) at 4°C for 15 min. CAV-9 (250 PFU) diluted in binding buffer (PBS supplemented with 2 mM CaCl2 and 1 mM MgCl2) was added to the cells (typically 0.5 × 106), and cells were incubated for 1 h at room temperature. Unbound viruses were removed by washing three times in binding buffer. Cells were then incubated with an appropriate dilution of CAV-9-neutralizing rabbit serum. Staining was visualized with FITC-conjugated swine anti-rabbit Ig. Fluorescence staining was analyzed as described above.
Virus binding assays in the presence of integrin αvβ3 or GRP78 antibodies.
Cells were harvested by gentle agitation and washed with blocking buffer at 4°C for 15 min. Tubes containing 0.5 × 106 cells were then incubated with either 2.5 μg of MAB1976-, 2.5 μg of MCA757G-, or 2.5 μg of GRP78-specific polyclonal serum in PBS for 1 h at 4°C. After three washes in binding buffer (PBS supplemented with 2 mM CaCl2 and 1 mM MgCl2), virus binding was assayed as described above.
RESULTS
GRP78, a receptor for CAV-9.
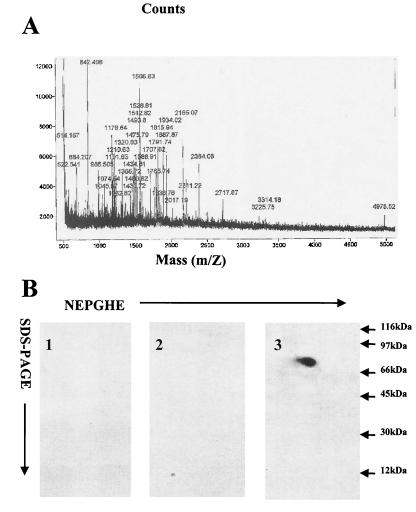
It has been known that CAV-9 forms a detergent-stable complex with a 70-kDa MHC-I-associated cell surface protein (MAP-70) (31). Virus-receptor complexes were isolated and analyzed by two-dimensional gel electrophoresis followed by SDS-PAGE. The 70-kDa receptor band was excised and subjected to tryptic digestion, and the resulting peptide mixtures were analyzed at the PAN Facility (University of Stanford) via a matrix-assisted laser desorption ionization mass spectrometer (Fig. 1A). The peptide maps were compared to the National Center for Biotechnology Information’s nr database using a Bayesian algorithm. The 70-kDa protein was identified as GRP78.
FIG. 1.
Identification of MAP-70. (A) Tryptic peptide mixture analysis via a matrix-assisted laser desorption ionization mass spectrometer. (B) Immunoblotting of protein with goat Igs followed by HRP-conjugated donkey anti-goat Ig (1), with only HRP-conjugated donkey anti-goat Ig (2), or with HRP-conjugated goat GRP78-specific serum (3). NEPHGE, nonequilibrium pH gradient gel electrophoresis.
To confirm that this protein was GRP78, immunoblotting of nitrocellulose membranes containing virus-receptor immunoprecipitates was performed with goat GRP78-specific serum-HRP. Control experiments with only goat Igs or HRP-conjugated donkey anti-goat Ig were also performed (Fig. 1B). Our results confirmed that this protein was GRP78.
Inhibition of CAV-9 binding.
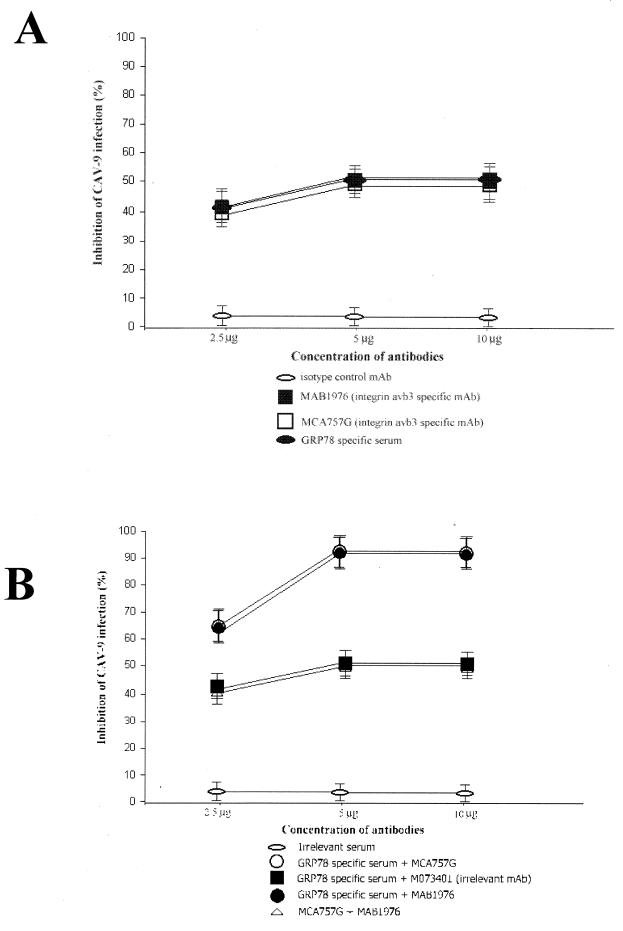
To verify the results obtained from our biochemical experiments, we proceeded to investigate whether we could inhibit virus infection by blocking its newly identified cellular receptor. Pretreatment of GMK cells with specific GRP78 polyclonal serum inhibited CAV-9 infection by 50%. When integrin αvβ3-specific MAbs MCA757G and MAB1976 either separately or in combination were used, they inhibited infection by 50%, but when a mixture of GRP78-specific serum with either of the integrin αvβ3-specific MAbs was used, it completely abolished infection. When a mixture of GRP-specific serum with an irrelevant MAb specific for the transferrin receptor (M073401) was used, the combination inhibited infection by 50%. In addition an irrelevant antiserum was used as a control and there was no infectivity inhibition (Fig. 2).
