Abstract
Despite the success of influenza virus vaccines in reducing severe illness, their efficacy is suboptimal. We describe here the immunogenicity and protective capacity of replication-incompetent influenza virus-like particles (VLPs) which were generated entirely from cDNAs and lacked either the entire NS gene (encoding both the NS1 and NS2 protein) or only the NS2 gene. In mammalian cells infected with NS gene-deficient VLPs, the nucleoprotein, but not other viral proteins including hemagglutinin (HA) and neuraminidase (NA), was detected. In contrast, cells infected with VLPs expressing NS1 but not NS2 (NS2 knockout) expressed multiple viral proteins, including HA and NA. When challenged with lethal doses of an antigenically homologous mouse-adapted influenza virus, 94% of mice vaccinated with the NS2-knockout VLPs survived, compared with less than 10% of those given the NS-deficient VLPs. These results demonstrate the potential of replication-incompetent NS2-knockout VLPs as novel influenza vaccines and perhaps also as vectors to express genes from entirely unrelated pathogens.
Influenza A virus causes appreciable morbidity and mortality in humans and domestic animals, resulting in large economic losses worldwide (14). The current method of immunization against influenza is parenteral administration of inactivated influenza virus vaccines. Although associated with a very low incidence of adverse reactions in healthy recipients, such vaccines do not efficiently elicit mucosal immunity or a cytotoxic T-cell response. They exhibit a 70 to 90% efficacy in reducing the incidence of clinical illness but fail to prevent influenza virus infection, warranting efforts to develop alternative vaccines.
Cold-adapted live attenuated vaccines have shown considerable promise in ongoing clinical trials, especially in young children, who are poor responders to inactivated vaccines due to the lack of immune memory of influenza virus (1). However, live vaccines have not consistently proved more efficacious than inactivated vaccines in adults (5), and the limited number of amino acid changes in vaccine strains has led to concern over the emergence of virulent revertants (10), although the phenotype of the cold-adapted vaccine is highly stable in clinical trials (2).
Recently, we (17) and others (8) established a system for generating infectious influenza virus entirely from cDNAs. Transfection of cells with plasmids containing cDNAs encoding all eight viral RNAs (vRNAs) of A/WSN/33 (H1N1) virus, controlled by RNA polymerase I promoter and terminator sequences, results in vRNA synthesis by cellular RNA polymerase I. Cotransfection of cells with plasmids for the synthesis of all viral structural proteins yields >107 infectious viruses per ml of supernatant (17). We also established a system for generating virus-like particles (VLPs) from plasmids that express all nine structural proteins and virus-like RNAs (18). These new capabilities have allowed us to consider the production of influenza virus vaccines that would elicit protective immunity without giving rise to infectious progeny. Here we describe replication-incompetent VLPs lacking their NS2 genes that are able to infect mammalian cells and protect mice against challenge with lethal doses of antigenically homologous influenza virus.
MATERIALS AND METHODS
Cells and viruses.
293T human embryonic kidney cells (a derivative of the 293 line into which the gene for simian virus 40 T antigen was inserted [4]) and Madin-Darby canine kidney (MDCK) cells were maintained in Dulbecco’s modified Eagle medium supplemented with 10% fetal calf serum and in minimum essential medium (MEM) containing 5% newborn calf serum, respectively. All cells were maintained at 37°C in 5% CO2. Influenza A/WSN/33 (H1N1) (WSN) virus was propagated in 10-day-old embryonated chicken eggs.
Plasmids.
All genes of the A/WSN/33 virus containing BsmBI sites were cloned into the BsmBI sites of the pHH21 vector, which contains the human RNA polymerase I promoter and the mouse RNA polymerase I terminator, separated by BsmBI sites, resulting in pPolIPA, pPolIPB1, pPolIPB2, pPolINP, pPolIHA, pPolINA, pPolIM, and pPolINS (17). The NS mutant, pPolINSΔSplice, was constructed as described earlier (16). Plasmids derived from pHH21 for the expression of vRNA are referred to as PolI constructs in this report. The plasmids for the expression of the hemagglutinin (HA) (pEWSN-HA), nucleoprotein (NP) (pCAGGS-WSN-NP0/14), neuraminidase (NA) (pCAGGS-WNA15), and M1 (pCAGGS-WSN-M1-2/1) proteins of WSN virus and the M2 (pEP24c), NS2 (pCANS2), PB1 (pcDNA774), PB2 (pcDNA762), and PA (pcDNA787) proteins of A/Puerto Rico/8/34 (H1N1) virus are described in a previous report (17).
