Abstract
The repertoire of human cytotoxic T-lymphocytes (CTL) in response to influenza A viruses has been shown to be directed towards multiple epitopes, with a dominant response to the HLA-A2-restricted M158–66 epitope. These studies, however, were performed with peripheral blood mononuclear cells (PBMC) of individuals selected randomly with respect to HLA phenotype or selected for the expression of one HLA allele without considering an influence of other HLA molecules. In addition, little information is available on the influence of HLA makeup on the overall CTL response against influenza viruses. Here, the influenza A virus-specific CTL response was investigated in groups of HLA-A and -B identical individuals. Between groups the individuals shared two or three of the four HLA-A and -B alleles. After in vitro stimulation of PBMC with influenza virus, the highest CTL activity was found in HLA-A2+ donors. A similar pattern was observed for the precursor frequency of virus-specific CTL (CTLp) ex vivo, with a higher CTLp frequency in HLA-A2-positive donors than in HLA-A2-negative donors, which were unable to recognize the immunodominant M158–66 epitope. In addition, CTL activity and frequency of CTLp for the individual influenza virus epitopes were determined. The frequency of CTLp specific for the HLA-B8-restricted epitope NP380–388 was threefold lower in HLA-B27-positive donors than in HLA-B27-negative donors. In addition, the frequency of CTLp specific for the HLA-A1-restricted epitope NP44–52 was threefold higher in HLA-A1-, -A2-, -B8-, and -B35-positive donors than in other donors, which was confirmed by measuring the CTL activity in vitro. These findings indicate that the epitope specificity of the CTL response is related to the phenotype of the other HLA molecules. Furthermore, the magnitude of the influenza virus-specific CTL response seems dependent on the HLA-A and -B phenotypes.
Influenza viruses are negative-sense RNA viruses that cause annual epidemics in the human population. Vaccination against influenza virus, aiming at the induction of virus neutralizing antibodies specific for the hemagglutinin (HA) and neuraminidase (NA) proteins, induces protective immunity when the HA and NA proteins of the vaccine strains closely resemble those of the circulating virus strains. Mutation of these proteins can result in viral escape from neutralizing antibodies (antigenic drift). In addition, occasionally new potentially pandemic influenza viruses emerge with novel HA and NA proteins, against which preexisting antibodies are absent in the human population (antigenic shift). In these cases, cytotoxic T lymphocytes (CTL) directed to more conserved internal proteins, such as nucleoprotein (NP), matrix protein, and polymerase proteins may contribute to protective immunity against these pandemic viruses (25, 32, 37, 49), although changes in these internal proteins causing loss of CTL recognition have also been described (33, 51).
Influenza virus-specific CTL-mediated immunity has been investigated in both humans and mice. In mice, the CTL response was found to be directed to a limited number of epitopes. This ability of the immune system to focus the T-cell responses to a limited number of epitopes is termed immunodominance (7, 8, 25, 52). In B6 mice the influenza A virus-specific CTL responses were directed against a number of H-2b-restricted immunodominant epitopes (3, 5, 6). Likewise, after an infection of lymphocytic choriomeningitis virus, the CTL response in mice was mainly directed against an epitope derived from the NP (50). Similar examples of immunodominance have been described for humans. In HLA-A2+ donors, the CTL response against influenza virus is predominantly directed to the HLA-A2-restricted epitope of the matrix protein (GILGFVFTL; M158–66) (2, 24, 25, 39). For Epstein-Barr virus (EBV) infections it was reported that the cellular response is focused towards HLA-A11- and HLA-B8-restricted epitopes (23, 46). Nevertheless, other studies have indicated that the CTL response after both acute (influenza virus) and chronic (human immunodeficiency virus [HIV]) viral infections can be directed to a large number of epitopes (9, 28, 45).
Several factors have been reported to contribute to the phenomenon of immunodominance, such as gamma interferon (IFN-γ) production, major histocompatibility complex (MHC) binding affinity of the epitopes, available T-cell receptor repertoire, epitope abundance, and antigen processing (1, 12, 13, 16, 21, 22, 38). However, the correlation between immunodominance and epitope-MHC binding affinity is presently under debate (15, 40). Several other factors that could possibly have an effect on immunodominance have not been investigated thoroughly, such as the influence of HLA phenotype and patient age, gender, and history of infection. The first evidence regarding the involvement of MHC phenotype in immunodominance was observed by Doherty et al., describing in mice a minimization of the H-2Db response in the presence of H-2Kk (18). Using modern tools for the measurement of CTL activity, these early findings were confirmed recently (4). In humans the role of HLA phenotype in immunodominance is still unclear. Tussey et al. described the absence of an HLA-B8-restricted response against the influenza A virus NP380–388 epitope in a HLA-B27+, HLA-B8+ donor (48) due to competition for overlapping epitopes in the endoplasmatic reticulum by HLA-B8 and HLA-B*2702, resulting in the suboptimal loading of HLA-B8.
