Abstract
The hydrodynamic diameters of native rotavirus particles, bovine RF and simian SA11 strains, were determined by quasielastic light scattering. By using this method and agarose gel electrophoresis, the Ca2+ dissociation constant, KCa, governing the transition from triple-layer particles (TLPs) to double-layer particles (DLPs), was shown to increase, at constant pH, as the temperature and/or the ionic strength of the incubation medium increased. We report the novel observation that, under physiological conditions, KCa values for both RF and SA11 rotaviruses were well above the intracytoplasmic Ca2+ concentrations of various cells, which may explain why TLP uncoating takes place within vesicles (possibly endosomes) during the entry process. A correlation between TLP uncoating and cell membrane permeabilization was found, as shown by the release of carboxyfluorescein (CF) from CF-loaded intestinal brush-border membrane vesicles. Conditions stabilizing the virion in the TLP form inhibited CF release, whereas conditions favoring the TLP-to-DLP transformation activated this process. We conclude that membrane permeabilization must be preceded by the loss of the outer-capsid proteins from trypsinized TLP and that physiological ionic strength is required for permeabilization to take place. Finally, the paper develops an alternative explanation for the mechanism of rotavirus entry, compatible with the Ca2+-dependent endocytic pathway. We propose that there must be an iterative process involving tight coupling in time between the lowering of endosomal Ca2+ concentration, virion decapsidation, and membrane permeabilization, which would cause the transcriptionally active DLPs to enter the cytoplasm of cells.
Recent advances in cryoelectron microscopy and icosahedral image reconstruction techniques have provided a detailed view of the three-dimensional structure of rotavirus, a nonenveloped member of the Reoviridae family (25, 29). Rotavirus has a triple-layered spherical structure. The core is composed of the VP2 innermost capsid protein containing a “subcore” formed by the genomic double-stranded RNA and minor proteins VP1 and VP3 (8, 9). The double-layered particle (DLP) is formed by the VP6 intermediate-capsid protein surrounding the core. Finally, the triple-layered particle (TLP) results from addition of the outermost shell formed by proteins VP7 and VP4. In the presence of trypsin, VP4 is cleaved in situ into two smaller proteins, VP5* and VP8*, conferring infectivity to the virus (12).
The critical Ca2+ concentration ([Ca2+]) governing the TLP-to-DLP transformation has been shown to be strain dependent. By using agar electrophoresis, Ruiz et al. (22) have shown that complete loss of the outer-capsid proteins from TLPs (TLP decapsidation) takes place in the nanomolar range of [Ca2+] (e.g., 600 nM for the RF strain and 10 to 20 nM for the SA11 strain) when a Ca-EGTA buffer containing 10 mM MOPS (morpholinepropanesulfonic acid) and 100 mM KCl at room temperature is used. Nevertheless, it should be emphasized that, in these conditions, at any given [Ca2+] value, the TLP-to-DLP transition observed by perpendicular light scattering was faster at 37°C than at 20°C for the RF strain (22). More recently, Ruiz et al. (20) found that TLP uncoating occurred “spontaneously” when TLPs, RF strain, were treated at 37°C with 200 mM Tris-chloride in the absence of added Ca2+. Such observations raise interesting new questions about the mechanism of TLP uncoating. One explanation is that either 200 mM Tris-chloride per se or a relatively high-ionic-strength solution has the capacity to induce loss of the rotavirus outer-capsid proteins. If this were the case, the conversion from the TLP to the DLP form could be expected to take place in the presence of relatively high, micromolar rather than nanomolar, [Ca2+].
When studying the mechanism(s) of rotavirus entry into cells, we previously showed that the time-dependent release of carboxyfluorescein (CF) from CF-loaded membrane vesicles isolated from various sources depends on the rotavirus particle form used (21). Only trypsinized TLP, RF strain, had the capacity to induce membrane permeabilization, but whether or not this reaction correlated with viral particle internalization, and hence infectivity, was not demonstrated. The strong inhibition of TLP-induced CF release caused by 1 mM Ca2+ has been interpreted as involving the formation of an inactive TLP-Ca2+ complex, where the fusogenic peptide existing in VP5* might be hidden (16). In contrast, the strong activation of CF release observed when TLP was either treated with EGTA or heated (4, 10) indicated a direct permeabilizing action of outer-capsid proteins VP7, VP5*, and/or VP8* when released to the medium (21). Cell membrane permeabilization thus seems to involve a direct interaction between the outer-layer rotavirus proteins and membrane lipids, although the exact mechanism involved has thus far remained unexplained. Such interactions between rotaviruses and glycosphingolipids (28) and between rotaviruses and liposomes have been shown to occur (17).
In the present paper, we introduce a new approach based on determining the hydrodynamic diameter of rotavirus particles by quasielastic light scattering (19). This method together with agarose gel electrophoresis (22) showed that the TLP-to-DLP transformation varied, at constant pH 7.2, as a function of three key variables: temperature, ionic strength, and [Ca2+]. We report the novel observation that, under physiological conditions, both RF and SA11 rotaviruses became uncoated at [Ca2+] values which were well above the intracytoplasmic [Ca2+] values of various cells. The data also show that conditions favoring TLP uncoating activated cell membrane permeabilization, whereas conditions stabilizing the virion in the TLP form inhibited this process. Taken as a whole, these results suggest that the uncoating of the RF and SA11 viruses occurs within the endosomal vesicle, which is in agreement with the Ca2+-dependent endocytic pathway proposed for rotavirus entry (7, 20, 23).
MATERIALS AND METHODS
Viruses.
The viruses used (bovine RF and simian SA11 strains) were multiplied in MA104 cells in the presence of trypsin (0.44 mg/ml; Sigma; type IX) (13). Virus particles were purified by centrifugation on a cesium chloride gradient according to the method of Sabara et al. (24). Particle suspensions were desalted by several cycles of dilution-centrifugation in a standard buffer (see below) at 100,000 × g for 90 min at 4°C and stored at 4°C. Viral protein concentration was measured by using the Bio-Rad protein assay kit, with serum globulin as the standard.