Generation of replication-incompetent influenza VLPs.
For generation of NS gene-deficient VLPs, all PolI constructs for eight RNA segments, excluding pPolINS, and nine protein expression constructs for structural proteins were mixed with transfection reagent (2 μl of Trans IT LT-1 [Panvera, Madison, Wis.] per μg of DNA), incubated at room temperature for 15 min, and added to 106 293T cells. Six hours later, the DNA transfection reagent mixture was replaced by Opti-MEM (Life Technologies, Rockville, Md.) containing 0.3% bovine serum albumin and 0.01% fetal calf serum. Forty-eight hours later, VLPs in the supernatant were harvested.
Immunostaining assay.
Sixteen hours after infection with influenza VLPs, cells were washed twice with phosphate-buffered saline (PBS) and fixed with 3.7% formaldehyde (in PBS) for 20 min at room temperature. Next, they were treated with 0.1% Triton X-100 and processed as described earlier (15). To examine the efficiency of VLP generation, 106 cells were infected with 0.1 ml of the culture supernatant of 293T cells and the number of NP-positive cells as detected by the immunostaining assay was determined at 16 h postinfection.
Immunization and protection tests.
Formalin-inactivated virus was prepared by first propagating WSN virus in MDCK cells. The virus-containing culture supernatant was then treated with 0.1% formalin at 4°C for a week. Inactivation of the virus was confirmed by inoculating the treated material into MDCK cells.
BALB/c mice (4-week-old females) were intranasally immunized with 50 or 100 μl of 16 hemagglutinating units (HAU) of formalin-inactivated virus or the replication-incompetent VLPs per ml three times at 3-week intervals. On the ninth week, four mice were sacrificed to obtain sera, trachea-lung washes, and nasal washes. One or three months after the last vaccination, immunized mice were challenged intranasally, under anesthesia, with 10 or 100 50% lethal doses (LD50) of the wild-type WSN virus. For determination of virus titers in lungs, lungs were harvested at day 3 and were homogenized and titrated on MDCK cells. The remaining animals were observed for clinical signs and symptoms of infection for 14 days after challenge.
Detection of virus-specific antibody.
Serum samples were examined for antibody by an enzyme-linked immunosorbent assay (ELISA) (11). In this assay, the wells were coated with purified WSN virus after treatment with 0.05 M Tris-HCl (pH 7.8) containing 0.5% Triton X-100 and 0.6 M KCl at room temperature and diluted in PBS. After incubation of virus-coated plates with test serum samples, bound antibody was detected with rabbit anti-mouse immunoglobulin A (IgA) (Kirkegaard & Perry Laboratories Inc., Gaithersburg, Md.) and goat anti-mouse IgG (Boehringer-Mannheim, Mannheim, Germany) conjugated to horseradish peroxidase.
RESULTS
Generation of replication-incompetent VLPs.