Although influenza virus-specific immunity mediated by CTL has been studied to a certain extent, in these studies peripheral blood mononuclear cells (PBMC) were obtained from donors selected for the presence of one particular HLA molecule, not controlling for HLA background, age, or history of infection. In the present study CTL immunity to influenza A virus and its individual epitopes was studied in donors with identical HLA-A and -B phenotypes and was compared with that of donors who share two or three out of the four HLA-A or -B alleles, enabling us to investigate the effect of HLA phenotype on the specificity and the magnitude of the CTL response. To this end the precursor frequency of CTL (CTLp) and CTL activity directed against influenza A virus and individual epitopes were determined ex vivo and after in vitro stimulation of PBMC with influenza virus, respectively.
MATERIALS AND METHODS
Human subjects.
A total of 18 healthy blood donors, between 35 and 50 years of age, were selected according to serological homology within the A locus and B locus of HLA class I molecules (Table 1). Genetic subtyping of the HLA-A and HLA-B loci was performed by using a commercial typing system (GenoVision, Vienna, Austria). PBMC were isolated by Lymphoprep (Nycomed, Norway) gradient centrifugation and were cryopreserved at −135°C. Serum samples were stored at −20°C and were used for serology (see below). All donors had serum antibodies against one or more influenza A virus strains (A/H1N1 or A/H3N2), measured by hemagglutination inhibition assay (HIA), indicative of one or more exposures to influenza virus in the past.
TABLE 1.
HLA-A and -B genotypes of the 18 donors
| Group | Donor | Genotype
|
|
|---|---|---|---|
| HLA-A | HLA-B | ||
| I | 1 | 0101, 0201 | 0801, 3501 |
| 2 | 0101, 0201 | 0801, 3501 | |
| 3 | 0101, 0201 | 0801, 3501 | |
| 4 | 0101, 0201 | 0801, 3501 | |
| II | 5 | 0101, 0201 | 0801, 2705 |
| 6 | 0101, 0201 | 0801, 2705 | |
| 7 | 0101, 0201 | 0801, 2705 | |
| 8 | 0101, 0201 | 0801, 2702 | |
| 9 | 0101, 0201 | 0801, 2705 | |
| III | 10 | 0101, 0301 | 0801, 3501 |
| 11 | 0101, 0301 | 0801, 3501 | |
| 12 | 0101, 0301 | 0801, 3501 | |
| 13 | 0101, 0301 | 0801, 3501 | |
| 14 | 0101, 0301 | 0801, 3501 | |
| 15 | 0101, 0301 | 0801, 3503 | |
| 16 | 0101, 0301 | 0801, 3503 | |
| IV | 17 | 0201, 0302 | 2705, 3501 |
| V | 18 | 0201, 0301 | 0801, 3501 |
Serology.
Serum samples were tested for the presence of influenza A virus-specific antibodies in the HIA according to standard methods (36, 41) using turkey erythrocytes. The sera were tested for antibodies against 10 influenza virus (A/H3N2) vaccine strains used since the emergence of these viruses in 1968 and for antibodies against 9 A/H1N1 strains, including the first isolate of H1N1 (A/Puerto Rico/8/34) and the latest H1N1 vaccine strain (A/New Caledonia/20/99). Ferret sera raised against the test antigens were used as positive controls.
Preparation of BLCL.
Autologous EBV-transformed B-lymphoblastoid cell lines (BLCL) of each donor was established by culturing 1 × 106 to 5 × 106 PBMC in 1 ml of culture supernatant from the EBV-producing cell line S594 in 24-well plates as previously described (43). No BLCL were established for donors 11 and 13
Influenza virus and peptides.
