Abstract
CD8+ T cells are a major component of the adaptive response of a host to infections by viruses and other intracellular pathogenic agents. However, because of the intrinsic immaturity of the immune system of neonatal animals, neonates are highly sensitive to a variety of pathogens and may be unable to respond in a protective manner. Here we explore whether a hyperattenuated strain of Listeria monocytogenes that can be used as a live vaccine vector in adults is safe and able to induce an effective response in neonates. We answer both questions affirmatively.
Naïve CD8+ T cells are stimulated to proliferate and to develop into cytotoxic effector T cells after recognition of short peptides associated with major histocompatibility complex (MHC) class I molecules on professional antigen-presenting cells (APCs). These effector cells are a major component of the adaptive response of a host to infections by viruses and other intracellular pathogenic agents. Because of the intrinsic immaturity of the immune system of neonatal animals, neonates are highly sensitive to a variety of these pathogens. Can young animals elicit an effective immune response to such agents? Following the early experiments of Billingham et al. (4), it was long believed that immunologically immature neonatal animals primarily became tolerized as a result of early antigen exposure. However, extensive subsequent research has shown that neonates, despite having reduced numbers of T cells (6, 21) and immature B and dendritic cells (17), nevertheless are able to generate Th1- and Th2-type CD4 responses (1, 2, 7). In addition, neonatal mice respond with cytotoxic T-lymphocyte (CTL) responses to attenuated herpes simplex virus (8), retrovirus (22), and DNA vaccines (12). Human infants produce a Th1-type response to measles immunization (11), and 36-h-old human neonates produced lasting Th1 responses to Mycobacterium bovis BCG vaccination (14). Maternally transmitted viruses have become a major health issue as increasing numbers of children are infected by human immunodeficiency virus type 1 (HIV-1), either by transmission of the virus in utero across the placenta or after mucosal exposure to infectious maternal blood or secretions. HIV-specific CTL activities have been seen in infants as young as 6 months of age (25). Early prophylactic or therapeutic immunization may be the best approach to reduce the incidence and severity of these diseases in this young population.
However, because of the intrinsic immaturity of the immune system during this stage of development, early postnatal immunization may not be effective and is not entirely without risk. Killed vaccines may not elicit strong responses and are ineffective in stimulating CD8+- T-cell responses necessary for protection against intracellular pathogens. Conversely, live vaccines may overwhelm the neonatal immune system and cause their own pathological consequences. For example, nef deletion mutants of simian immunodeficiency virus are attenuated in adult macaques and protect these animals against subsequent pathogenic virus infection (5). However, immunization of neonatal macaques with these viruses has resulted in viremia and death (3).
We have developed a hyperattenuated strain of Listeria monocytogenes that can be used as a live vaccine vector when engineered to stably express foreign antigens (10, 19, 24). Immunization of adult mice with a gag strain of this organism induced strong CTL responses and long-lasting systemic and mucosal protection against challenge with a surrogate for HIV, a gag recombinant of vaccinia virus (19). This recombinant was used, since HIV cannot infect mice. In view of the important question of safety of a live vaccine in neonatal animals, we here explore this issue with the attenuated strain of Listeria. For immunological comparison, 7-day-old mouse neonates correspond approximately to newborn humans (6). We will show that this vector is both safe and able to initiate a protective CD8+-cytolytic-T-cell response in these young animals.
The attenuated strain of Listeria is safe in neonatal mice.
Since neonatal animals are highly sensitive to infection by a variety of pathogenic microorganisms and viruses, it was important to determine whether the attenuated strain of Listeria that we developed as a vaccine vector in adult mice could also be used safely in neonatal animals. The L. monocytogenes dal dat mutant is a double-deletion mutant of L. monocytogenes in which large portions of the alanine racemase gene (dal) and d-amino acid aminotransferase gene (dat) have been removed in frame (24). In laboratory media and in animals, growth of this double-deletion mutant requires d-alanine for cell wall synthesis and survival. The 50% lethal dose (LD50) of the L. monocytogenes dal dat double mutant in adult female BALB/c mice following intravenous or intraperitoneal (i.p.) infection is >8 × 108. If the mutant is injected in the presence of 10 mg of d-alanine, the LD50 is somewhat lower. The L. monocytogenes dal dat gag mutant, produced by stable integration of the HIV-1 gag gene into the chromosome of the double mutant, has an LD50 of ∼5 × 108 in d-alanine. The LD50 of wild-type L. monocytogenes strain 10403S is approximately 104, and the LD50 of the L. monocytogenes gag recombinant is ∼5 × 107 (9).