Buffers.
All experiments were carried out in a 20 mM HEPES-10 mM Tris buffer adjusted to pH 7.2 with Tris base (standard buffer). Two main classes of buffer were used: (i) high-ionic-strength (HIS) buffers, where the standard buffer was supplemented with either 50 or 200 mM monovalent, neutral salt (NaCl, KCl, Tris-Cl, or KSCN was used with essentially identical results) and (ii) low-ionic-strength (LIS) buffers, where equimolecular d-sorbitol replaced the monovalent salt.
For the [Ca2+] values in the nanomolar range, the desired concentration of free Ca2+ was fixed by using Ca-EGTA buffer as described by Tsien and Pozzan (27). For [Ca2+] values in the micromolar range, the buffer was supplemented with CaCl2 in the range of 2 μM to 1 mM or was not supplemented. No attempt was made to fix the concentration of free Ca2+ by using Ca-EGTA buffers, since these buffers have no calcium buffering capacity in this range (27). The minimum [Ca2+] values present as contaminants in each individual experiment (on average, 5 μM) were calculated from the Ca2+ impurity content of the reagents used, a procedure recommended by M. Claret (Orsay, France; personal communication). CaCl2 was added on top of this to obtain the nominal micromolar values of [Ca2+] illustrated in the figures. Because of the large [Ca2+] scale used in these experiments, the error introduced by applying this procedure can be considered to be negligible from 10 μM Ca2+ upward.
In experiments involving isolated brush border membrane vesicles, the membrane buffer consisted of the standard buffer supplemented with d-sorbitol to a total of 500 mosM and adjusted to pH 7.5 with Tris base (13).
Size determination of the rotavirus particles by quasielastic light scattering.
Stokes radii of rotavirus particles were determined by using a model N4 MD submicron particle analyzer (Coulter, Hialeah, Fla.). Viral particles suspended in a given buffer at a final protein concentration of 240 μg/ml were observed by light scattering at 20°C. Diffusion constants were derived from photon correlation spectroscopy. We used the Coulter N4 size distribution processor (SDP), which is based on the CONTIN program for constrained solutions of linear equations (19). The reported diameter data were those derived from the SDP analysis.
Membrane vesicle loading and fluorescence measurements.
Brush border membrane vesicles from adult pig jejunum were prepared according to the magnesium precipitation method of Hauser et al. (14). The final vesicle preparations were resuspended at about 17 mg of protein/ml in the membrane buffer and then stored until use in liquid nitrogen in 250-μl batches. Charging of vesicles with CF and the CF release assays were performed as described previously (21).
Electrophoresis.
The TLP-to-DLP transformation was followed by electrophoresis in 0.6% agarose gels in a 10 mM MOPS-5 mM Tris buffer (pH 7.1) as described previously (22). Gels were stained with either silver complexes with a Bio-Rad Silver Stain Plus kit or Coomassie blue (22).
RESULTS
Hydrodynamic diameters of viral particles.
The hydrodynamic diameters of purified TLPs and DLPs suspended in the standard buffer were measured at 20°C by quasielastic light scattering (19). The results indicate statistically different diameters for the TLPs (127 ± 14 nm, n = 22, for the RF strain and 132 ± 15 nm, n = 42, for the SA11 strain) and the DLPs (83 ± 9 nm, n = 27, for the RF strain and 98 ± 8 nm, n = 32, for the SA11 strain).
Treatment of purified TLPs with 10 mM EGTA caused the apparent diameter to drop to values statistically indistinguishable from those of purified DLPs, which can be taken as evidence of complete loss of the outer-capsid proteins from TLPs. EGTA-induced TLP decapsidation was not affected by the ionic strength of the medium.
High [Ca2+] blocks heat-induced TLP uncoating for the RF strain.
Estes et al. (10) previously reported that the infectivity of the SA11 strain dropped by 80% after the strain was heated for 5 min at 50°C, which they interpreted as suggesting that a heat-labile capsid protein is required for infectivity. However, these results can be interpreted as being due not necessarily to increased denaturation of an outer-capsid protein in situ but rather to TLP uncoating to yield free DLPs, which by themselves are noninfective (4, 22).
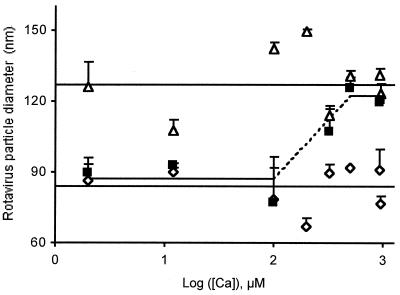
To study the effect of [Ca2+] on heat-dependent rotavirus uncoating, purified TLPs, RF strain, were suspended in the standard buffer supplemented with 50 mM NaCl and between 2 μM and 1 mM CaCl2. After incubation in the conditions indicated in Fig. 1, the diameters of the particles were analyzed by quasielastic light scattering at 20°C. The results show that, when TLPs were kept at room temperature, their diameters were constant, irrespective of the [Ca2+] in the medium (Fig. 1). Essentially identical results were obtained both when these diameters were calculated as the arithmetic mean of all the experimental points available (127 ± 14 nm; n = 22) and as the y intercept of the regression line calculated by using these points (122 ± 6 nm).
FIG. 1.
Calcium concentrations above 300 μM prevent heat-induced uncoating of rotavirus strain RF in media of high ionic strength. TLPs suspended in the standard buffer supplemented with 50 mM NaCl and [Ca2+] ranging from 2 μM to 1 mM were treated as follows: (i) no treatment, standing at 20°C (Δ); (ii) heat treatment at 60°C for 90 s (▪); and (iii) treatment with 10 mM EGTA at 20°C (⋄). After each of these treatments, appropriate aliquots were used for particle size determination by quasielastic light scattering at 20°C. The results are presented as particle diameters ± standard deviations (n = 2 to 6 determinations per point) as a function of the logarithm of the [Ca2+] in the incubation medium. The upper and lower solid lines correspond, respectively, to the arithmetic means of the TLP and DLP diameters. With the heat-treated TLPs (▪) the two statistically different horizontal segments were found to be separated by a region (broken line) where particle size determination is impossible, so that no experimental points can be shown here. The explanation for this anomaly and further details are given in the text.