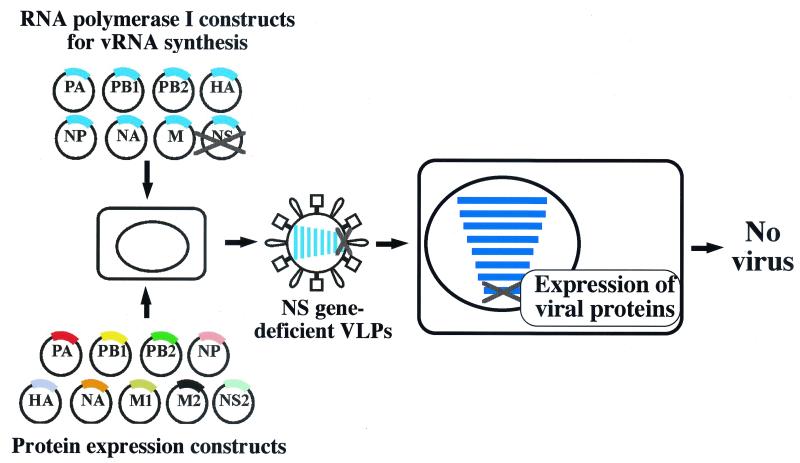
We initially chose to delete the entire NS gene as a means to produce VLPs that would infect cells and express immunogenic proteins without generating progeny particles in infected cells. NS2 protein, which is encoded by a spliced mRNA (12), is essential for influenza virus replication (19), and the absence of this protein was expected to inhibit production of infectious progeny (16). We therefore transfected 293T cells with nine protein expression plasmids as well as with those for the production of viral RNA segments that encoded all genes of the influenza WSN virus, excluding the NS gene (Fig. 1). Forty-eight hours posttransfection, the supernatants of 293T cell cultures were harvested and used to infect MDCK cells. None of the VLP-infected cells produced progeny virus. Furthermore, we confirmed the lack of production of replication-competent viruses by passaging the replication-incompetent VLPs three times in MDCK cells in three separate experiments (data not shown).
FIG. 1.
Schematic diagram of a system for generating NS gene-deficient VLPs. 293T cells were transfected with plasmids expressing all viral structural proteins and seven RNA polymerase I plasmids for vRNA synthesis, omitting pPolI-WSN-NS. Forty-eight hours after transfection, supernatants derived from transfected cells were used to infect MDCK cells. Progeny virus was not produced from the VLP-infected cells.
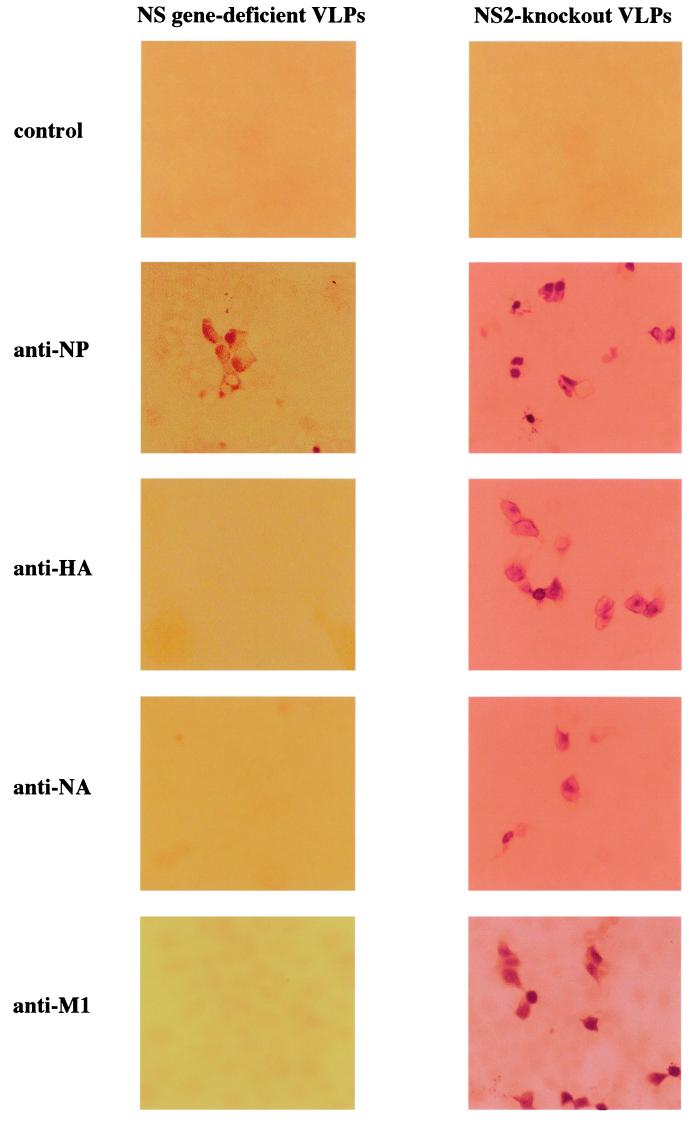
The efficiency of VLP generation and the extent of viral protein expression in VLP-infected cells were assessed by fixing cells at 16 h postinfection and examining them for expression of NP, HA, NA, and M1. The titer of the NS gene-deficient VLPs in the culture supernatant of 293T cells was approximately 104 infectious VLPs/ml, measured by counting NP-expressing cells. By immunostaining, the NP protein was readily detected in the infected cells, but HA, NA, and M1 were not (Fig. 2, left panels).
