Abstract
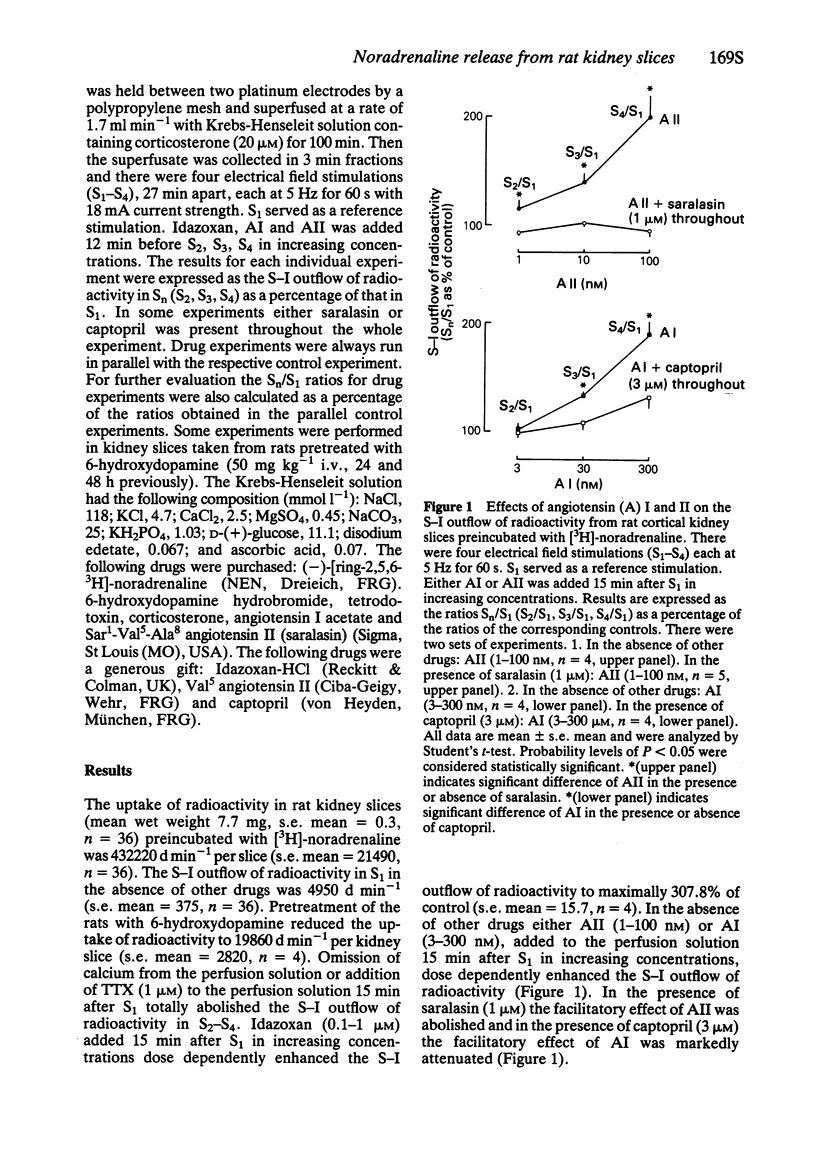
Rat kidney slices were incubated with [3H]-noradrenaline and placed into a superfusion chamber between two platinum electrodes. The kidney slices accumulated and stored radioactivity. In kidney slices taken from rats whose sympathetic nerve terminals were destroyed by pretreatment with 6-hydroxydopamine accumulation of radioactivity was abolished. The α2-adrenoceptor antagonist idazoxan (0.1-1 μM) enhanced but tetrodotoxin (TTX, 1 μM) or omission of calcium from the superfusion solution abolished the stimulation induced (S-I) outflow of radioactivity. Angiotensin (A) I (3-300 nM) and AII (1-100 nM) enhanced S-I outflow of radioactivity. The effect of AI was markedly attenuated by the angiotensin converting enzyme inhibitor captopril (3 μM) and that of AII was blocked by the AII receptor antagonist saralasin (1 μM). These results suggest that the kidney slice preparation is a valid technique to study modulation of renal noradrenaline release. Endogenous noradrenaline released from sympathetic nerves in rat kidney slices activates prejunctional α2-adrenoceptors to inhibit its own release. AII, which can also be formed locally from AI in these kidney slices, activates prejunctional AII receptors to facilitate renal noradrenaline release.
Keywords: kidney, noradrenaline release, prejunctional receptors, angiotensin
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kostrzewa R. M., Jacobowitz D. M. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev. 1974 Sep;26(3):199–288. [PubMed] [Google Scholar]
- Langer S. Z. The metabolism of (3H)noradrenaline released by electrical stimulation from the isolated nictitating membrane of the cat and from the vas deferens of the rat. J Physiol. 1970 Jul;208(3):515–546. doi: 10.1113/jphysiol.1970.sp009135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Limberger N., Starke K. Transmitter release patterns of noradrenergic, dopaminergic and cholinergic axons in rabbit brain slices during short pulse trains, and the operation of presynaptic autoreceptors. Naunyn Schmiedebergs Arch Pharmacol. 1988 Dec;338(6):632–643. doi: 10.1007/BF00165627. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Rump L. C., Majewski H. Beta adrenoceptor facilitation of norepinephrine release is not dependent on local angiotensin II formation in the rat isolated kidney. J Pharmacol Exp Ther. 1987 Dec;243(3):1107–1112. [PubMed] [Google Scholar]
- Rump L. C. Modulation of renal noradrenaline release by prejunctional receptor systems in vitro. Clin Exp Pharmacol Physiol. 1987 May;14(5):423–428. doi: 10.1111/j.1440-1681.1987.tb00993.x. [DOI] [PubMed] [Google Scholar]