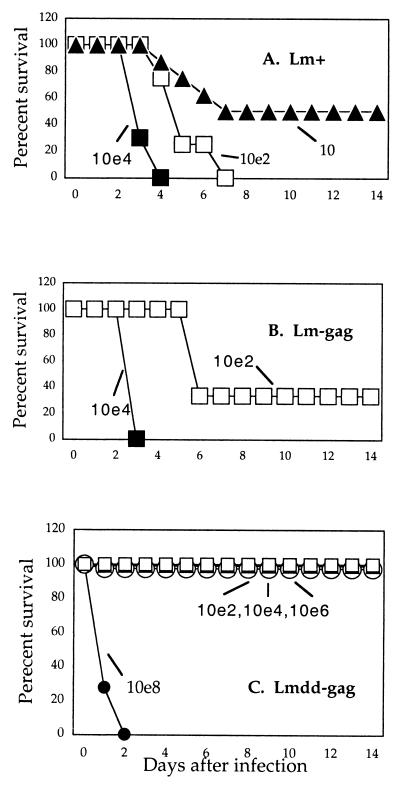
In the following experiments, we examined the virulence of the three different strains of Listeria following i.p. infection of 3- to 5-day-old neonatal BALB/c mice. Figure 1A shows that the LD50 of wild-type L. monocytogenes in the neonatal mice was approximately 10 bacteria. Injection of 102 of these organisms caused death of all pups within 7 days, and 104 caused death earlier. The gag recombinant of the wild-type strain (Fig. 1B) was slightly less lethal, with an LD50 for this strain of approximately 102, and 104 of these bacteria caused the death of all pups. Conversely, neither 102 nor 104 of the attenuated strain (Fig. 1C) caused any deaths of neonates, and more striking, in more than 30 neonates tested, 106 of the L. monocytogenes dal dat gag mutant also caused no deaths. A dose of 108 of all strains, including the hyperattenuated strain, was lethal for all animals, presumably partly because of the massive dose of bacterial products introduced. We examined the persistence of these bacteria in the neonates, and as seen earlier in adult mice (24), when a dose of 106 attenuated L. monocytogenes dal dat gag mutant was administered i.p. to neonates, few viable bacteria could be recovered from animals 3 days after infection and no bacteria could be recovered 4 days after infection (not shown).
FIG. 1.
Survival of neonatal mice after infection with three different strains of Listeria. The three strains used were the wild-type L. monocytogenes (Lm+) (A), the HIV-gag recombinant of the wild-type strain (Lm-gag) (B), and the HIV-gag recombinant of the attenuated strain of L. monocytogenes (Lmdd-gag) (C). Three-to-five-day-old pups were infected i.p. with 30 μl of inoculum containing various numbers of bacteria (101 [10], 102 [10e2], 104 [10e4], etc.). In order to initiate infection and secrete sufficient Gag protein to elicit an immune response, the inoculum of the attenuated Listeria contained 0.3 mg of d-alanine. Because this compound is the unnatural isomer of l-alanine, it is not used or stored by the host but is eliminated from the system. For appropriate comparisons, the same diluent containing d-alanine was used for all three bacterial infections. Most treatment groups contained five to eight neonatal mice.
The attenuated strain of Listeria induces a CD8+-cytolytic-T-cell response in neonates.
Because of the immaturity of the immune system of the newborn, the immunological consequence of an infection is difficult to predict (23). L. monocytogenes is a strong inducer of type 1 immunity and has long been studied as a paradigm of cell-mediated immune responses (13). We therefore examined whether adult mice derived from animals infected neonatally with the gag recombinant of the attenuated strain of Listeria retained memory of the infection in the form of CD8+ T cells specific for the Gag antigen. Such cells might be protective in the event of a subsequent HIV infection or might enhance protection during an ongoing infection.