FIG. 2.
Expression of viral proteins in cells infected with VLPs. MDCK cells were infected with NS gene-deficient VLPs or NS2-knockout VLPs, and immunostaining assays were performed at 16 h postinfection. VLP-infected MDCK cells, processed as others with the exception of primary antibody, were used as a control.
Since NS1 stimulates the translation of viral mRNA (3, 6, 20), we reasoned that its absence might inhibit normal expression of viral proteins. Thus, to produce influenza VLPs expressing immunogenic viral proteins in cells, we next attempted to generate VLPs encoding NS1, but not NS2, which is encoded by spliced mRNA derived from the NS segment (12). To this end, we transfected 293T cells with pPolI-WSN-NSΔSplice (Fig. 3), which contained only the NS1 coding region, and the remaining RNA polymerase I constructs and protein expression plasmids. Although no progeny virus was produced from cells infected with the NS2-knockout VLPs (data not shown), they expressed the four viral proteins tested (i.e., NP, HA, NA, and M1) (Fig. 2, right panels). At 48 h posttransfection, the titer of these VLPs, measured by counting NP-positive cells, was approximately 105 infectious VLPs/ml. These results establish the requirement for NS1 protein in translational regulation of viral mRNAs and its importance in viral replication.
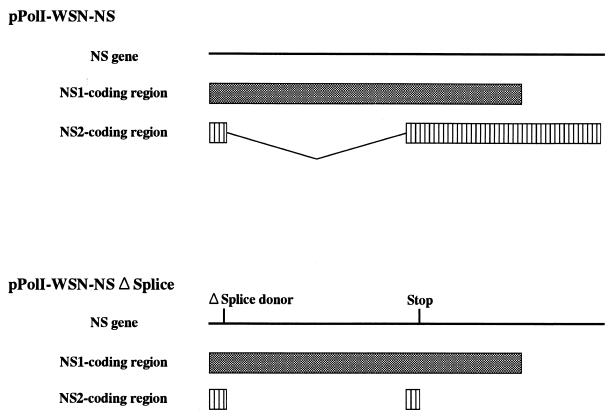
FIG. 3.
Schematic representation of RNA polymerase I constructs for NS vRNA synthesis. Solid bars represent NS vRNAs synthesized by RNA polymerase I. Translation products are shown as boxes (shaded boxes, NS1; hatched boxes, NS2). pPolI-WSN-NS, PolI plasmid encoding the wild-type NS gene; pPolI-WSN-NSΔSplice, PolI plasmid encoding a mutant NS gene. “ΔSplice donor” and “Stop” indicate the splice donor sequence (G-to-A alteration at position 56) and introduction of a stop signal at codon 17 (C to A at position 548) in the NS2 reading frame.
Antibody responses of mice immunized with NS gene-deficient or NS2-knockout VLPs.