Sucrose gradient-purified influenza A virus (H3N2) Resvir-9, a reassortant between A/Puerto Rico/8/34 (H1N1) and A/Nanchang/933/95 (H3N2), was used for the infection of PBMC or BLCL. The virus was selected for this study because it contains all known influenza A virus CTL epitopes, in contrast to other virus strains (51). The infectious virus titer (109 50% tissue culture infectious doses) was determined in cell culture using Madin-Darby-Canine-Kidney (MDCK) cells as indicator cells, as described previously (42). All peptides (Table 2) corresponding to influenza A virus CTL epitopes were manufactured, high-performance liquid chromatography purified, and analyzed by mass spectrometry (Eurogentec, Seraing, Belgium). Peptides were dissolved in dimethyl sulfoxide at 5.0 μg/ml, diluted in RPMI 1640 (Life Technologies, Rockville, Md.) to 100 μM, and stored at −20°C.
TABLE 2.
Influenza virus CTL epitopes used in this study
| Epitope | Antigen (amino acid positions) | Restriction element | Reference |
|---|---|---|---|
| CTELKLSDY | NP (44–52) | HLA-A1 | 17 |
| VSDGGPNLY | PB1 (591–599) | HLA-A1 | 17 |
| GILGFVFTL | M1 (58–66) | HLA-A*0201 | 2, 39 |
| AIMDKNIIL | NS1 (122–130) | HLA-A*0201 | 33 |
| ILRGSVAHK | NP (265–273) | HLA-A3 | 17 |
| ELRSRYWAI | NP (380–388) | HLA-B*0801 | 47 |
| ADRGLLRDIa | NP (263–271) | HLA-B8 | 44 |
| SRYWAIRTR | NP (383–391) | HLA-B*2705 | 48, 27 |
| RRSGAAGAAVK | NP (174–184) | HLA-B27 | 29 |
| ASCMGLIY | M1 (128–135) | HLA-B*3501 | 19 |
Influenza B virus epitope.
Infected PBMC for the stimulation of influenza virus-specific CTL.
In order to validate the antigen-presenting capacity of PBMC, cells (106/ml) from two HLA-A2-negative, HLA-A1-positive donors were infected with influenza virus (Resvir-9) at a multiplicity of infection (MOI) of 3 in RPMI 1640 supplemented with 10% fetal calf serum, glutamin (2 mM), streptomycin (100 μg/ml), and penicillin (100 IU/ml) (R10F). After 1 h of incubation at 37°C, the cells were pelleted and resuspended in RPMI 1640 medium supplemented with 10% pooled human AB serum, glutamin (2 mM), streptomycin (100 μg/ml), penicillin (100 IU/ml), and 2-mercaptoethanol (20 μM) (R10H) at a concentration of 2 × 106 cells/ml and distributed in a 96-well U-bottom plate (100 μl). In duplicate wells various numbers of cells of an HLA-A2+, HLA-A1-restricted T-cell clone (A1/NP), specific for the influenza A virus NP44–52 epitope, were added and incubated for 5 h at 37°C. Next, GolgiStop (Pharmingen, San Diego, Calif.) was added to each well and the cells were incubated for a subsequent 6 h at 37°C. The staining of intracellular IFN-γ was performed as recommended by the manufacturer by using a Cytofix/Cytoperm kit (Pharmingen), phycoerythrin-conjugated anti-IFN-γ monoclonal antibody (MAb) (559326; Pharmingen), mouse anti-human-HLA-A2 MAb (Biotest AG, Dreieich, Germany), and a fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse MAb (F0313; Dako, Glostup, Denmark) at previously determined optimal MAb concentrations. The percentage of HLA-A2+, IFN-γ+ cells of the A1/NP clone was determined by flow cytometry. A1/NP stimulated with peptide-loaded BLCL and uninfected PBMC were included as positive and negative controls, respectively. The assay was done in duplicate and the results were calculated from the averages of duplicate wells.
In vitro stimulation of PBMC with influenza virus.
PBMC were resuspended at 106/ml in R10F and infected with influenza A virus (Resvir-9) at an MOI of 3 for 1 h at 37°C. After centrifugation the stimulator PBMC were resuspended in R10H and added to uninfected responder PBMC in a 25-cm2-diameter flask at a ratio of 1:1, which was found to be optimal. A total of 10 × 106 PBMC were incubated for 2 days at 37°C before recombinant interleukin 2 was added (50 U/ml). Following a subsequent 7-day incubation at 37°C the cells were harvested, analyzed by flow cytometry, and used as effector cells in the CTL assay.