FIG. 2.
Inhibition of CAV-9 infectivity by antibodies against its cellular receptors. Shown are percentages of inhibition of CAV-9 infectivity of GMK cells in the presence of MAbs (A) and combinations of MAbs (B) at concentrations of 2.5, 5, and 10 μg. Error bars, standard deviations over a number of independent experiments.
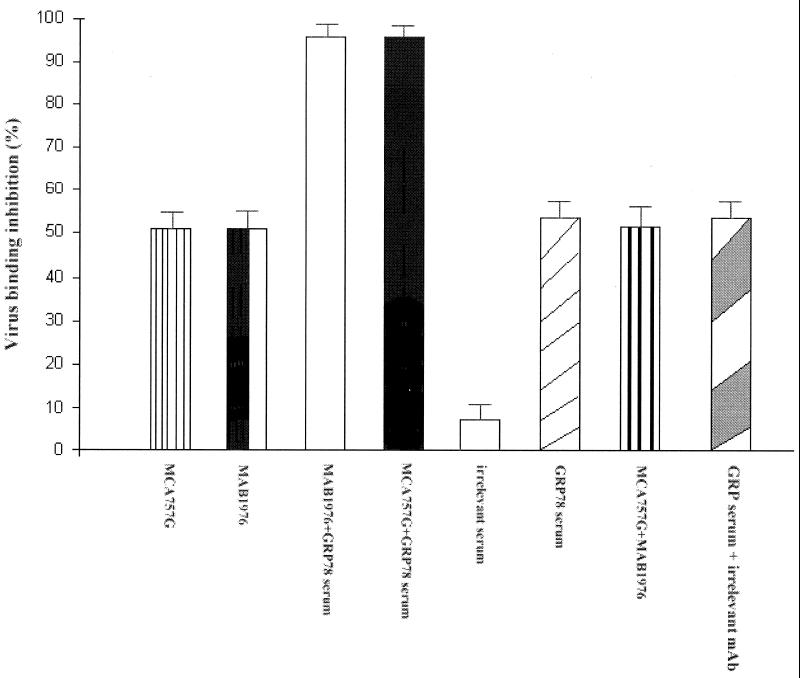
To confirm that this inhibition was due to prevention of CAV-9 binding and that GRP78 acts as a cellular receptor for CAV-9, we semiquantified by flow cytometry the degree of virus binding in the presence of GRP78 and integrin αvβ3-specific antibodies. Our results showed that, in the presence of GRP78-specific serum, the virus binding was reduced by 50% and that, in the presence of integrin-specific MAb MCA757G or MAB1976, binding was also reduced by 50% (Fig. 3). When a combination of GRP78-specific serum and integrin αvβ3-specific MAb MCA757G or MAB1976 was used, complete inhibition was observed. As a control a combination of GRP78-specific serum with irrelevant MAb M073401 (transferrin receptor specific) was used; this showed a 50% reduction in binding. The use of an irrelevant serum did not inhibit virus binding (Fig. 3). Taken together these findings strongly suggest that inhibition of CAV-9 infection is due to a block in binding by integrin αvβ3- and GRP78-specific antibodies.
FIG. 3.
Inhibition of CAV-9 binding by antibodies against its cellular receptors. Shown are percentages of inhibition of CAV-9 binding to GMK cells. The inhibition of CAV-9 binding to GMK cells was measured in the presence of MAB1976 (integrin αvβ3 specific), MCA757G (integrin αvβ3 specific), and combinations of GRP78-specific serum with MAB1976, MCA757G, or MCA757G, and also GRP78-specific serum with irrelevant antibody M073401 (transferrin receptor specific). Inhibition in the presence of an irrelevant serum was also tested. Error bars, standard deviations over a number of independent experiments.
CAV-9 and GRP78 interactions investigated by FRET.
The FRET technique, a biophysical method probing the proximity of molecules on the cell surface under conditions very close to the physiological state of the cells, was employed in order to verify CAV-9 and GRP78 interactions. We measured FRET in terms of dequenching of donor fluorescence after complete photobleaching of the acceptor fluorophore. Increased donor fluorescence after destruction of the acceptor indicated that donor fluorescence was quenched in the presence of the acceptor because of energy transfer. We tested the energy transfer efficiency in our system using, as a positive control, the energy transfer from MAbs Cy3-W6/32 and Cy5-MCA1115 to two different epitopes on MHC-I molecules (Table 1), which showed that the maximum energy transfer efficiency (E) was 38% ± 1.5% (under idealized FRET conditions a maximum E of 40.96% is achieved). A negative control involving an irrelevant surface receptor such as the transferrin receptor and GRP78 was performed by testing the energy transfer between Cy3-M073401 specific for the transferrin receptor and Cy5-GRP78-specific Fab; the resulting E was 1% ± 1.5%.
TABLE 1.