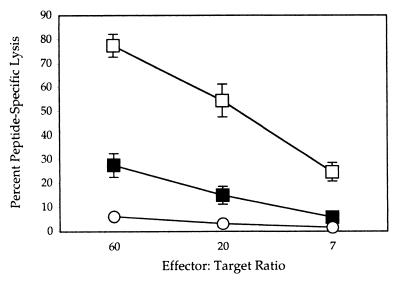
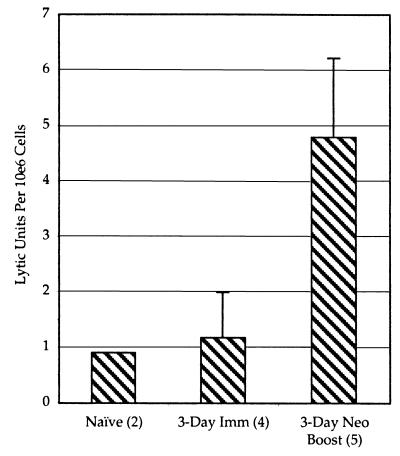
Cells obtained from neonatally immunized mice were tested for Gag-specific cytolytic activity. Figure 2 shows the result of a chromium release assay in which splenocytes isolated 2 to 3 months after a single neonatal infection were assayed on target cells pulsed with the Kd-specific MHC class I Gag peptide (16). Specific lysis by these splenocytes was 5 times stronger than exhibited by cells from control litters of neonates that had not been immunized, showing that a type 1 immune reaction had occurred in response to the infection. Additional evidence that immunity rather than tolerance was elicited is shown by the CTL activities of neonatally infected mice examined 2 months after a booster dose when the mice were 19 days old. In this case, high CTL activities were elicited from all animals examined. As an additional test for the presence of memory T cells, neonatally immunized mice were given booster doses as adults and examined just 3 days later. At 3 days, a naïve animal is unable to mount a significant immune response, which usually can be detected only 5 days after infection, peaking at 7 to 9 days. In this experiment we found that while the lytic activity of control mice immunized only as adults and assayed 3 days later was similar to that of completely naïve animals, neonatally infected animals treated similarly had 5 times the lytic activity of naïve animals (Fig. 3). This showed that the neonatally infected mice possessed primed T cells that could respond rapidly to a second infection.
FIG. 2.
Neonatal immunization with attenuated Listeria gag mutant elicits spleen cells with cytolytic activity. Splenocytes were isolated 2 to 3 months after neonatal infection with 106 L. monocytogenes dal dat gag mutant, stimulated in culture in the presence of the Kd-specific MHC class I Gag peptide (amino acids 197 to 205) (16) for 6 days and assayed on chromium-labeled peptide-pulsed P815 cells following previously published procedures (9). In the in vitro culture, APCs were splenocytes from naive adult same-gender mice. They were loaded with 5 to 10 μM peptide for 60 min, irradiated, and used at a ratio of 3 or 4 lymphocytes per APC. Targets for these assays were peptide-pulsed P815. Uninfected neonates (open circles), neonates infected at 5 days (solid squares), or neonates infected at 5 days and given booster doses 14 days later (open squares) were assayed 85 days after birth. The data are means and standard deviations of the means (error bars) for groups of seven, four, and six mice, respectively.
FIG. 3.
Neonatal immunization with attenuated L. monocytogenes gag mutant generates a pool of rapidly responding memory cells. The lytic activity of splenocytes isolated from uninfected adults (Naïve; n = 2), from adults infected with the L. monocytogenes dal dat gag mutant 3 days prior to splenocyte isolation (3-Day Imm; n = 4), or from adults infected initially as neonates and then given a booster dose as adults 3 days prior to splenocyte isolation (3-Day Neo Boost; n = 5) were assayed by a chromium release assay as described in the legend to Fig. 2. To compare multiple independent experiments, the data are presented in lytic units, which is the number of effector cells per 106 (10e6) total cells that can lyse 15% of 2 × 104 target cells. The data are means with standard deviations of the means (error bars).