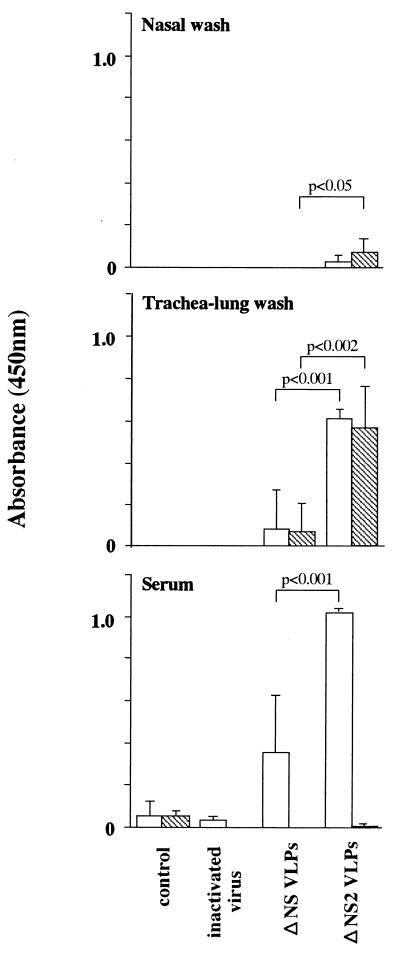
To test the efficacy of replication-incompetent particles as a vaccine, we first performed a pilot experiment by intranasally inoculating mice with 16 HAU of the NS2-knockout VLPs once or twice at 2-week intervals. When immunized mice were challenged with 100 LD50 of the wild-type WSN virus 2 weeks after the last vaccination, their survival rates were 25 or 75%, respectively. Based on these results, we intranasally inoculated mice with 16 HAU of the NS gene-deficient or NS2-knockout VLPs three times at 3-week intervals. IgG and IgA were measured in sera, trachea-lung washes, and nasal washes of immunized mice with an ELISA (Fig. 4). Both IgG and IgA production in nasal and trachea-lung washes was significantly higher in mice immunized with NS2-knockout VLPs than with NS gene-deficient VLPs. The IgA response was negligible in serum, regardless of the type of VLPs used for immunization, but IgG production was clearly higher in mice inoculated with the NS2-knockout VLPs. None of the sera from immunized mice had detectable hemagglutination inhibition antibodies. Neither the culture supernatant lacking VLPs (control) nor the inactivated WSN virus elicited a significant antibody response. Thus, antibody responses were induced more efficiently with the NS2-knockout VLPs that produced multiple proteins.
FIG. 4.
Virus-specific antibodies in samples from vaccinated mice. Samples (sera, trachea-lung washes, and nasal washes) from six mice from each group were obtained before virus challenge. IgG and IgA in the samples of individual mice were detected by ELISA, as described in Materials and Methods. Results are expressed as the mean absorbances (± standard deviations) of undiluted samples (trachea-lung and nasal washes) or 1:16 diluted samples (sera). Differences between responses to NS gene-deficient and NS2-knockout VLPs were tested for statistical significance by Student’s t test.
Protective efficacy of replication-incompetent VLPs.
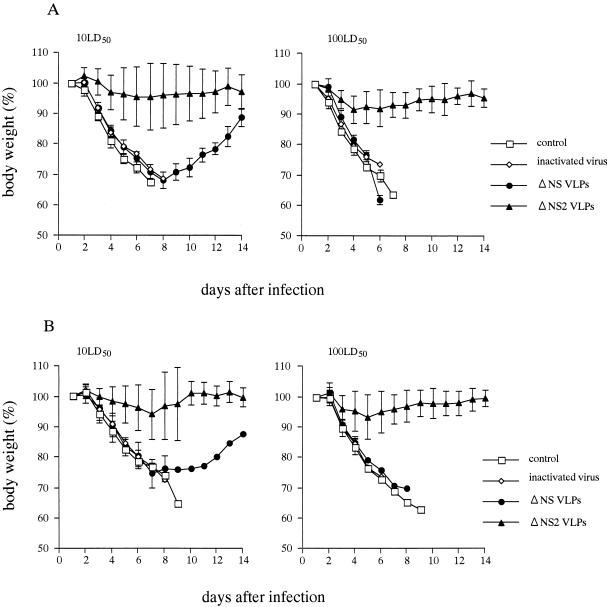
Mice immunized with either NS gene-deficient or NS2-knockout VLPs were challenged with 10 or 100 LD50 of the wild-type WSN virus 1 month after the last vaccination. In contrast to the fate of control mice and mice receiving inactivated virus or NS gene-deficient VLPs, those immunized with the NS2-knockout particles were protected against lethal challenge with WSN virus (Table 1). Eight of nine mice in the NS2-knockout group survived even when challenged 3 months after the last vaccination. Moreover, their body weights were not appreciably affected by virus challenge, in contrast to the other vaccination groups, whose weights decreased rapidly postchallenge (Fig. 5). We also determined the virus titers in the lungs of mice. Both control mice and mice immunized with inactivated virus or NS gene-deficient VLPs had more than 107 PFU in lung tissue after challenge with 10 or 100 LD50 of wild-type WSN virus. In contrast, mice immunized with NS2-knockout VLPs had >100-fold lower titers in lungs after challenge with the same doses (Table 1). We conclude that the NS2-knockout VLPs can effectively protect mice against lethal influenza virus infection.