CTL assay.
HLA-A- and HLA-B-matched BLCL (106) were incubated in R10F in the presence of 5 μM peptide (Table 2) and were used as target cells. In addition, BLCL were infected with influenza A virus (Resvir-9) at an MOI of 1 or were left untreated and used as positive and negative controls, respectively. In order to rule out any bystander activation, every CTL assay contained target cells loaded with an HLA-B8-restricted influenza B virus epitope (NP263–271; Table 2). After incubation for 16 h at 37°C, target cells were washed once in serum-free medium and 5 × 105 target cells were labeled for 1 h at 37°C with 50 μCi of Na2[51Cr]O4 in RPMI 1640 medium. The cells were washed three times in R10F and resuspended at 105 cells/ml. Effector cells were transferred to 96-well V-bottom plates at a concentration of 1 × 105 and 5 × 104 cells/100 μl, and 50 μl of the different target cells were added (effector:target cell ratio [E:T ratio] of 20:1 and 10:1). Furthermore, target cells were lysed with 100 μl of 10% Triton X-100 or incubated with R10F to determine the maximum and spontaneous release. Following 4 h of incubation at 37°C, the supernatants were harvested (Skatron Instruments, Sterling, Va.) and radioactivity was measured by gamma counting. The percentage of specific lysis was calculated with the following formula: [(experimental release − spontaneous release)/(maximum release − spontaneous release)] × 100. The data are presented as the average specific lysis of at least three wells.
Detection of CTL specific for individual influenza virus epitopes by ELISPOT assay.
A 96-well Silent Screen Plate (Life Technologies) was coated with 7.5 μg of anti-IFN-γ MAb 1-DIK (Mabtech, Stockholm, Sweden)/ml in 100 μl of sodium-bicarbonate buffer (0.1 M) overnight at 4°C. The plates were washed three times with phosphate-buffered saline (PBS) and blocked with R10H for 2 h at 37°C. For the detection of epitope-specific CD8+ T cells, PBMC were incubated at a density of 2.5 × 105 cells/well in 150 μl of R10H in the presence of HLA-compatible peptides (10 μM) in quadruplicate wells. The specificity of the ELISPOT assay for the enumeration of epitope-specific CD8+ T cells was previously validated by others (31). Cells treated with phytohemagglutinin (1 μg/ml) (Roche Diagnostics, Mannheim, Germany) and untreated cells were used as positive and negative controls, respectively. After 6 h at 37°C the wells were washed six times with PBS-0.05% Tween 20 (PBST) (Sigma Chemical Co., St. Louis, Mo.). Detection of secreted IFN-γ was done with 100 μl of biotinylated anti-IFN-γ MAb 7-B6-1 (1.0 μg/ml; Mabtech) overnight at 4°C. After the plates were washed three times with PBST, 100 μl of a 1:1,000 diluted streptavidin labeled with alkalin phosphatase was added for 1 h at room temperature. The plates were subsequently washed three times and 100 μl of a phosphatase substrate, BCIP/NBT (Kirkegaard & Perry Laboratories, Gaithersburg, Md.), was added per well. After an incubation of 1 h at room temperature the reaction was terminated by washing the plates three times with water. The frequency of peptide-specific CTLp was based on the number of spots obtained in two independent experiments, counted by two individuals, and given as the number of CTLp per 2.5 × 105 PBMC for peptide-specific cells or percentage of CTLp within the CD8+ T-cell fraction.
Detection of virus-specific CTL by ELISPOT assay.
Since it has been shown that NK cells are specifically activated by influenza virus-infected cells (34), it was necessary to isolate the CD8+ T lymphocytes, followed by the depletion of CD16+ cells, using Dynabeads (see below) in order to exclude IFN-γ spots produced by CD4+ T cells or NK cells. The CD3+, CD8+, CD16-negative cells (>98% pure) were incubated with 4 × 104 autologous influenza A virus-infected BLCL or uninfected BLCL for 90 min at 37°C in a 96-well V-bottom plate following centrifugation for 1 min at 140 × g. For donor 11 and 13 no autologous BLCL were available, and therefore HLA-A- and HLA-B-matched BLCL from donor 10 were used. A total number of 2 × 104 or 1 × 104 effector cells was used, and the assay was performed in quadruplicate. After preincubation the cells were transferred to a plate coated with anti-IFN-γ MAb 1-DIK (see above) and incubated for an additional 5 h at 37°C. Virus-specific CD8+ T cells were visualized as described above, and the spots were counted by two individuals. The frequency of virus-specific CTLp was calculated from the number of specific spots of multiple experiments and given as the percentage of influenza A virus-specific CD8+ T cells within the total CD8 population.