Energy transfer efficiency values for donor-acceptor pairsa
| Donor (Cy3) | Acceptor (Cy5)/cell line | Mean E ± SD (%) |
|---|---|---|
| MHC-I | β2-microglobulin/GMK | 38 ± 1.5 |
| Transferrin receptor | GRP78/GMK | 1.0 ± 1.5 |
| GRP78 | CAV-9/GMK | 25 ± 0.5 |
| CAV-9 | GRP78/GMK | 23 ± 1.0 |
| MHC-I | GRP78/GMK | 21 ± 0.8 |
| MHC-I | GRP78 (in the presence of CAV-9)/GMK | 31 ± 0.5 |
| MHC-I | GRP78/Daudi | 0.5 ± 0.5 |
| MHC-I | GRP78/Daudi-MHC+ | 25 ± 1.5 |
Energy transfer between different pairs was detected from the increase in donor fluorescence after acceptor photobleaching. Data are from a number of independent experiments.
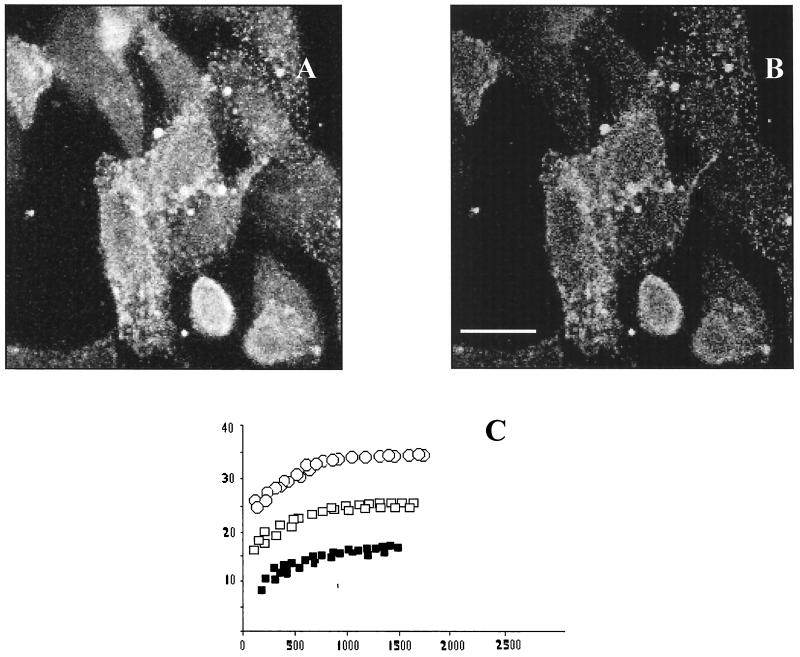
We measured FRET between GRP78 (using a Cy3-labeled GRP78 goat-specific Fab) and CAV-9 particles (using Cy5-labeled rabbit-specific CAV-9 Fab) on GMK cells. Large dequenching was observed once the Cy5 was photobleached (E = 25% ± 0.5%) (Fig. 4), suggesting that CAV-9 and GRP78 associate on the cell surface. We similarly examined FRET from the reverse direction. From the Cy3 rabbit-specific CAV-9 Fab and the Cy5-GRP78 goat-specific Fab, the energy efficiencies in both cases were approximately the same (Table 1).
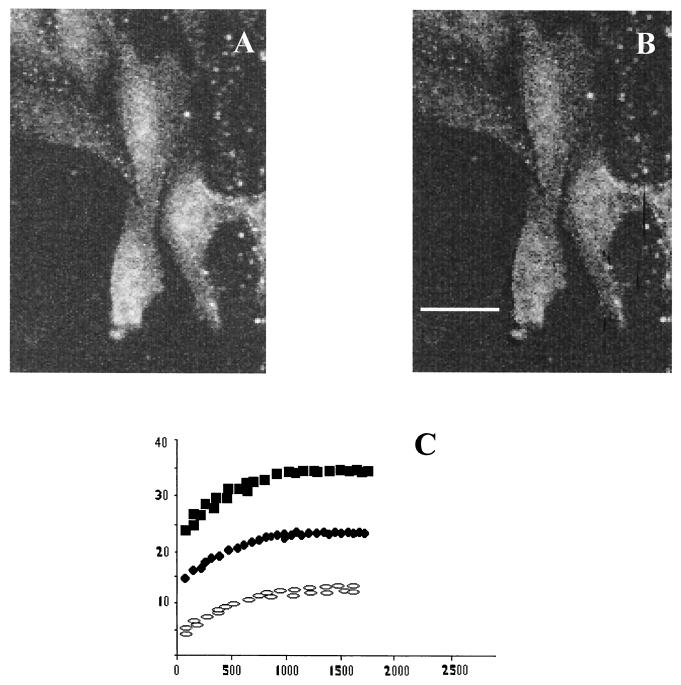
FIG. 4.
FRET measurements for CAV-9 and GRP78. Energy transfer between CAV-9 and GRP78 can be detected from the increase in donor fluorescence after acceptor photobleaching. (A) Donor (Cy3) image after acceptor photobleaching. (B) E image. (C) E as a function of the fluorescence at donor/acceptor antibody ratios of 1:1 (solid squares), 1:2 (open squares), and 1:4 (open circles). Scale bar, 10 μm.
The high values of energy transfer confirmed the mutual proximity of the virus with GRP78 molecules, thus verifying that GRP78 molecules and CAV-9 interact on the cell surface. Control experiments were performed with the Cy3-GRP78-specific Fab and the Cy5-rabbit-specific CAV-9 Fab without the presence of CAV-9 particles. The results showed that the energy transfer efficiency was 2% ± 0.5%, thus verifying that there was no antibody interaction.
To rule out the possibility that the observed FRET between GRP78 and CAV-9 was due to randomly distributed and not clustered molecules, we decided to test the theory of Kenworthy and Edidin (15) and measure the dependence of FRET on donor and acceptor surface density. Thus we varied the ratio of donors and acceptors used to label the proteins of interest (Fig. 4C). E was found to be independent of acceptor density, to be sensitive to the donor/acceptor ratio, and not to go to zero at low surface density, thus suggesting that the FRET values observed were due to clustered molecules and not random associations.