TABLE 1.
Protection against lethal virus challenge in immunized micea
| Time of virus challenge after the last vaccination and immunogen | No. of survivors/no. testedb
|
Virus titer in lungs [log10(PFU/g)]
|
||
|---|---|---|---|---|
| 10 LD50 | 100 LD50 | 10 LD50 | 100 LD50 | |
| 1 month | ||||
| Control | 0/9 | 0/9 | 7.9 ± 0.4 | 7.7 ± 0.5 |
| Inactivated virus | 0/9 | 0/9 | 7.5 ± 0.1 | 7.2 ± 0.3 |
| NS gene-deficient VLPs | 2/9 | 0/9 | 7.8 ± 0.6 | 7.6 ± 0.5 |
| NS2-knockout VLPs | 9/9 | 9/9 | 5.1 ± 1.2c | 5.2 ± 1.0c |
| 3 months | ||||
| Control | 0/9 | 0/9 | 7.3 ± 0.2 | 7.0 ± 0.2 |
| Inactivated virus | 0/9 | 0/9 | 7.4 ± 0.2 | 7.1 ± 0.3 |
| NS gene-deficient VLPs | 1/9 | 0/9 | 7.1 ± 0.8 | 7.2 ± 0.3 |
| NS2-knockout VLPs | 8/9 | 8/9 | 3.7 ± 1.3d | 4.0 ± 1.1e |
Mice were challenged intranasally, under anesthesia, with 10 or 100 LD50 of the wild-type WSN virus at 1 or 3 months after the last vaccination. Virus titers in the lungs were determined 3 days after challenge.
Mice were monitored for 14 days after challenge.
Significantly different from the above three groups (P < 0.001) by Student’s t test.
Significantly different from the above three groups (P < 0.02) by Student’s t test.
Significantly different from the above three groups (P < 0.003) by Student’s t test.
FIG. 5.
Body weights of immunized mice after challenge with wild-type virus. Control mice and immunized mice with either NS gene-deficient or NS2-knockout VLPs or inactivated virus were challenged with 10 or 100 LD50 at 1 month (A) or 3 months (B) after the last vaccination.
DISCUSSION
We have evaluated two types of replication-incompetent influenza VLPs for their immunogenicity in a mouse model. Mice vaccinated intranasally with the NS2-knockout VLPs, but not those given NS gene-deficient VLPs, were protected against lethal infection by wild-type influenza virus. We attribute these results to the greater expression of viral proteins in host cells infected with the NS2-knockout construct, which likely stimulated an immune response sufficient to prevent infection of mucosal surfaces.
The NS2 protein mediates the nuclear transport of viral RNP (16, 19). We have demonstrated that the NS2-knockout VLPs did not produce infectious progeny virus (16), substantiating the importance of this protein in the viral life cycle. In contrast, influenza A virus can replicate in the absence of NS1 in interferon-deficient hosts (9). Although NS1 is shown to translationally regulate viral mRNAs in vitro (3, 6, 20), direct evidence for this effect in the context of influenza virus infection was lacking. Here we show that multiple viral proteins, except for NP, are poorly expressed in cells infected with NS gene-deficient VLPs, in contrast to their abundant expression in cells infected with the NS2-knockout VLPs (Fig. 2). Thus, the NS1 protein clearly enhances the expression of late viral proteins and therefore plays an important role in the life cycle of influenza A viruses. Consistent with our finding, Enami and Enami (7) have reported that translation of HA and M1 proteins was significantly reduced in host cells infected with an NS1 mutant lacking the C-terminal portion of the NS1 protein.