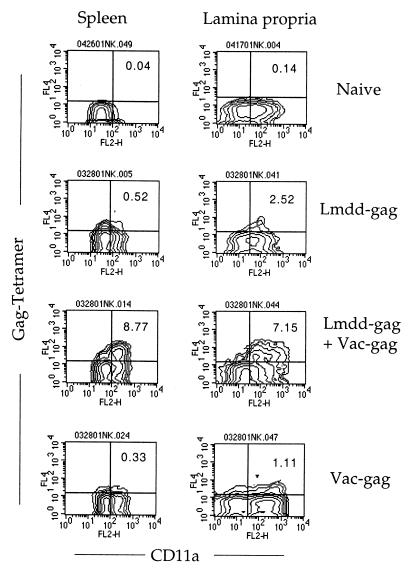
To determine whether this response was associated with the presence of a pool of Gag-specific memory cells with T-cell receptors capable of binding Kd-Gag epitope-labeled MHC class I molecules, we assessed the Gag tetramer staining of cells by flow cytometry. To enhance the frequency of such cells, we examined neonates that had been primed at 5 days of age and then given a booster dose 2 weeks later. In Fig. 4 we examine the memory T cells found in spleen and lamina propria taken from these mice after resting for 120 days. They are compared with naïve adults of the same sex and age. In the experiment shown, 0.52% of the LFA-1-positive CD8+ splenic T cells bound the H-2Kd-Gag tetramers, a 13-fold increase over the nonspecific binding (0.04%) of the control cells. Lamina propria contained 2.52% of such cells, an 18-fold increase over naive. Their abundance in lamina propria suggests that these memory cells may be widely distributed in the animal (15, 18, 20).
FIG. 4.
Infection of neonates leads to a pool of tetramer-positive Gag-specific memory CD8+ T cells that can expand rapidly following virus infection. Neonatal mice were primed by infection when they were 5 days old with 106 L. monocytogenes dal dat gag mutant as described in the legend to Fig. 1 and then given a booster dose 14 days later (Lmdd-gag). The mice were allowed to rest to day 120. Lymphocytes were then isolated 6 days after some of these mice were challenged with 5 × 106 vaccinia virus-gag (Lmdd-gag + Vac-gag). Controls were neonates not immunized at all (Naive) or neonates that were challenged only as adults with vaccinia virus-gag (Vac-gag). Splenocytes and lamina propria lymphocytes were stained for three-color flow cytometry with fluorescein isothiocyanate-labeled CD8a, phycoerythrin-labeled LFA-1 (CD11a), and APC-labeled H-2Kd-Gag peptide/MHC tetramers. CD8+ cells were positively gated and then examined for tetramers and expression of LFA-1. Similar results were obtained when CD8+ splenocytes were first isolated by negative selection prior to analysis.
We wanted to determine how this memory T-cell subset would respond to challenge by infection with an HIV-1 gag-expressing virus. Figure 4 shows that as a result of challenge with recombinant vaccinia virus expressing the gag gene (vaccina virus-gag) 6 days earlier, there was a further 17-fold increase of tetramer-positive cells in the CD8+-T-cell subset of the spleen and a threefold increase in tetramer-positive cells among the CD8+ cells of lamina propria. By contrast, vaccinia virus-gag infection of healthy adult mice resulted in the induction of an effector population that contained only 0.33% Gag-tetramer-positive cells among the splenic CD8+ T cells and 1.11% tetramer-positive cells among the lamina propria CD8+ T cells. Thus, the memory pool after neonatal Listeria infection was as large or larger than that induced during the peak of a primary response in adults to vaccinia infection.
Neonatally immunized mice show protection against virus challenge.
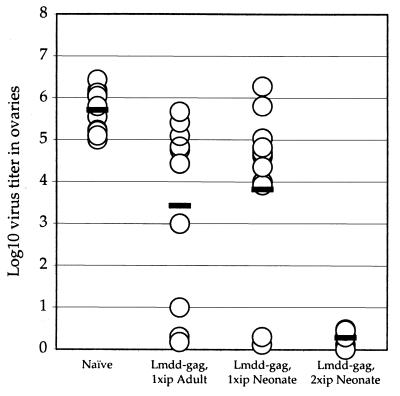
A test of the efficacy of a cell-mediated immune response is to demonstrate protection against challenge by an agent expressing the pertinent antigen. At 2 to 3 months after immunization, mice were challenged systemically with 5 × 106 recombinant vaccinia virus expressing HIV-1 Gag. Six days later, the mice were sacrificed and their ovaries were assayed for virus content. When adult mice grown from litters of control, nonimmunized neonates were infected with vaccinia virus-gag, 6 days after infection these animals had a median vaccinia virus titer in their ovaries of 5.7 × 105 (Fig. 5). However, adults grown from neonates that had been immunized once at 3 to 5 days of age showed a titer of 3.8 × 103, a 2-log decrease in ovarian vaccinia virus titers. Some animals were completely protected, and some showed no protection. The wide variation in responses may in part be related to the uncertainty of the precise volume of Listeria vaccine retained without leakage of inoculum from the infected pups. Neonates that had been immunized once and then given a booster dose 14 days later showed complete protection (less than 101) against the vaccinia virus-gag challenge. That this complete protection did not result simply as a consequence of the booster dose at 14 days is shown by the fact that control mice immunized once as young adults showed, like once-immunized neonates, only partial protection (Fig. 5).