GRP78 and MHC-I interactions.
A previous study from our laboratory indicated that CAV-9, in addition to integrin αvβ3, utilized an MHC-I-associated protein (MAP-70) as a receptor molecule (31). These results were obtained on the basis of experiments involving solubilization of GMK cell membranes in the presence of mild detergents and immunoisolation by means of MAbs. This approach, however, suffers from inherit limitations due to the possible effects of detergents which may cause changes in the composition of the interacting proteins. In this study we identified MAP-70 as GRP78 and set out to confirm on GMK cells whether GRP78 and MHC-I molecules associate using the FRET methodology, which is a noninvasive technique that allows us to study receptor interactions on the cell membrane. High FRET values were obtained from the Cy3-W6/32 probe and the Cy5-GRP78-specific Fab (E = 21% ± 0.8%) (Fig. 5), thus showing that GRP78 and MHC-I molecules interact on the cell membrane. To rule out the possibility that the FRET observed was due to random distribution, we varied the ratio of donors and acceptors used to label the proteins of interest (Fig. 5C). E was found to be independent of acceptor density, to be sensitive to the donor/acceptor ratio, and not to go to zero at low surface density, thus suggesting that the FRET values observed were due to clustered molecules and not random associations.
FIG. 5.
FRET measurements for MHC-I and GRP78. Energy transfer between MHC-I and GRP78 can be detected from the increase in donor fluorescence after acceptor photobleaching. (A) Donor (Cy3) image after acceptor photobleaching. (B) E image. (C) E as a function of the fluorescence of donor/acceptor antibody ratios of 1:1 (open ellipse), 1:2 (filled circles), and 1:4 (filled squares). Scale bar, 10 μm.
Daudi cells, a B-lymphoma cell line that is deficient in the expression of β2-microglobulin and that thus lacks MHC-I molecules at the surface, were used as a negative control. The E value obtained from Cy3-W6/32- and Cy5-GRP78-specific Fab fragments (0.5% ± 0.5%) showed as expected no interaction. Daudi-MHC+ cells transfected with the β2-microglobulin gene and therefore expressing MHC-I molecules at the cell surface were also used (20). High FRET values were obtained from Cy3-W6/32- and Cy5-GRP78-specific Fab fragments (E = 25% ± 1.5%). This confirmed that MHC-I molecules and GRP78 interact at the cell surface (Table 1).
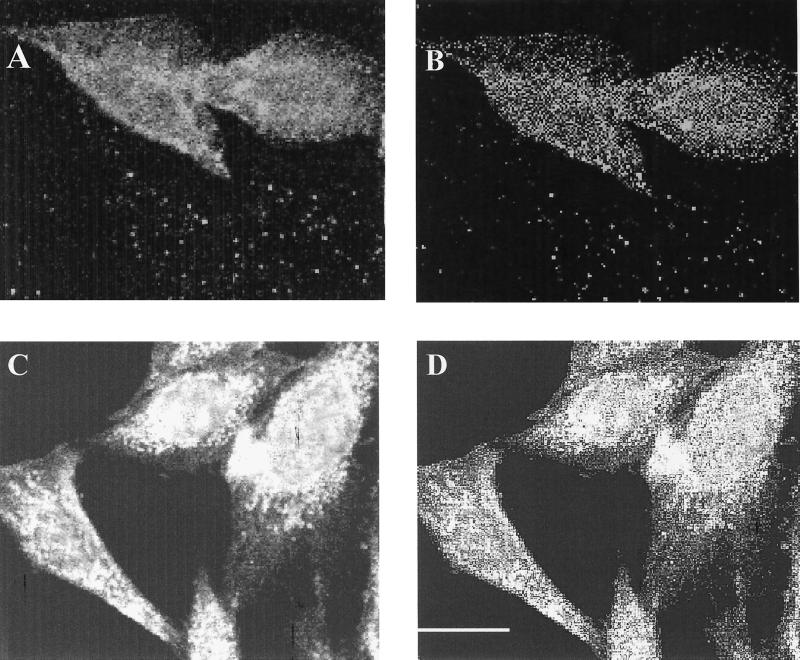
Although we verified that MHC-I and GRP78 associate at the cell surface, we also wanted to study MHC-I and GRP78 associations before and after CAV-9 infection to elucidate whether the virus, upon infection, cross-links these two molecules and thus promotes increased associations and clustering. Therefore we performed FRET experiments with MHC-I and GRP78 in the presence and absence of CAV-9 particles. The E value for GMK cells from Cy3-W6/32- and Cy5-GRP78-specific Fab fragments was 21% ± 0.8% (Fig. 6A and B), whereas after the addition of virus particles E increased to 31% ± 0.5% (Fig. 6C and D), thus confirming that the virus promotes MHC-I and GRP78 clustering.
FIG. 6.
MHC-I and GRP78 interactions in the absence and presence of virus. Measurement of energy transfer on GMK cells between MHC-I and GRP78 in the absence (A and B) and presence (C and D) of CAV-9 particles. The energy transfer can be detected from the increase in donor fluorescence after acceptor photobleaching. (A and B) Measurements in the absence of CAV-9, where the donor (Cy3) image after acceptor photobleaching (A) and the E image (B) are shown. (C and D) Measurements in the presence of CAV-9, where the donor (Cy3) image after acceptor photobleaching (C) and the E image (D) are shown. Scale bar, 10 μm.
MHC-I involvement in the CAV-9 internalization process.