Characterization, depletion, and isolation of T cells from PBMC.
Depletion and isolation of CD4+ or CD8+ T cells was performed using DYNABEADS M-450 (111.05 and 111.07; Dynal, Oslo, Norway) according to the manufacturer’s protocol. The depletion of CD16+ cells was done with pan-anti-mouse immunoglobulin G-labeled Dynabeads (110.22; Dynal) coupled to mouse anti-human-CD16 MAb (30621A; Pharmingen). The success of the procedure was monitored by flow cytometry, using mouse MAb against human CD3 (CD3-RPE; Dako), CD4 (CD4-RPE; Dako), CD8 (CD8-FITC; Dako) and CD16 (CD16; Dako). Flow cytometry was also used to determine the percentage of CD3+, CD4+, and CD8+ cells in each donor before and after stimulation of PBMC with influenza virus.
Statistical analysis.
Results are presented as means ± standard deviations (SD). Statistical significance was determined by using a Student’s t test, and a P value of <0.05 was considered statistically significant. Donors with a genotypic mismatch for a certain HLA class I allele were excluded from the data analysis.
RESULTS
Infected PBMC as antigen-presenting cells for the stimulation of influenza virus-specific CTL.
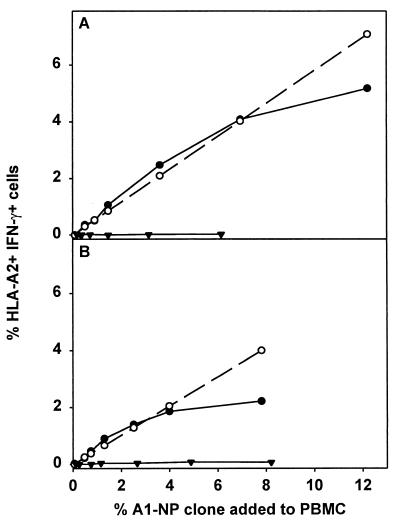
Using an HLA-A1-restricted T-cell clone (A1/NP) specific for NP44–52, the antigen-presenting capacity of influenza virus-infected PBMC was evaluated. First it was demonstrated that a maximum of 58.1% of the cells of the A1/NP clone were activated after stimulation with peptide-loaded HLA-A1+ BLCL. This percentage was used to calculate the expected percentage of IFN-γ-producing cells of the A1/NP clone after stimulation with virus-infected HLA-A1+ PBMC (Fig. 1, dotted line), which was compared with the percentage of cells that were actually activated (Fig. 1, solid circle). Influenza virus-infected PBMC of donor A activated cells of the A1/NP clone to a similar extent as peptide-loaded BLCL when the A1/NP cells constituted up to 4% of the PBMC population (Fig. 1A). At higher cell numbers of the A1/NP clone the antigen-presenting capacity of infected PBMC was no longer sufficient for the activation of all A1/NP cells. Influenza virus-infected PBMC of donor B activated the A1/NP cells when they constituted up to 2% of the total PBMC population (Fig. 1B).
FIG. 1.
Ability of influenza virus-infected PBMC to activate an influenza virus-specific CTL clone. Influenza virus-infected (Resvir-9, MOI = 3) PBMC or uninfected PBMC from two HLA-A1-positive, HLA-A2-negative donors (A and B) were incubated in the presence of serially diluted HLA-A2+ A1/NP clone. The percentage of IFN-γ-positive cells within the A1/NP population was determined by flow cytometry by using HLA-A2-specific antibodies (▾). The calculated percentage of IFN-γ-producing cells of A1/NP clone, determined after multiplying the maximal percentage of IFN-γ-positive A1/NP cells after stimulation with NP44–52 (5 μM)-loaded HLA-A1-positive, HLA-A2-negative BLCL (58.1%) with the percentage of cells of the A1/NP clone present within the total PBMC population, was plotted (dotted lines). When the experimental value (percentage of IFN-γ-positive cells) is similar to the calculated value, the virus-infected PBMC are able to stimulate the cells of the A1/NP clone. Uninfected PBMC did not induce IFN-γ production at any percentage of cells of the A1/NP clone (•). The results represent the averages of duplicate wells.