When the TLPs were heated at 60°C for 90 s in the presence of variable [Ca2+], the complex result in Fig. 1 was obtained. At [Ca2+] values equal to or greater than 500 μM, the particle diameter (122 ± 13 nm; n = 9) was not affected by heating (Fig. 1), in agreement with the tenet that high [Ca2+] stabilizes the virion in the TLP form (4, 23). In contrast, at [Ca2+] values equal to or smaller than 100 μM, the particle diameter (87 ± 11 nm; n = 13) dropped to that characterizing the DLP (Fig. 1), indicating that complete loss of the outer-capsid proteins can occur at [Ca2+] values as high as 100 μM. At [Ca2+] values ranging from 100 to 500 μM, which is clearly the region where the TLP-to-DLP transition occurs, it was impossible to determine precise particle diameters, probably due to the incomplete loss of the TLP outer layer. The mixture of TLPs and DLPs existing in these conditions is impossible to resolve using quasielastic light scattering because the two types of particles are too close in size and cannot be distinguished if present as polydisperse mixtures (19). Given this limitation of the light scattering technique, the sigmoid curve theoretically linking the two branches of the titration curve cannot be established and is simply illustrated in Fig. 1. The value of the equilibrium constant, KCa, was estimated as the middle point between the two extreme [Ca2+] values mentioned above, representing, respectively, the fully capsidated and the fully decapsidated particles. Consequently, in conditions of 90-s heating at 60°C, we assigned a value of 300 μM to KCa60°C in the presence of 50 mM NaCl HIS buffer (Table 1). To confirm the validity of these interpretations, 10 mM EGTA was added to the samples at the end of each experiment to make the concentration of free Ca2+ negligible. As expected, the particle diameter dropped significantly, approaching that of the DLP (84 ± 10 nm; n = 20), irrespective of the total [Ca2+] present initially (Fig. 1).
TABLE 1.
At a constant pH of 7.2, KCa, defining the rotavirus TLP-to-DLP transformation, is dependent on the rotavirus strain, the temperature, and the ionic strengtha
| Rotavirus strain | Temp (°C) | Concn of salt added (mM) | Approximate KCa |
|---|---|---|---|
| RF | 4 | No | ≤1 μM |
| 20 | No | ≤1 μM | |
| 37 | No | ≤1 μM | |
| 60 | No | 100 μM | |
| 60 | 50 | 300 μM | |
| 4 | 200 | ≤1 μM | |
| 20 | 200 | ≤1 μM | |
| 37 | 200 | 20 μM | |
| SA11 | 20 | 200 | 18 nM |
| 37 | 200 | 700 nM |
TLPs were incubated at the indicated temperatures in the standard buffer supplemented with a monovalent salt at the indicated concentrations or not supplemented (no). NaCl, KCl, Tris-Cl, and KSCN were used as the salt, with similar results. When no salt was added, the same result was obtained both when 500 mM sorbitol was used as a substitute for the salt (iso-osmotic conditions) and when no sorbitol was added (hypo-osmotic conditions). The KCa values are derived from both quasielastic light scattering and agarose gel electrophoresis analyses, which give semiquantitatively identical results. Further details are given in the text.
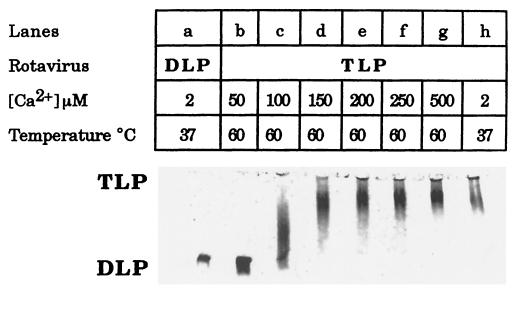
The calcium- and heat-dependent rotavirus uncoating was further studied in a LIS buffer using agar electrophoresis. Heat treatment at 60°C for 90 s of the Ca2+-treated TLPs in LIS buffer had no effect on the particle size as long as [Ca2+] was greater than 150 μM (Fig. 2, lanes d to g). At intermediate [Ca2+] values of around 100 μM, a long smear appeared, joining the TLP and DLP bands (Fig. 2, lane c). This smear probably represents incomplete loss of the TLP outer layer (22). But complete loss of the outer-capsid proteins was evident from 50 μM [Ca2+] downward (Fig. 2, lane b). In these conditions, KCa60°C was assigned a value of 100 μM in the presence of a LIS buffer (Table 1). The shift toward the micromolar range was even greater, approaching a KCa60°C of 300 μM, when the incubation buffer was supplemented with 50 mM NaCl (Fig. 1).
FIG. 2.
Calcium concentrations above 50 μM prevent heat-induced uncoating of rotavirus strain RF in media of low ionic strength. Analysis was by agar electrophoresis. TLPs were suspended in the standard buffer supplemented with 500 mM sorbitol and the indicated nominal calcium concentrations. Before analysis by agar gel electrophoresis at room temperature, the particles were incubated at 37°C for 5 min and then heated for an additional 90 s at 60°C (lanes b to g) or not heated (lanes a and h) as shown. TLPs and DLPs were at 1.9 and 2.4 μg of protein per well, respectively.
Ionic strength- and temperature-induced KCa shifts in TLP uncoating. (i) RF strain.