Since we had already established that CAV-9 utilized GRP78 in its attachment process and that GRP78 was physically associated with MHC-I, we set out to investigate whether MHC-I was also involved in the CAV-9 infectious cycle.
To verify the relationship between MHC-I molecules and CAV-9, we preincubated GMK cells with different well-characterized MAbs to MHC-I (W6/32) and to β2-microglobulin (MCA1115), a combination of these MAbs, and isotype control antibodies. The productive infection could be assayed by plaque assays. Our results showed that the MAbs inhibited virus infection by 85%, whereas the isotype control antibodies showed no inhibition (Fig. 7). A flow-cytometric study in the presence of W6/32, MCA1115, and isotype control antibodies showed no inhibition of the virus binding (Fig. 7E).
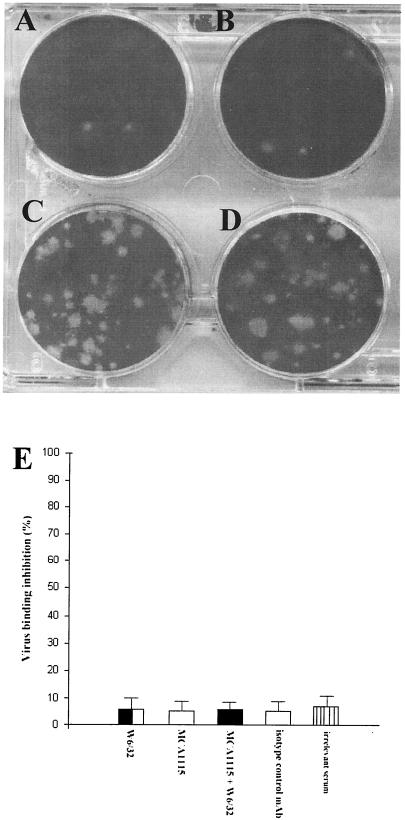
FIG. 7.
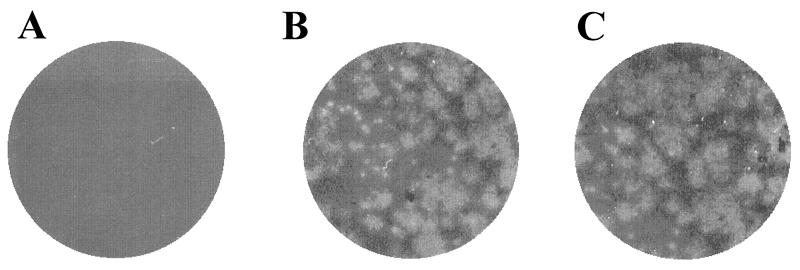
Role of MHC-I in the CAV-9 infectious cycle. Experiments testing the inhibition of CAV-9 infection on GMK cells were performed. The following were used: MCA1115 (β2-microglobulin-specific MAb) (A), W6/32 (MHC-I-specific MAb) (B), an isotype control MAb (C), and infection with CAV-9 particles (D). These plates are representative of a number of independent experiments. CAV-9 binding to GMK cells in the presence of MCA1115, W6/32, an isotype control MAb, an irrelevant serum, and also a combination of W6/32 and MCA1115 was tested by flow-cytometric analysis. Binding was assayed by incubation with CAV-9 rabbit-specific serum and developed using an appropriate dilution of FITC-conjugated swine anti-rabbit Ig. (E) Percentage inhibition of CAV-9 binding to GMK cells. Error bars are calculated from standard deviations over a number of independent experiments.
These results were in good agreement with previous studies, which have shown that CAV-9 and MHC-I do not physically associate and that MAbs specific for MHC-I inhibit virus infectivity but do not inhibit virus binding. Therefore the MHC-I function has been proposed to be involved with a postattachment stage of the virus infectious cycle (33).
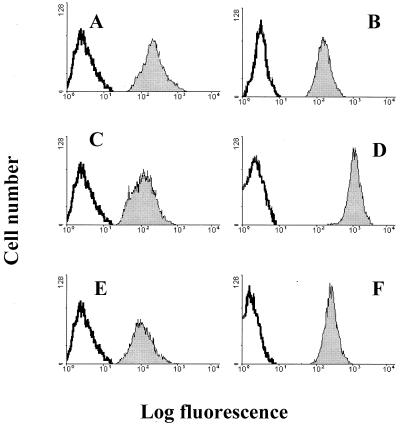
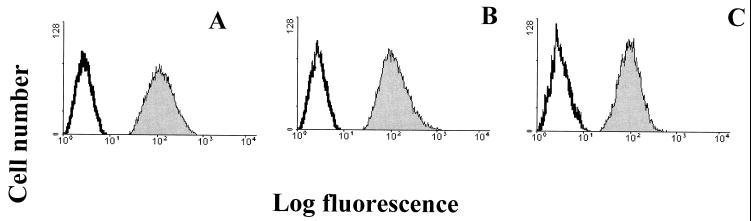
To determine whether indeed MHC-I was used in a postattachment step of the virus infectious cycle, possibly involved in the entry of the virus into the cell, Daudi cells that are deficient in the expression of β2-microglobulin and that thus lack MHC-I expression and Daudi-MHC+ cells transfected with the β2-microglobulin gene and therefore expressing MHC-I molecules at the cell surface were used in this study (20). Both Daudi and Daudi-MHC+ cells, when tested by flow-cytometric analysis, revealed the presence of integrin αvβ3 and GRP78 cell surface expression (Fig. 8).
FIG. 8.