CD8+ phenotype of effector cells in CTL assays.
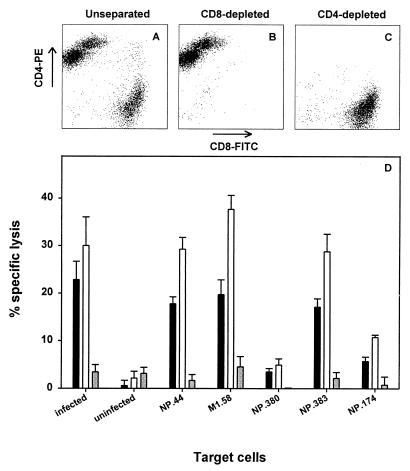
To assess whether in vitro-expanded CD8+ T cells were responsible for the lysis of influenza A virus-infected or peptide-loaded target cells, PBMC of one donor were stimulated with influenza virus, followed by a CTL assay against HLA-matched target cells infected with influenza virus or loaded with a number of different peptides. Prior to the CTL assay the effector cells were divided into three portions. One portion was used to deplete CD4+ T cells, from a second portion the CD8+ T cells were depleted, and a third portion was used as unseparated control cells. The depletion of either CD4+ or CD8+ T cells was confirmed by flow cytometry (Fig. 2). The three effector cell populations were added to target cells at an E:T ratio of 10:1. The lysis of virus-infected target cells and peptide-loaded target cells increased after depletion of CD4+ T cells compared to that of the unseparated control effector cell population. However, after depletion of CD8+ T cells it was found that the specific lysis of the target cells was reduced to background levels (Fig. 2), indicating that the CD8+ T cells were responsible for the lytic activity of virus-stimulated bulk cultures.
FIG. 2.
CD8+ T cells mediate CTL activity of PBMC after in vitro stimulation with influenza virus. Uninfected PBMC (donor 7) were stimulated in vitro with influenza virus-infected PBMC (Resvir-9, MOI = 3) at a ratio of 1:1, and virus-specific T cells were expanded for 9 days. Subsequently the effector cells were divided into three portions: CD4 depleted (panel C and open bars in panel D), CD8 depleted (panel B and grey bars in panel D), or unseparated (panel A and black bars in panel D). Confirmation of depletion was done by flow cytometry (>99% pure). The effector cells were added to Na2[51Cr]O4-labeled target cells (BLCL) at an E:T ratio of 10:1. BLCL were infected with influenza A virus (Resvir-9, MOI = 1), left uninfected, or pulsed with 5 μM HLA-matched viral peptide (as indicated). Results are given as the averages of triplicate wells ± SD.
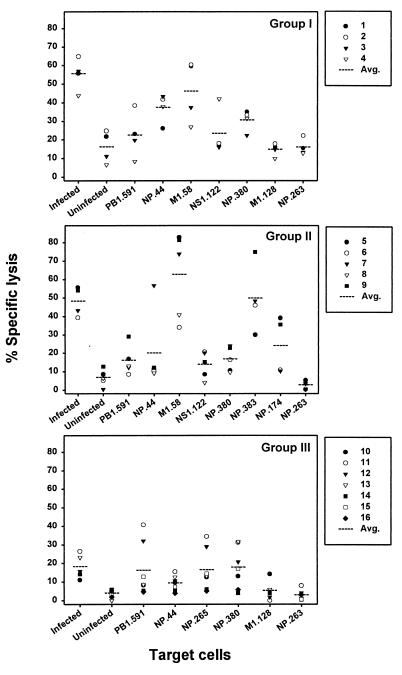
CTL responses to influenza virus and epitopes in HLA class I defined donors.