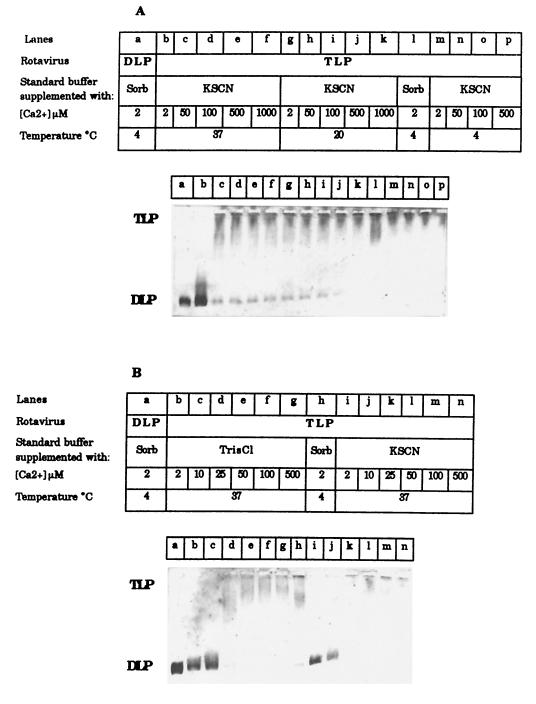
By using quasielastic light scattering and varying the temperature and ionic composition of the media in the presence of only traces (micromolar) of Ca2+, the diameter of TLPs (127 nm) was found to drop to that characterizing the DLPs (83 nm), both when (i) particles suspended in LIS buffer were incubated for 90 s at 60°C but not at 37°C and (ii) particles suspended in 200 mM monovalent salts HIS buffer were incubated for 5 min at 37°C but not at 20°C. Similar results were obtained when the TLP-to-DLP transition was studied using agar electrophoresis. Incubation of TLP at either 4°C (Fig. 3A, lane l) or 37°C (Fig. 2, lane h) in LIS buffer did not induce loss of the outer-capsid proteins. Similar stabilization of the TLP form was observed when Ca-treated TLPs were incubated at either 4°C (Fig. 3A, lanes m to p) or 20°C (Fig. 3A, lanes g to k) in HIS buffer, irrespective of the [Ca2+] used. Identical results were obtained when the salt used to increase the ionic strength was 200 mM KSCN, NaCl, KCl, or Tris-Cl. In all the study conditions, KCa values should be in the range expected for nanomolar rather than micromolar [Ca2+] (Table 1). On the other hand, at 37°C, a change in particle size was apparent below a 50 μM [Ca2+] (Fig. 3A, lanes b to f), the complete TLP uncoating being evident at 10 μM (but not at 25 μM) [Ca2+], independent of the presence of any added salt (Fig. 3B). On the basis of these results, KCa37°C values of about 20 μM were estimated in media of high ionic strength (Table 1). Again, KCa37°C appears to shift gradually from the nanomolar to the micromolar range as the ionic strength of the incubation medium increases. The results also indicate that, at constant high ionic strength, KCa increases from ≤1 μM at 20°C to 20 μM at 37°C (Table 1).
FIG. 3.
Effect of ionic strength, calcium concentration, and temperature on the TLP-to-DLP transition for the RF strain. Analysis was by agar electrophoresis. TLPs were pretreated for 5 min at the indicated temperatures in the standard buffer supplemented with either 500 mM sorbitol (Sorb) or a mixture of 100 mM sorbitol and 200 mM monovalent salt (either KSCN or Tris-Cl) in the presence of the indicated nominal calcium concentrations. TLPs and DLPs were at 1.6 and 1.2 μg of protein per well, respectively.
(ii) SA11 strain.
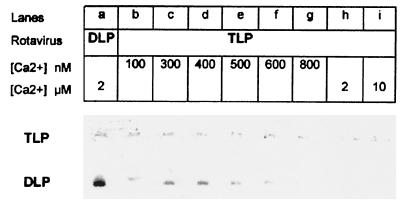
Purified TLPs, SA11 strain, were suspended in 200 mM NaCl HIS buffer supplemented with 1 mM CaCl2 and variable EGTA concentrations to obtain concentrations of free Ca2+ varying between 10 and 800 nM. When TLPs were kept at 20°C, their diameter, as measured by quasielastic light scattering, was constant (132 ± 16 nm; n = 35) at [Ca2+] values equal to or greater than 20 nM. In contrast, at [Ca2+] values equal to or smaller than 15 nM, the particle diameter dropped to that characterizing the DLP (97 ± 9 nm; n = 16). Consequently, a value of 18 nM was assigned to KCa20°C (Table 1). Incubation at 37°C for 5 min of calcium-treated TLPs in the range from 10 to 350 nM [Ca2+] induced the TLP diameter to decrease to that of DLPs. Again, a temperature-induced KCa shift was observed when the TLP-to-DLP transition was studied with the SA11 strain. Here, however, the shift was evident in the nanomolar range. Similar conclusions were deduced using agar electrophoresis. After incubation at 37°C for 15 min, the complete loss of the outer-capsid proteins was evident from 600 nM [Ca2+] downward (Fig. 4). As long as [Ca2+] was greater than 800 nM, the virus was stabilized in the TLP form. In these conditions, KCa37°C was assigned a value of 700 nM in the presence of 200 mM NaCl HIS buffer. Taken together, these results show that, at high ionic strength, increasing the temperature from a nonphysiological to a physiological value caused KCa to increase from 18 nM at 20°C to 700 nM at 37°C.
FIG. 4.
Effect of calcium concentration on rotavirus SA11 uncoating. Analysis was by agar electrophoresis. TLPs were pretreated for 15 min at 37°C in the standard buffer supplemented with 200 mM NaCl, 1 mM CaCl2, and variable EGTA concentrations to obtain the indicated nanomolar concentrations of free Ca2+. For the [Ca2+] in the micromolar range, the particles were pretreated as described in Fig. 2. TLPs and DLPs were each at 1.4 μg of protein per well, respectively.
Relationship between TLP uncoating and CF release reactions.