Expression of GRP78 and αvβ3 on Daudi and GMK cells. Shown is flow-cytometric analysis of integrin αvβ3 and GRP78 expression on GMK, Daudi, and Daudi-MHC+ cells. Control GMK, Daudi, and Daudi-MHC+ cells were incubated with FITC-conjugated rabbit anti-mouse IgG. GMK cells were incubated with integrin αvβ3-specific MAb MAB1976 (A) and GRP78-specific serum (B). Daudi cells were incubated with integrin αvβ3-specific MAb MAB1976 (C) and GRP78-specific serum (D). Daudi-MHC+ cells were incubated with integrin αvβ3-specific MAb MAB1976 (E) and GRP78-specific serum (F). The histograms display relative cell numbers as a function of relative fluorescence intensities.
Infectivity assays utilizing Daudi and Daudi-MHC+ cells showed that, although CAV-9 could not infect Daudi cells, the Daudi-MHC+ cells were permissive to virus infection. To exclude the possibility that Daudi cells were infected by the virus, CAV-9 particles (107 PFU) were added to Daudi cells (106 cells); Daudi-MHC+ cells (106 cells) and GMK cells (106 cells) were used as a control. These cells were incubated for different time periods, and, for each time period, the cells were frozen and thawed to release CAV-9 particles that may have been produced. The cell lysate was added to GMK cells, which were then assayed for the presence of virus by plaque formation. The data showed no plaque formation on GMK cells when Daudi cell lysate had been added. In contrast, plaques formed on GMK cells when Daudi-MHC+ lysate or GMK lysate was added (Fig. 9).
FIG. 9.
Infectivity assay. Shown are the results of plaque assay of GMK cells when Daudi cell lysate (A), Daudi-MHC+ (B), and GMK lysate (C) were added.
To determine whether the lack of infectivity that we had observed was due to lack of virus binding or internalization, we assayed for CAV-9 binding by flow cytometry. Daudi and Daudi-MHC+ cells, as well as GMK cells, which were used as a control, were utilized in this study. Our results showed that CAV-9 particles exhibited high-efficiency binding to Daudi, Daudi-MHC+, and GMK cells (Fig. 10).
FIG. 10.
nCAV-9 binding on MHC+ and MHC− cells. Flow-cytometric analysis of CAV-9 binding to cells. GMK cells, Daudi cells, and Daudi-MHC+ cells were incubated without CAV-9 particles, as a control. CAV-9 binding on GMK cells (A), Daudi cells (B), and Daudi-MHC+ cells (C) was then assayed. Virus binding was assayed by incubation with CAV-9-specific serum and detected using an appropriate dilution of FITC-conjugated swine anti-rabbit Ig. The histograms display relative cell numbers as a function of relative fluorescence intensities.
This demonstrates that, although CAV-9 binding is supported on all these cells since they express integrin αvβ3 and also GRP78 molecules, the failure of Daudi cells to support viral infection resulted from a deficiency in the cell entry process caused by the absence of MHC-I expression on the cell surface.
DISCUSSION
Over the years cell infection by virus seems to have evolved into a multistep process, where some viruses initially attach to the cell membrane via one of the host cell’s surface molecules and subsequently utilize a different cell surface molecule for internalization.
Entry of viruses into cells is a complex, multistep process, and for several viruses cell attachment and internalization have been shown to be distinct steps requiring different cell surface receptors. In addition, it is possible that viruses can enter cells by alternative pathways, again requiring different molecules.
An example of viruses using different cell surface receptors is adeno-associated virus 2, which binds to heparan sulfate but which also requires human fibroblast growth factor receptor 1 for efficient cell entry (19). Also CAV-21 binds to CD55 but requires intracellular adhesion molecule 1 (ICAM-1) for cell entry (25).
In this work we have studied the infectious process of CAV-9 attachment and cell entry. CAV-9 is a virus genetically related to coxsackie B viruses and associated with autoimmune IDDM (22, 23). Elucidation of the mechanisms of CAV-9 infectious process could give us an understanding of the various stages of the pathogenesis of IDDM. Using two-dimensional electrophoresis and peptide mass fingerprinting, we have identified MAP-70 as GRP78, a glucose-regulated protein belonging to the HSP70 family of proteins, as a coreceptor molecule for CAV-9. HSPs have been implicated as chaperones in antigen presentation and have been shown to deliver viral peptides to MHC-I molecules in the endoplasmic reticulum (ER) lumen (5). Although they are mostly intracellular, their existence on the cell surface has also been suggested (12). Several studies have suggested that certain molecular chaperones, such as Hsp60 (14, 26, 27), Hsp70 (11, 18, 29, 30, 34), and Hsp90 (28), exist on the cell surface. Their function on the cell surface is still unknown, but several HSPs have shown the ability to carry tumor antigens and generate protective immunity. HSPs have also been shown to carry viral peptides (5), minor histocompatibility, and model antigens (1) and have even been implicated in allograft rejection (4).
Using FRET in this study we provide evidence that MHC-I molecules associate on the cell surface with GRP78. Furthermore, we showed that CAV-9, in addition to integrin αvβ3, uses GRP78 as another receptor molecule. When GMK cells were pretreated with specific GRP78 polyclonal serum, they inhibited CAV-9 infection by 50%, whereas, when a combination of GRP78 polyclonal serum and the integrin αvβ3-specific MAb was used, CAV-9 infectivity was completely abolished, suggesting that GRP78 and integrin αvβ3 are the main CAV-9 attachment receptors. In addition, cell entry and internalization were shown to be distinct steps from the CAV-9 infectious cycle. GRP78 and integrin αvβ3 were found to be responsible for virus binding, whereas MHC-I molecules were found to be crucial for virus cell entry, as shown by the failure of MHC-I-negative cells to support CAV-9 infection.