Influenza viruses have been used as carriers to express genes or portions of genes from entirely unrelated infectious agents (13, 21–24). They offer numerous advantages in this regard. Not only do they stimulate strong cell-mediated and humoral immune responses, but they also afford a wide array of virion surface HA and NA proteins (i.e., 15 HA and 9 NA subtypes plus their epidemic variants), allowing repeated immunization of the same target population. Thus, the replication-incompetent influenza VLPs described here might prove useful as vaccine vectors, permitting expression of foreign proteins or immunogenic epitopes. This potential is especially appealing for vaccination against human immunodeficiency virus, foot-and-mouth disease virus, and other infections, where any reversion of the live virus to a wild type is absolutely unacceptable or where the efficacy of inactivated vaccines may be limited due to limited induction of mucosal immunity and cytotoxic T-lymphocyte responses.
In summary, we show that replication-incompetent NS2-knockout VLPs administered via the respiratory mucosal route can protect mice against otherwise lethal influenza virus challenge. Such particles efficiently elicited mucosal as well as systemic immune responses that were sufficient to reduce initial infection of mucosal surfaces, in contrast to inactivated influenza virus vaccines. The newfound ability to manipulate the viral genome should lead to the development of vaccines that can safely prevent diseases that continue to afflict us with regularity.
Acknowledgments
We thank Lisa Arendt, Krisna Wells, and Martha McGregor for excellent technical assistance and John Gilbert for editing the manuscript. Automated sequencing was performed at the University of Wisconsin—Madison Biotechnology Center.
Support for this work was provided by U.S. Public Health Service research grants and by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and Welfare, Japan. T.W. is the recipient of a research fellowship of the Japan Society for the Promotion of Science for Young Scientists. S.W. is the recipient of the Japan Society for Promotion of Science Postdoctoral Fellowship for Research Abroad.
REFERENCES
- 1.Belshe, R. B., P. M. Mendelman, J. Treanor, J. King, W. C. Gruber, P. Piedra, D. I. Bernstein, F. G. Hayden, K. Kotloff, K. Zangwill, D. Iacuzio, and M. Wolff. 1998. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine in children. N. Engl. J. Med. 338:1405–1412. [DOI] [PubMed] [Google Scholar]
- 2.Cha, T. A., K. Kao, J. Zhao, P. E. Fast, P. M. Mendelman, and A. Arvin. 2000. Genotypic stability of cold-adapted influenza virus vaccine in an efficacy clinical trial. J. Clin. Microbiol. 38:839–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Luna, S., P. Fortes, A. Beloso, and J. Ortin. 1995. Influenza virus NS1 protein enhances the rate of translation initiation of viral mRNAs. J. Virol. 69:2427–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DuBridge, R. B., P. Tang, H. C. Hsia, P. M. Leong, J. H. Miller, and M. P. Calos. 1987. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol. Cell. Biol. 7:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards, K. M., W. D. Dupont, M. K. Westrich, W. D. Plummer, Jr., P. S. Palmer, and P. F. Wright. 1994. A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J. Infect. Dis. 169:68–76. [DOI] [PubMed] [Google Scholar]
- 6.Enami, K., T. A. Sato, S. Nakada, and M. Enami. 1994. Influenza virus NS1 protein stimulates translation of the M1 protein. J. Virol. 68:1432–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enami, M., and K. Enami. 2000. Characterization of influenza virus NS1 protein by using a novel helper-virus-free reverse genetic system. J. Virol. 74:5556–5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fodor, E., L. Devenish, O. G. Engelhardt, P. Palese, G. G. Brownlee, and A. Garcia-Sastre. 1999. Rescue of influenza A virus from recombinant DNA. J. Virol. 73:704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Sastre, A., A. Egorov, D. Matassov, S. Brandt, D. E. Levy, J. E. Durbin, P. Palese, and T. Muster. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324–330. [DOI] [PubMed] [Google Scholar]