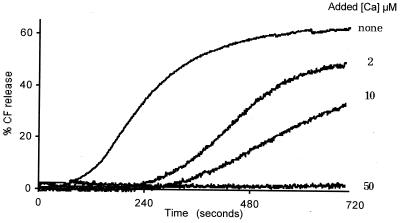
In an attempt to determine a correlation with the virus decapsidation reactions described earlier, TLP-induced CF release was studied at 37°C as a function of [Ca2+] present in the membrane vesicle incubation medium. The experiments were performed with trypsinized TLP, the RF strain, and pig jejunal brush border membrane vesicles suspended in the membrane buffer supplemented with 200 mM Tris-Cl and variable [Ca2+]. The time-dependent CF release reaction observed in the absence of added calcium (nominal [Ca2+] of about 5 μM; Fig. 5) illustrates the sigmoidal kinetics characterizing trypsinized TLPs, RF strain (21). Addition of an extra 50 μM Ca2+ resulted in complete inhibition (Fig. 5). Addition of Ca2+ at intermediate concentrations gave partial inhibitions, indicating that the lag period increased with [Ca2+], exhibiting values of 70, 240, and 340 s for additional CaCl2 at 0, 2, and 10 μM, respectively. Inversely, both the apparent rate constant (given by the slope of the curve [17, 21]) and the amplitude decreased as [Ca2+] increased. These results indicate that conditions stabilizing the virion in the TLP form inhibited CF release, whereas conditions favoring outer-capsid protein loss activated this process. Consequently, KCa was assigned a value of 30 μM, equivalent to that estimated by agar electrophoresis in similar conditions (KCa of 20 μM; Table 1).
FIG. 5.
Effect of the calcium present in the membrane vesicle incubation medium on time-dependent rotavirus-induced CF release. CF-loaded vesicles were incubated at 37°C in the membrane buffer supplemented with 200 mM Tris-Cl in the absence (none) or the presence of micromolar concentrations of extra CaCl2, as indicated. Purified TLPs, RF strain, kept at 4°C were added at time zero. The final vesicular and viral protein concentrations were 6 and 13 μg/ml, respectively.
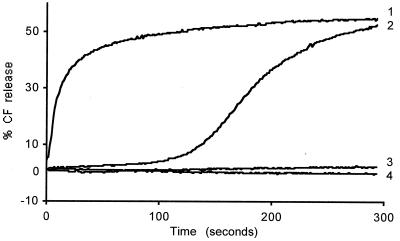
To verify that virions stabilized in the TLP form are inactive, additional experiments were performed using low-calcium buffers (e.g., 5 μM nominal [Ca2+]). TLPs were added to CF-loaded vesicles incubated with either the membrane buffer supplemented with 200 mM Tris-chloride at 20°C or a similar but salt-free sorbitol buffer at 37°C. In both situations, CF release was fully inhibited (Fig. 6, curve 4), confirming that stabilization of the outer capsid prevents membrane permeabilization.
FIG. 6.
Effect of the ionic strength of the incubation medium on CF release induced by either intact or predecapsidated TLPs. CF-loaded vesicles were incubated at 37°C in the membrane buffer supplemented with either 500 mM sorbitol (curves 3 and 4) or 100 mM sorbitol plus 200 mM Tris-Cl (curves 1 and 2). At time zero, TLPs, strain RF, were added either directly (curves 2 and 4) or after treatment with 10 mM EGTA (curves 1 and 3). The final vesicular and viral protein concentrations were 8.5 and 18.8 μg/ml, respectively.
The conditions demonstrating the existence of a close correlation between TLP uncoating and CF release are shown in Table 22.
TABLE 2.
Relationship among the conditions governing the rotavirus TLP-to-DLP transformation and the vesicle membrane CF release reactionsa
| [Ca2+] (μM) | Temp (°C) | Salt supplement | TLP-to-DLP transition | CF release |
|---|---|---|---|---|
| 2–1,000 | 20 | ± | No | No |
| 2–10 | 37 | Yes | Yes | Yes |
| >10, <50 | 37 | Yes | Partial | Yes |
| 50–1,000 | 37 | Yes | No | No |
| 2–1,000 | 37 | No | No | No |
The TLP-to-DLP transition for the RF strain was studied using both quasielastic light scattering and agarose gel electrophoresis. In CF release experiments, membrane vesicles were incubated at either 20 or 37°C in the membrane buffer supplemented (yes) with 100 mM sorbitol plus 200 mM Tris-Cl or not supplemented (no) in the presence of extra Ca2+ at the indicated concentrations. When 200 mM Tris-Cl was not added, 500 mM d-sorbitol was used to maintain iso-osmotic conditions (13). ±, identical results were obtained in either the presence or absence of added salt. Purified TLPs, RF strain, were added directly to the Ca2+-treated membrane vesicle incubation medium, as described for Fig. 5.
Role of the ionic strength of the medium in membrane permeabilization.
To better understand the permeabilizing role of the outer-capsid proteins, CF release was studied by using either intact or previously decapsidated TLPs and media of either high or low ionic strength. Full virion decapsidation, yielding DLP plus the free outer-shell proteins, was achieved by treating the TLPs with 10 mM EGTA independently of the ionic strength of the medium.
In agreement with previous results (21), the CF release reaction in media of high ionic strength (Tris-Cl, HIS buffer) was found to exhibit either sigmoidal or hyperbolic kinetics, depending on whether intact or fully decapsidated TLPs were used (Fig. 6, curves 2 and 1, respectively). In contrast, total absence of CF release was observed when either intact or fully decapsidated virus particles were added to membrane vesicles in LIS buffers (Fig. 6, curves 4 and 3), indicating that membrane permeabilization by the free outer-capsid proteins strictly requires a vesicle incubation medium with high ionic strength.