In view of our findings we suggest a model wherein CAV-9 utilizes integrin αvβ3 and GRP78 as receptor molecules for attachment. Once the virus has attached to the cell surface it utilizes MHC-I, which is associated with GRP78, as means of internalization. Interestingly, earlier studies have shown that cross-linking of MHC-I molecules using antibodies induces internalization via small uncoated plasma membrane invaginations (13, 17). We believe that CAV-9 has evolved to take advantage of this property of MHC-I. By attaching to GRP78, an MHC-I-associated protein, the virus cross-links GRP78 and class I, inducing internalization and thus gaining access to the cell’s machinery.
The fact that in this study we have implicated the heat shock family of proteins as viral receptors forces us to take a closer look at this multitalented family of molecules. Their intrinsic association with MHC and antigen presentation, even the fact that they are encoded from within the MHC loci, suggests that they are somehow associated with antigen presentation. The fact that they can elicit CD8 T cells possibly suggests that they are remnants of an ancient system of host defense, a progenitor of MHC-I, that viruses have evolved over millions of years to evade and utilize. It would be interesting to take a closer look at the involvement of HSPs in the attachment and internalization processes of other viruses that are known to utilize MHC-I.
Acknowledgments
We thank Dick Winant (Stanford PAN Facility) for help with mass spectroscopy.
This work was supported by Diabetes United Kingdom.
REFERENCES
- 1.Arnold, D. F. S., H. Rammensee, and H. Schild. 1995. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunisation with the heat shock protein gp96. J. Exp. Med. 162:3757–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastiaens, P. I., and T. M. Jovin. 1996. Microspectroscopic imaging tracks the intracellular processing of a signal transduction protein: fluorescent-labeled protein kinase C beta 1. Proc. Natl. Acad. Sci. USA 93:8407–8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastiaens, P. I., I. V. Majoul, P. J. Verveer, H. D. Soling, and T. M. Jovin. 1996. Imaging the intracellular trafficking and state of the AB5 quaternary structure of cholera toxin. EMBO 15:4246–4253. [PMC free article] [PubMed] [Google Scholar]
- 4.Birk, O. S., S. L. Gur, D. Elias, R. Margalit, F. Mor, P. Carmi, J. Bockova, D. M. Altmann, and I. R. Cohen. 1999. The 60-kDa heat shock protein modulates allograft rejection. Proc. Natl. Acad. Sci. USA 96:5159–5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciupitu, A. M., M. Petersson, and C. L. O’Donnell. 1998. Immunization with a lymphocytic choriomeningitis virus peptide mixed with heat shock protein 70 results in protective antiviral immunity and specific cytotoxic T cells. J. Exp. Med. 187:685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clegg, R. M. 1995. Fluorescence resonance energy transfer. Curr. Opin. Biotechnol. 6:103–110. [DOI] [PubMed] [Google Scholar]
- 7.Clegg, R. M. 1996. Fluorescence resonance energy transfer (FRET), p.179–252. In X. F. Wang and B. Herman (ed.), Fluorescence imaging spectroscopy and microscopy. John Wiley, New York, N.Y.
- 8.Gething, M. J. 1999. Role and regulation of the ER chaperone BiP. Semin. Cell Dev. Biol. 10:465–472. [DOI] [PubMed] [Google Scholar]
- 9.Gething, M. J., and J. Sambrook. 1992. Protein folding in the cell. Nature 355:33–35. [DOI] [PubMed] [Google Scholar]
- 10.Grist, N. R., and D. Reid. 1988. General pathogenicity and epidemiology, p.221–239. In M. Berdinelli and H. Friedman (ed.), Coxsackieviruses, a general update. Plenum Press, New York, N.Y.
- 11.Guzhova, I. V., A. C. V. Arnholdt, Z. A. Darieva, A. V. Kinev, E. B. Lasunskaia, K. Nilsson, V. M. Bozhkov, A. P. Voronin, and B. A. Margulis. 1998. Effects of exogenous stress protein 70 on the functional properties of human promonocytes through binding to cell surface and internalisation. Cell Stress Chaperones 3:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirai, I., N. Sato, W. Qi, S. Ohtani, T. Torigoe, and K. Kikuchi. 1998. Localization of pNT22 70 kDa heat shock cognate-like protein in the plasma membrane. Cell Struct. Funct. 23:153–158. [DOI] [PubMed] [Google Scholar]
- 13.Huet, C., J. F. Ash, and S. J. Singer. 1980. The antibody induced clustering and endocytosis of HLA antigens on cultured fibroblasts. Cell 21:429–432. [DOI] [PubMed] [Google Scholar]
- 14.Kaur, I., S. D. Voss, R. S. Gupta, K. Schell, P. Fisch, and P. M. Sondel. 1993. Human peripheral γδ T cells recognize hsp60 molecules on Daudi Burkitt’s lymphoma cells. J. Immunol. 150:475–478. [PubMed] [Google Scholar]