- 10.Herlocher, M. L., H. F. Maassab, and R. G. Webster. 1993. Molecular and biological changes in the cold-adapted “master strain” A/AA/6/60 (H2N2) influenza virus. Proc. Natl. Acad. Sci. USA 90:6032–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kida, H., L. E. Brown, and R. G. Webster. 1982. Biological activity of monoclonal antibodies to operationally defined antigenic regions on the hemagglutinin molecule of A/Seal/Massachusetts/1/80 (H7N7) influenza virus. Virology 122:38–47. [DOI] [PubMed] [Google Scholar]
- 12.Lamb, R. A., P. W. Choppin, R. M. Chanock, and C. J. Lai. 1980. Mapping of the two overlapping genes for polypeptides NS1 and NS2 on RNA segment 8 of influenza virus genome. Proc. Natl. Acad. Sci. USA 77:1857–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyahira, Y., A. Garcia-Sastre, D. Rodriguez, J. R. Rodriguez, K. Murata, M. Tsuji, P. Palese, M. Esteban, F. Zavala, and R. S. Nussenzweig. 1998. Recombinant viruses expressing a human malaria antigen can elicit potentially protective immune CD8+ responses in mice. Proc. Natl. Acad. Sci. USA 95:3954–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy, B. R., and R. G. Webster. 1996. Orthomyxoviruses, p.1397–1445. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 15.Neumann, G., M. R. Castrucci, and Y. Kawaoka. 1997. Nuclear import and export of influenza virus nucleoprotein. J. Virol. 71:9690–9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neumann, G., M. T. Hughes, and Y. Kawaoka. 2000. Influenza A virus NS2 protein mediates vRNP nuclear export through NES-independent interaction with hCRM1. EMBO J. 19:6751–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neumann, G., T. Watanabe, H. Ito, S. Watanabe, H. Goto, P. Gao, M. Hughes, D. R. Perez, R. Donis, E. Hoffmann, G. Hobom, and Y. Kawaoka. 1999. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl. Acad. Sci. USA 96:9345–9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann, G., T. Watanabe, and Y. Kawaoka. 2000. Plasmid-driven formation of influenza virus-like particles. J. Virol. 74:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Neill, R. E., J. Talon, and P. Palese. 1998. The influenza virus NEP (NS2 protein) mediates the nuclear export of viral ribonucleoproteins. EMBO J. 17:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park, Y. W., and M. G. Katze. 1995. Translational control by influenza virus. Identification of cis-acting sequences and trans-acting factors which may regulate selective viral mRNA translation. J. Biol. Chem. 270:28433–28439. [DOI] [PubMed] [Google Scholar]
- 21.Restifo, N. P., D. R. Surman, H. Zheng, P. Palese, S. A. Rosenberg, and A. Garcia-Sastre. 1998. Transfectant influenza A viruses are effective recombinant immunogens in the treatment of experimental cancer. Virology 249:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staczek, J., H. E. Gilleland, L. B. Gilleland, R. N. Harty, A. Garcia-Sastre, O. G. Engelhardt, and P. Palese. 1998. A chimeric influenza virus expressing an epitope of outer membrane protein F of Pseudomonas aeruginosa affords protecion against challenge with P. aeruginosa in a murine model of chronic pulmonary infection. J. Virol. 66:3990–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strobel, I., M. Krumbholz, A. Menke, E. Hoffmann, P. R. Dunbar, A. Bender, G. Hobom, A. Steinkasserer, G. Schuler, and R. Grassmann. 2000. Efficient expression of the tumor-associated antigen MAGE-3 in human dendritic cells, using an avian influenza virus vector. Hum. Gene Ther. 11:2207–2218. [DOI] [PubMed] [Google Scholar]
- 24.Zhou, Y., M. Konig, G. Hobom, and E. Neumeier. 1998. Membrane-anchored incorporation of a foreign protein in recombinant influenza virions. Virology 246:83–94. [DOI] [PubMed] [Google Scholar]