DISCUSSION
We introduce a new approach based on determining the hydrodynamic diameters of rotavirus particles by quasielastic light scattering (19). We show that, for both classes of particles (TLP and DLP) and independently of the strain (SA11 and RF), diameters are about 30% larger than those observed by Yeager et al. (29) using cryoelectron microscopy. In principle, the larger size observed with light scattering can be explained in terms of the water of hydration, which is included in the hydrodynamic diameter measurement as it stems directly from the Stokes-Einstein equation used for the calculation (19). By using both this method and agarose gel electrophoresis, we show that the Ca2+ dissociation constant, KCa, governing the rotavirus TLP-to-DLP transition is modulated by the ionic strength and temperature. We do not consider pH as a variable, all the experiments having been performed at pH 7.2. However, other authors have shown, for instance, that an alkali treatment increasing the pH to 11.2 causes specific release of VP4 from the SA11 strain (1).
As summarized in Table 1, KCa appears to increase gradually as the temperature or ionic strength of the incubation medium or both increase. According to Ruiz et al. (22), complete loss of the outer-capsid proteins of rotavirus RF and SA11 strains takes place at around 600 nM and 10 to 20 nM [Ca2+], respectively, when TLPs are incubated at 20°C in a buffer containing 100 mM KCl. Our data show that similar results (KCa20°C of 1 μM or lower for the RF strain and 18 nM for the SA11 strain) are obtained at 20°C, but not at 37°C (Table 1). We further show that similar KCa values for the RF strain can be obtained, either at low temperatures (4 to 20°C), irrespective of the ionic strength, or at 37°C, but only in the presence of LIS buffers. At 37°C, increasing the ionic strength (200 mM salt) caused KCa37°C to increase to 20 μM, indicating that a relatively high ionic strength can by itself favor TLP uncoating. Further increases in the KCa values occurred when the temperature was raised to 60°C; these increases varied from about 100 to 300 μM, depending on whether LIS or HIS buffer was used. These last results also demonstrate for the first time that TLP uncoating, which can be observed at 60°C, can be prevented by treating the particles with relatively high [Ca2+]. Taken as a whole, it seems clear that KCa can vary quite drastically, depending on the experimental conditions. The most salient feature is that increasing both the ionic strength and the temperature to physiological values caused KCa to increase to 20 μM for the RF strain and 700 nM for the SA11 strain, further indicating that KCa is specific to the rotavirus strain. Surprisingly, the KCa values for the SA11 strain are 30 to 50 times smaller than those for the RF strain in similar conditions (Table 1).
One question that should be considered is how the present in vitro study can be related to the in vivo situation. We have seen that, in vitro, increasing both the ionic strength and the temperature to physiological values can induce uncoating of the free, soluble TLPs but that complete loss of the outer-capsid proteins is prevented at [Ca2+] values above 800 nM for the SA11 strain and 25 μM for the RF strain. Accordingly, when free in the intestinal lumen, rotavirus can never be decapsidated because the bulk of the intraluminal fluid exhibits [Ca2+] values in the millimolar range. On the other hand, the SA11 and RF strains might be thought to be decapsidated within an intracellular compartment, probably in endocytic vesicles (15, 20). It has been proposed that various calcium transport systems present in the endosomal membrane contribute to equilibration of the endosomal [Ca2+] at the level of intracellular [Ca2+] ([Ca2+]i ≈ 100 to 450 nM [2, 5]), thereby facilitating virion uncoating (20, 23). Using MA104 cells, Cuadras et al. (5) determined the basal [Ca2+]i to be 280 nM. More recently, Brunet et al. (2) have shown that the [Ca2+]i value of 137 nM in noninfected human intestinal epithelial Caco-2 cells progressively increased in Caco-2 cells infected with rotavirus RRV to reach values on the order of 450 nM at 18 h postinfection. All of these [Ca2+]i values are well below the values required for rotavirus uncoating at 37°C but not at 20°C. Taken as a whole, our results show that, under physiological conditions, equilibration of intraendosomal [Ca2+] to the level of [Ca2+]i can favor the uncoating process of virions, strengthening the hypothesis that the Ca2+-dependent endocytosis pathway (7, 20, 23) could be the prevailing mode for rotavirus entry.
In the second part of the present study, we established the existence of a close correlation between the rotavirus outer-layer release process and the mechanism of membrane permeabilization by rotavirus (Table 2). All of the above observations, which were not fully understood at first, can now be rationalized in the light of our present results. Intact, trypsinized TLPs are inactive as concerns CF release, and because we already know that both untrypsinized TLPs and DLPs are also inactive in themselves (17, 21), we conclude that it is only in conditions permitting outer-capsid protein loss that CF release can take place. Ruiz et al. (22) have shown that the kinetics of the TLP-to-DLP transition observed in real time by perpendicular light scattering were faster in the presence of a 300 nM [Ca2+] than in the presence of a 1 μM [Ca2+] for the RF strain when using a Ca-EGTA-10 mM MOPS-100 mM KCl buffer at 37°C. Therefore, the CF release sigmoidal kinetics induced by trypsinized virions can be thought to reflect the kinetics of the TLP-to-DLP transition reaction. We propose that the lag period may represent the time required for TLPs to initiate the uncoating process and that the emerging free outer-capsid proteins, by binding to the membrane, could be directly responsible for permeabilization, as evidenced by the rapid increase in CF release. The observation that the CF release kinetics became hyperbolic (without a lag period) when EGTA-treated TLPs are used confirms that only the free outer-shell proteins were able to induce membrane permeabilization. We previously showed that suspensions of the outer-capsid proteins, either in the presence (EGTA-treated TLPs) or absence (the high-speed supernatant of EGTA-treated TLPs) of DLPs induced CF release hyperbolic kinetics if TLP was trypsinized but did not cause any CF release if TLPs were untrypsinized (21). It has also been reported that trypsinized and EDTA-treated rotavirus induced CF release from preloaded liposomes (17) and maintained the ability to mediate fusion from without in cholesterol-treated cells (11).