- 15.Kenworthy, A. K., and M. Edidin. 1998. Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of <100 Å using imaging fluorescence resonance energy transfer. J. Cell Biol. 142:69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenworthy, A. K., and M. Edidin. 1998. Imaging fluorescence resonance energy transfer as probe of membrane organisation and molecular associations of GPI-anchored proteins, p.37–49. In M. H. Gelb (ed.), Methods in molecular biology. Humana Press Inc., Totowa, N.J. [DOI] [PubMed]
- 17.Machy, P., A. Truneh, D. Gennaro, and S. Hoffstein. 1987. Endocytosis and de novo expression of MHC class I: kinetics and ultrastructural studies. Eur. J. Cell Biol. 45:126–131. [PubMed] [Google Scholar]
- 18.Multhoff, G., C. Otzler, L. Jennen, J. Schmidt, J. Ellwart, and R. Issels. 1997. Heat shock protein 72 on tumour cells. J. Immunol. 158:4341–4350. [PubMed] [Google Scholar]
- 19.Qing, K., C. Mah, J. Hansen, S. Zhou, V. Dwarki, and A. Srivastava. 1999. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat. Med 1:71–77. [DOI] [PubMed] [Google Scholar]
- 20.Quillet, A., F. Presse, C. Marchiol-Fournigault, A. Harel-Bellan, M. Benbunan, H. Ploegh, and D. Fradelizi. 1988. Increased resistance to non-MHC-restricted cytotoxicity related to HLA A, B expression: direct demonstration using b2-microglobulin-transfected Daudi cells. J. Immunol. 141:17–20. [PubMed] [Google Scholar]
- 21.Roivainen, M., L. Piirainen, T. Hovi, I. Virtanen, T. Riikonen, J. Heino, and T. Hyypia. 1994. Entry of coxsackievirus A9 into host cells: specific interactions with avb3 integrin, the vitronectin receptor. Virology 203:307–312. [DOI] [PubMed] [Google Scholar]
- 22.Roivanen, M., M. Knip, H. Hyoty, P. Kulmala, M. Hiltunen, P. Vahasalo, T. Hovi, and H. K. Akerblo. 1998. Several different enterovirus serotypes can be associated with prediabetic autoimmune episodes and onset of overt IDDM. Childhood Diabetes in Finland (DiMe) study group. J. Med. Virol. 56:74–78. [DOI] [PubMed] [Google Scholar]
- 23.Roivanen, M., S. Rasilainen, P. Ylipaasto, R. Nissinen, J. Ustinov, L. Bouwens, D. L. Eizirik, T. Hovi, and T. Otonkoski. 2000. Mechanisms of coxsackievirus-induced damage to human pancreatic b-cells. J. Clin. Endocrinol. Metab. 85:432–440. [DOI] [PubMed] [Google Scholar]
- 24.Schneider-Schaulies, J. 2000. Cellular receptors for viruses: links to tropism and pathogenesis. J. Gen. Virol. 81:1413–1429. [DOI] [PubMed] [Google Scholar]
- 25.Shaffren, D. R., R. D. Barry, D. J. Dorahy, R. A. Ingham, G. F. Burns, and R. D. Barry. 1997. Coxsackievirus A21 binds to decay-accelarating factor but requires intracellular adhesion molecule 1 for cell entry. J. Virol. 71:4736–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soltys, B. J., and R. S. Gupta. 1996. Immunoelectron microscopic localization of the 60 kDa heat shock chaperonin protein (Hsp60) in mammalian cells. Exp. Cell Res. 222:16–27. [DOI] [PubMed] [Google Scholar]
- 27.Soltys, B. J., and R. S. Gupta. 1997. Cell surface localisation of the 60 kDa heat shock chaperonin protein (hsp60) in mammalian cells. Cell Biol. Int. 21:315–320. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava, P. K., H. Udono, N. E. Blachere, and Z. Li. 1994. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics 39:93–98. [DOI] [PubMed] [Google Scholar]
- 29.Takashima, S., N. Sato, A. Kishi, Y. Tamura, I. Hirai, T. Torigoe, A. Yagihashi, S. S. S. Takahashi, R. Kudo, and K. Kikuchi. 1996. Involvement of peptide antigens in the cytotoxicity between 70 kDa heat shock cognate protein-like molecule and CD3+, CD4−,CD8−, TCRab− killer T-cells. J. Immunol. 157:3391–3395. [PubMed] [Google Scholar]
- 30.Tamura, Y., N. Tsuboi, N. Sato, and K. Kikuchi. 1993. 70 kDa heat shock cognate protein is a transformation-associated antigen and possible target for the host’s anti-tumour immunity. J. Immunol. 151:5516–5524. [PubMed] [Google Scholar]
- 31.Triantafilou, M., K. Triantafilou, and K. M. Wilson. 2000. A 70 kDa MHC-class-I associated protein (MAP-70), identified as a receptor molecule for coxsackievirus A9 cell attachment. Hum. Immunol. 61:867–878. [DOI] [PubMed] [Google Scholar]
- 32.Triantafilou, M., K. Triantafilou, K. M. Wilson, Y. Takada, and N. Fernandez. 2000. High affinity interactions of coxsackievirus A9 with integrin avb3 (CD51/61) require the CYDMKTTC sequence of b3 but do not require the RGD sequence of the CAV-9 VP1 protein. Hum. Immunol. 61:453–459. [DOI] [PubMed] [Google Scholar]
- 33.Triantafilou, M., K. Triantafilou, K. M. Wilson, Y. Takada, and N. Fernandez. 1999. Involvement of b2-microglobulin and integrin avb3 molecules in the coxsackievirus A9 infectious cycle. J. Gen. Virol. 80:2591–2600. [DOI] [PubMed] [Google Scholar]
- 34.Tsuboi, N., M. Ishikawa, Y. Tamura, S. Takayama, H. Tobioka, A. Matsuura, K. Hirayoshi, K. Nagata, N. Sato, and K. Kikuchi. 1994. Monoclonal antibody specifically reacting against 73-kilodalton heat shock protein: possible expression on mammalian cell surface. Hybridoma 48:2798–2804. [DOI] [PubMed] [Google Scholar]
- 35.Wu, P., and L. Brand. 1994. Resonance energy transfer: methods and applications. Anal. Biochem. 218:1–13. [DOI] [PubMed] [Google Scholar]