FIG. 5.
Adult mice show protection against challenge by vaccinia virus-gag after neonatal immunization with attenuated Listeria gag mutant. Neonatal mice were either not immunized (Naïve; n = 11) or they were immunized at day 5 with 106 L. monocytogenes dal dat gag mutant (Lmdd-gag, 1xip Neonate; n = 15) or immunized at day 5 and given a booster dose 14 days later with 106 L. monocytogenes dal dat gag mutant (Lmdd-gag, 2xip Neonate; n = 6). A control group of young adult mice was immunized once with L. monocytogenes dal dat gag mutant (Lmdd-gag, 1xip Adult, n = 11). At 2 to 3 months after the last infections, mice were challenged with 5 × 106 recombinant vaccinia virus expressing HIV-1 Gag and 6 days later assayed for virus titer in ovaries as described previously (19). The mean of each set of data is shown by a black bar.
Conclusions.
On the basis of early observations, it was concluded that the neonatal period was a phase during development in which the immature immune system was learning to recognize self and that antigens delivered during this period would be treated as self and tolerize the individual. However, several investigators questioned whether this tolerization was not more a technical issue, reflecting predominantly the low numbers of naïve T cells at this developmental stage and the resultant often ineffectual ratios of APCs to T cells achieved during immunization of such animals. The form in which the antigen is provided, and consequently the APCs recruited, also affected the response. It was demonstrated that adult animals showed a similar response when analogous tolerizing immunization protocols were used (7, 21, 22). Thus, neonates did respond to immunization when antigens were administered appropriately.
Infection by intracellular agents that take up residence and multiply in the cytoplasm of their host cells, including viruses and certain bacteria and parasitic pathogens, demand a strong Th1 response by the host to achieve their successful clearance. Neonatal animals elicit both Th1- and Th2-type CD4 responses, but there is some bias toward Th2 (2). L. monocytogenes is a gram-positive intracellular bacterium that has long been studied as a model agent for the induction of Th1-type cell-mediated immunity in adult mice (13). CD8+ T cells induced by this organism are protective. We wished to know whether this organism was capable of inducing such a protective Th1-type response in neonates and whether it could be used as a vaccine vector for animals at this stage of development. Our experiments demonstrated that Listeria was able to elicit a protective type 1 immune response to a foreign antigen in neonatal mice.
However, since Listeria is a pathogen, we first examined whether an attenuated strain of this organism could be safely administered to neonates. In the presence of 0.3 mg of d-alanine, required for the initiation of infection and the early survival of this attenuated organism, as many as 106 gag recombinant attenuated Listeria were found to be completely avirulent in 3- to 5-day-old neonatal mice, while as few as 10 wild-type Listeria resulted in the death of half the infected animals. Virtually all of the attenuated bacteria were no longer viable by the third day after infection.
When we examined the adult animals 1 to 2 months after neonatal infection, we found that their splenic lymphocytes showed moderate Gag-specific CTL activities. If these animals were given a booster dose with a second infection 2 weeks after the first, their CTL activities reached high levels, indicating no evidence of tolerization by the vaccine. These CTL activities were shown to provide functional immunity by challenging the adult animals with infection by a gag recombinant vaccinia virus. In this case, the neonatally immunized animals showed partial protection against virus replication. Neonatally immunized animals that were given a booster dose at 3 weeks were fully protected against this challenge. Examination of the levels of the Gag-specific T-cell subset in these animals showed a persistent memory level severalfold higher than seen in naïve mice, particularly in lamina propria. This memory level increased dramatically following virus challenge.