Interestingly, our results also indicate that there was no CF release when EGTA-treated TLPs were added to membrane vesicles in LIS buffers. This lack of response in media of low ionic strength suggests that the membrane itself plays a key role in the permeabilization reaction, although little is known at present about the possible mechanism(s) involved. In experiments with porcine intestinal brush border membranes, Ohyashiki et al. (18) observed that increasing the KCl concentration in the extravesicular medium caused a rearrangement of the membrane lipids, resulting in an increase in the overall membrane fluidity. On the basis of our complete results, we conclude that membrane permeabilization must be preceded by both trypsinization and uncoating of TLPs and that the permeabilization process requires high (physiological) ionic strength.
From the present study, we cannot deduce the exact role played by each of the trypsinized, outer-layer proteins (VP7, VP5*, and VP8*) in the membrane permeabilization process. Nevertheless, definite progress in this direction has recently been made. We earlier reported that virus-like particles (VLP) containing VP2, VP6, and VP7 but lacking VP4 could cause membrane permeabilization as soon as VLP2, -6, and -7 were both trypsinized and decapsidated (3). It was further suggested that the permeabilizing agent(s) must be one or several peptides resulting from cleavage products of VP7, once dissociated from TLPs or VLP2, -6, and -7 (3). Also, we previously reported that the CF release reaction was partially inhibited when an anti-VP5* antibody, but not an anti-VP8* antibody, was preincubated with trypsinized TLPs (21). More recently, Denisova et al. (6) demonstrated that the purified VP5* protein was able to permeabilize liposomes in the absence of other rotavirus proteins and that the permeabilizing effect was fully abolished by various anti-VP5* antibodies (6). These reports indicated quite strongly that each of the two (trypsinized) free outer-layer proteins, VP5* and VP7, could independently cause membrane permeabilization. It is not clear why two proteins as different as VP5* and VP7 can each have a similar membrane-destabilizing effect. The same question applies to NSP4, a nonstructural rotavirus protein recently shown to have membrane permeabilization properties (26). Mention should also be made of a study indicating that antibodies to VP7 as well as antibodies to VP5* were found to inhibit cell-cell fusion (11). As suggested by these authors (11), there seems to be a close relationship between the mechanisms of syncytium formation and rotavirus entry into the cells.
In conclusion, the inability of rotavirus to be decapsidated in the intestinal lumen but its ability to be uncoated within endosomes may provide further evidence for the Ca2+-dependent endocytic pathway during the rotavirus entry process (7, 20, 23). To date, there have been two hypothetical models that describe how endocytosis of trypsinized TLP leads to the delivery of transcriptionally active DLP into the cytoplasm of cells. However, these models differ with respect to the mechanisms of both permeabilization and lysis of endosomal membranes. The model of Ruiz et al. (20) hypothesized that equilibration of endosomal [Ca2+] to [Ca2+]i favored full virion uncoating and that the free outer-capsid proteins were able to permeabilize, and then to lyse, the endosomal membranes. More recently, Dowling et al. (7) demonstrated that the purified VP5* protein had permeabilizing but not lytic effects. Accordingly, these authors proposed a model in which virion-bound VP5*, by selectively permeabilizing the early endosomal membrane, could accelerate the lowering of endosomal [Ca2+] and that the concomitant uncoating of virions could release VP7-cleaved peptides with permeabilizing and lytic functions (7). The latter model, however, appears to be incompatible with our findings that TLP uncoating occurs before, and not after, membrane permeabilization. We postulate, therefore, that there must be tight coupling in time between loss of the outer-capsid proteins, membrane permeabilization, and membrane lysis, all of which need to take place in the endocytic vesicle for rotavirus infection to occur. The present paper offers an alternative explanation for the mechanism of rotavirus entry, compatible with the Ca2+-dependent endocytic pathway. We propose that, after infectious rotavirus enters into early endosomal vesicles, the progressive decrease of endosomal [Ca2+] favors progressive virion decapsidation and subsequent permeabilization of endosomal membrane. This permeabilization reaction would in turn accelerate the lowering of endosomal [Ca2+], and hence virion decapsidation and membrane permeabilization. This three-step process (lowering of [Ca2+], uncoating, and permeabilization) would be reiterated until the transcriptionally active DLP is delivered into the cytoplasm of cells. Whether or not VP7-cleaved peptides are responsible for endosomal lysis, as suggested in the model of Dowling et al. (7), cannot be deduced from the present study.
Acknowledgments
We thank our colleague Jacqueline Cotte-Lafitte for assistance in the viral production. We also thank Sheila Carrodus for help in preparing the manuscript.
This work was supported in part by the Institut National de la Santé et de la Recherche Médicale (INSERM), by the Fondation pour la Recherche Médicale, Paris, France, by the INCO Program of the European Economic Community (grant ERB-3514-PL-950019), and the Ministère Français de l’Education Nationale, de la Recherche et de la Technologie (grant MENRT-PRFMMIP).
REFERENCES
- 1.Anthony, I. D., S. Bullivant, S. Dayal, A. R. Bellamy, and J. A. Berriman. 1991. Rotavirus spike structure and polypeptide composition. J. Virol. 65:4334–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunet, J. P., J. Cotte-Laffitte, C. Linxe, A. M. Quero, M. Géniteau-Legendre, and A. Servin. 2000. Rotavirus infection induces an increase in intracellular calcium concentration in human intestinal epithelial cells: role in microvillar actin alteration. J. Virol. 74:2323–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charpilienne, A., M. J. Abad, F. Michelangeli, F. Alvarado, M. Vasseur, J. Cohen, and M. C. Ruiz. 1997. Solubilized and cleaved VP7, the outer glycoprotein of rotavirus, induces permeabilization of cell membrane vesicles. J. Gen. Virol. 78:1367–1371. [DOI] [PubMed] [Google Scholar]
- 4.Cohen, J., J. Laporte, A. Charpilienne, and R. Scherrer. 1979. Activation of rotavirus RNA polymerase by calcium chelation. Arch. Virol. 60:177–186. [DOI] [PubMed] [Google Scholar]
- 5.Cuadras, M. A., C. F. Arias, and S. Lopez. 1997. Rotaviruses induce an early membrane permeabilization of MA 104 cells and do not require a low intracellular Ca2+ concentration to initiate their replication cycle. J. Virol. 71:9065–9074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denisova, E., W. Dowling, R. LaMonica, R. Shaw, S. Scarlata, F. Ruggeri, and E. R. Mackow. 1999. Rotavirus capsid protein VP5* permeabilizes membranes. J. Virol. 73:3147–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dowling, W., E. Denisova, R. Lamonica, and E. R. Mackow. 2000. Selective membrane permeabilization by the rotavirus VP5* protein is abrogated by mutations in an internal hydrophobic domain. J. Virol. 74:6368–6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estes, M. K. 1994. Rotaviruses: molecular biology, p.1281–1290. In R. G. Webster and A. Granoff (ed.), Encyclopedia of virology. Academic Press, Harcourt Brace & Co., New York, N.Y.