It will be of interest to study further how the neonatal animal handles infection by a microbe like Listeria. In adults, the host response is characterized by a complex interplay between innate and adaptive immune elements, which likely will differ in the immunologically immature system of the neonate. Early events after Listeria infection include the induction of the cytokines interleukin 12, gamma interferon (γ-IFN), and tumor necrosis factor alpha (TNF-α), which lead to the killing of most of the bacteria in activated macrophages and neutrophils. However, sterilizing immunity requires the participation of CD8+ T cells. The mechanisms by which these cells mediate immunity continue to be explored. For protection against L. monocytogenes infections, perforin-dependent and CD95/CD95L-mediated pathways of cytolysis play a role, but neither is essential (27). Likewise, several cytokines secreted by CD8+ T cells, IFN-γ and TNF-α, are also dispensable individually in the development of resistance to Listeria (26). It therefore appears that protection depends on multiple, redundant effector pathways, although there may be other as-yet-unknown mechanisms. Nevertheless, the necessary effectors appear to be present and activated in neonatal mice immunized with the attenuated Listeria recombinant. Analogous results were obtained after infection of neonatal mice with a similarly crippled viral vector, herpes simplex virus DISC, which induced a CTL response and protection against the parent virus (8), although induction of a response against a foreign antigen was not examined. Our results demonstrate that the attenuated Listeria vector is capable of safely eliciting a Th1-type response in neonates that can result in subsequent protection against challenge and therefore may have potential use as a vaccine in young animals. It will be important to test this concept in primates.
Acknowledgments
We appreciate the input of Judy Lieberman, who first suggested the problem, and the expert technical assistance of Natalia Kovaltchouk, who performed the experiment shown in Fig. 1.
This work was supported in part by Public Health Services grant AI-42509 and an award from the University of Pennsylvania Research Foundation.
REFERENCES
- 1.Adkins, B., Y. Bu, E. Cepero, and R. Perez. 2000. Exclusive Th2 primary effector function in spleens but mixed Th1/Th2 function in lymph nodes of murine neonates. J. Immunol. 164:2347–2353. [DOI] [PubMed] [Google Scholar]
- 2.Adkins, B., Y. Bu, and P. Guevara. 2001. The generation of Th memory in neonates versus adults: prolonged primary Th2 effector function and impaired development of Th1 memory effector function in murine neonates. J. Immunol. 166:918–925. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T. W., Y. S. Jeong, D. Pennick, R. Bronson, M. F. Greene, and R. M. Ruprecht. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 267:1820–1825. [DOI] [PubMed] [Google Scholar]
- 4.Billingham, R. E., L. Brent, and P. B. Medawar. 1953. Actively acquired tolerance of foreign cells. Nature 172:603–606. [DOI] [PubMed] [Google Scholar]
- 5.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nefgene. Science 258:1938–1941. [DOI] [PubMed] [Google Scholar]
- 6.Fadel, S., and M. Sarzotti. 2000. Cellular immune responses in neonates. Int. Rev. Immunol. 19:173–193. [DOI] [PubMed] [Google Scholar]
- 7.Forsthuber, T., H. C. Yip, and P. V. Lehmann. 1996. Induction of TH1 and TH2 immunity in neonatal mice. Science 271:1728–1730. [DOI] [PubMed] [Google Scholar]
- 8.Franchini, M., C. Abril, C. Schwerdel, C. Ruedl, M. Ackermann, and M. Suter. 2001. Protective T-cell-based immunity induced in neonatal mice by a single replicative cycle of herpes simplex virus. J. Virol. 75:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frankel, F. R., S. Hedge, J. Lieberman, and Y. Paterson. 1995. Induction of cell-mediated immune responses to human immunodeficiency virus type 1 Gag protein by using Listeria monocytogenes as a live vaccine vector. J. Immunol. 155:4775–4782. [PubMed] [Google Scholar]
- 10.Friedman, R. S., F. R. Frankel, Z. Xu, and J. Lieberman. 2000. Induction of human immunodeficiency virus (HIV)-specific CD8 T-cell responses by Listeria monocytogenes and a hyperattenuated Listeria strain engineered to express HIV antigens. J. Virol. 74:9987–9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gans, H. A., Y. Maldonado, L. L. Yasukawa, J. Beeler, S. Audet, M. M. Rinki, R. DeHovitz, and A. M. Arvin. 1999. IL-12, IFN-gamma, and T cell proliferation to measles in immunized infants. J. Immunol. 162:5569–5575. [PubMed] [Google Scholar]
- 12.Hassett, D. E., J. Zhang, M. Slifka, and J. L. Whitton. 2000. Immune responses following neonatal DNA vaccination are long-lived, abundant, and qualitatively similar to those induced by conventional immunization. J. Virol. 74:2620–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufmann, S. H. E. 1993. Immunity to intracellular bacteria. Annu. Rev. Immunol. 11:129–163. [DOI] [PubMed] [Google Scholar]
- 14.Marchant, A., T. Goetghebuer, M. O. Ota, I. Wolfe, S. J. Ceesay, D. De Groote, T. Corrah, S. Bennett, J. Wheeler, K. Huygen, P. Aaby, K. P. McAdam, and M. J. Newport. 1999. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J. Immunol. 163:2249–2255. [PubMed] [Google Scholar]
- 15.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413–2417. [DOI] [PubMed] [Google Scholar]
- 16.Mata, M., P. J. Travers, Q. Liu, F. R. Frankel, and Y. Paterson. 1998. The MHC class I-restricted immune response to HIV-gag in BALB/c mice selects a single epitope that does not have a predictable MHC-binding motif and binds to Kd through interactions between a glutamine at P3 and pocket D. J. Immunol. 161:2985–2993. [PubMed] [Google Scholar]
- 17.Muthukkumar, S., J. Goldstein, and K. E. Stein. 2000. The ability of B cells and dendritic cells to present antigen increases during ontogeny. J. Immunol. 165:4803–4813. [DOI] [PubMed] [Google Scholar]
- 18.Pope, C., S. K. Kim, A. Marzo, K. Williams, J. Jiang, H. Shen, and L. Lefrancois. 2001. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166:3402–3409. [DOI] [PubMed] [Google Scholar]
- 19.Rayevskaya, M. V., and F. R. Frankel. 2001. Systemic immunity and mucosal immunity are induced against human immunodeficiency virus Gag protein in mice by a new hyperattenuated strain of Listeria monocytogenes. J. Virol. 75:2786–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinhardt, R. L., A. Khoruts, R. Merica, T. Zell, and M. K. Jenkins. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410:101–105. [DOI] [PubMed] [Google Scholar]
- 21.Ridge, J. P., E. J. Fuchs, and P. Matzinger. 1996. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science 271:1723–1726. [DOI] [PubMed] [Google Scholar]
- 22.Sarzotti, M., D. S. Robbins, and P. M. Hoffman. 1996. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science 271:1726–1728. [DOI] [PubMed] [Google Scholar]
- 23.Siegrist, C. A. 2000. Vaccination in the neonatal period and early infancy. Int. Rev. Immunol. 19:195–219. [DOI] [PubMed] [Google Scholar]
- 24.Thompson, R. J., H. G. A. Bouwer, D. A. Portnoy, and F. R. Frankel. 1998. Pathogenicity and immunogenicity of a Listeria monocytogenes strain that requires d-alanine for growth. Infect. Immun. 66:3552–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wasik, T. J., P. P. Jagodzinski, E. M. Hyjek, J. Wustner, G. Trinchieri, H. W. Lischner, and D. Kozbor. 1997. Diminished HIV-specific CTL activity is associated with lower type 1 and enhanced type 2 responses to HIV-specific peptides during perinatal HIV infection. J. Immunol. 158:6029–6036. [PubMed] [Google Scholar]
- 26.White, D. W., V. P. Badovinac, G. Kollias, and J. T. Harty. 2000. Cutting edge: antilisterial activity of CD8+ T cells derived from TNF-deficient and TNF/perforin double-deficient mice. J. Immunol. 165:5–9. [DOI] [PubMed] [Google Scholar]
- 27.White, D. W., and J. T. Harty. 1998. Perforin-deficient CD8+ T cells provide immunity to Listeria monocytogenes by a mechanism that is independent of CD95 and IFN-gamma but requires TNF-alpha. J. Immunol. 160:898–905. [PubMed] [Google Scholar]