- 9.Estes, M. K., and J. Cohen. 1989. Rotavirus gene structure and function. Microbiol. Rev. 53:410–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estes, M. K., D. Y. Graham, E. M. Smith, and C. P. Gerba. 1979. Rotavirus stability and inactivation. J. Gen. Virol. 43:403–409. [DOI] [PubMed] [Google Scholar]
- 11.Falconer, M. M., J. M. Gilbert, A. M. Roper, H. B. Greenberg, and J. S. Gavora. 1995. Rotavirus-induced fusion from without in tissue culture cells. J. Virol. 69:5582–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham, D. Y., and M. K. Estes. 1980. Proteolytic enhancement of rotavirus infectivity: biological mechanisms. Virology 101:432–439. [DOI] [PubMed] [Google Scholar]
- 13.Halaihel, N., V. Liévin, F. Alvarado, and M. Vasseur. 2000. Rotavirus infection impairs intestinal brush border membrane Na+-solute cotransport activities in young rabbits. Am. J. Physiol. 279:G587–G596. [DOI] [PubMed] [Google Scholar]
- 14.Hauser, H., K. Howell, R. M. C. Dawson, and D. E. Boyer. 1980. Rabbit small intestinal brush border membrane preparation and lipid composition. Biochim. Biophys. Acta 602:567–577. [DOI] [PubMed] [Google Scholar]
- 15.Ludert, J. E., F. Michelangeli, F. Gil, F. Liprandi, and J. Esparza. 1987. Penetration and uncoating of rotaviruses in cultured cells. Intervirology 27:95–101. [DOI] [PubMed] [Google Scholar]
- 16.Mackow, E. R., R. D. Shaw, S. M. Matsui, P. T. Vo, M. N. Dang, and H. B. Greenberg. 1988. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc. Natl. Acad. Sci. USA 85:645–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nandi, P., A. Charpilienne, and J. Cohen. 1992. Interaction of rotavirus particles with liposomes. J. Virol. 66:3363–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohyashiki, K., M. Kodera, and T. Mohri. 1985. Inhibitory effects of monovalent cations on the Ca2+-induced aggregation of porcine intestinal brush border membranes. J. Biochem. 98:1441–1446. [DOI] [PubMed] [Google Scholar]
- 19.Ollivon, M., A. Walter, and R. Blumenthal. 1986. Sizing and separation of liposomes, biological vesicles, and viruses by high-performance liquid chromatography. Anal. Biochem. 152:262–274. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz, M. C., M. J. Abad, A. Charpilienne, J. Cohen, and F. Michelangeli. 1997. Cell lines susceptible to infection are permeabilized by cleaved and solubilized outer layer proteins of rotavirus. J. Gen. Virol. 78:2883–2893. [DOI] [PubMed] [Google Scholar]
- 21.Ruiz, M. C., S. R. Alonso-Torre, A. Charpilienne, M. Vasseur, F. Michelangeli, J. Cohen, and F. Alvarado. 1994. Rotavirus interaction with isolated membrane vesicles. J. Virol. 68:4009–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz, M. C., A. Charpilienne, F. Liprandi, R. Gajardo, F. Michelangeli, and J. Cohen. 1996. The concentration of Ca2+ that solubilizes outer capsid proteins from rotavirus particles is dependent on the strain. J. Virol. 70:4877–4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiz, M. C., J. Cohen, and F. Michelangeli. 2000. Role of Ca2+ in the replication and pathogenesis of rotavirus and other viral infections. Cell Calcium 28:137–149. [DOI] [PubMed] [Google Scholar]
- 24.Sabara, M., J. E. Gilchrist, G. R. Hudson, and L. A. Babiuk. 1985. Preliminary characterization of an epitope involved in neutralization and cell attachment that is located on the major bovine rotavirus glycoprotein. J. Virol. 53:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw, A. L., R. Rothnagel, D. Chen, R. F. Ramig, W. Chiu, and B. V. V. Prasad. 1993. Three-dimensional visualization of the rotavirus hemagglutinin structure. Cell 74:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian, P., J. M. Ball, C. Q. Zeng, and M. K. Estes. 1996. The rotavirus nonstructural glycoprotein NSP4 possesses membrane destabilization activity. J. Virol. 70:6973–6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsien, R., and R. Pozzan. 1989. Measurements of cytosolic free Ca2+ with Quin 2. Methods Enzymol. 172:230–263. [DOI] [PubMed] [Google Scholar]
- 28.Willoughby, R. E., R. H. Yolken, and R. L. Schnaar. 1990. Rotaviruses specifically bind to the neutral glycosphingolipid asialo-GM1. J. Virol. 64:4830–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeager, M. J., A. Berriman, T. S. Baker, and A. R. Bellamy. 1994. Three-dimensional structure of the rotavirus haemagglutinin VP4 by cryo-electron microscopy and difference map analysis. EMBO J. 13:1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]