The CTL responses specific for influenza A virus or viral epitopes were compared within a group of HLA-A and -B identical donors or between groups of donors mismatched for one HLA molecule. The percentage of specific lysis for HLA-A1-, HLA-A2-, HLA-B8-, HLA-B35-positive donors revealed two donors (donors 1 and 2) with the highest percentages of specific lysis against target cells loaded with the HLA-A2-restricted M158–66 epitope (Fig. 3, group I). In donor 3 the highest response was directed towards NP44–52 (HLA-A1), while donor 4 demonstrated the highest response towards NS1122–130, a second HLA-A2-restricted epitope. In the second group, containing HLA-A1-, HLA-A2-, HLA-B8-, HLA-B27-positive donors, the response was dominated by the M158–66 epitope in four out of five donors (Fig. 3, group II). One donor (donor 6) showed a greater response against the HLA-B27-restricted NP383–391 epitope. HLA-A1-, HLA-A3-, HLA-B8-, HLA-B35-positive donors within group III (Table 1) demonstrated a more diverse response against the different epitopes tested. From the seven donors within group III, three donors showed the highest response to the HLA-B8-restricted epitope NP380–388 (Fig. 3, group III). Furthermore, donor 14 had the highest response to the HLA-A3-restricted epitope NP265–273, donor 10 had the highest response against the HLA-B35-restricted epitope M1128–135, and finally donors 11 and 12 responded best to the HLA-A1-restricted epitope PB1591–599. Of the remaining two donors, donor 17, an HLA-A2-, HLA-A3-, HLA-B8-, HLA-B27-positive donor, had the highest percentage of specific lysis against the HLA-B27-restricted epitope NP174–184, whereas donor 18, an HLA-A2-, HLA-A3-, HLA-B27-, HLA-B35-positive donor, showed the highest response to the NP380–388 epitope (data not shown). Overall, the M158–66-specific response was found to be dominant in 7 out of 11 HLA-A2-positive donors compared with the response against the other epitopes.
FIG. 3.
Influenza virus- and epitope-specific CTL activity in PBMC from donors with identical HLA-A and -B phenotypes. PBMC were stimulated with influenza virus, and the resulting effector cells were tested for CTL activity against radioactively labeled HLA-A- and HLA-B-matched target cells (BLCL), either infected with influenza virus (Resvir-9, MOI = 1), left uninfected, or loaded with 5 μM peptide (as indicated) at an E:T ratio of 20:1. The three groups correspond to HLA-A1-, HLA-A2-, HLA-B8-, HLA-B35-positive donors (group I); HLA-A1-, HLA-A2-, HLA-B8-, HLA-B27-positive donors (group II); and HLA-A1-, HLA-A3-, HLA-B8-, HLA-B35-positive donors (group III). The percentage of specific lysis was calculated from at least three wells, and the average CTL activity for a given target cell within a group is plotted (dotted line). The CTL activity against virus-infected BLCL and HLA-B27 peptide-pulsed BLCL were not included for donor 8 (HLA-B*2702+). Furthermore, donors 15 and 16 (HLA-B*3503+) were excluded from the calculation of CTL activity specific for virus-infected BLCL and HLA-B*3501-restricted epitope M1128–135-pulsed BLCL.
For HLA-A1, -A2, and -B27 the response to multiple peptides presented by one HLA molecule could be investigated. It was found that for HLA-A2 the response against M158–66 was higher than the response to NS1122–130 in 10 out of 11 cases (Fig. 3). For the HLA-B27-restricted epitopes (donor 8 was excluded due to its HLA-B*2702 genotype), three donors demonstrated the highest response to NP383–391 and two donors to NP174–184. Finally, the preferred recognition of the two HLA-A1-restricted epitopes, NP44–52 and PB1591–599, was evenly distributed over the donors.
Comparison of the magnitude of the CTL response against virus-infected target cells between the groups of donors revealed a lower average percentage of specific lysis of target cells in group III (P < 0.001; Fig. 3). This inferior recognition of target cells was not caused by a difference in infectibility of the three target cells (data not shown). Therefore, the lower percentage of specific lysis observed for donors within group III is likely to be caused by a reduction in the number of influenza virus-specific CTL after in vitro stimulation of PBMC or a reduced lytic capacity of the respective CTL. When the magnitude of the response against the different influenza virus epitopes presented by shared HLA molecules (A1, B8) was compared between the three donor groups, no difference was found for the HLA-A1-restricted epitope PB1591–599 (Fig. 3). The second HLA-A1-restricted epitope, NP44–52, had a higher response in group I than in both groups II and III. The average percentage of specific lysis specific for the HLA-B8-restricted NP380–388 epitope was lower in the donors from group II (HLA-B27+) than in the donors from groups I and III. The response against the only known B35 epitope was very low, and only two donors (10 and 13) showed a response (>5% specific lysis) against the M1128–135 epitope. As expected, none of the donors had a response against the HLA-B8-restricted irrelevant influenza B virus epitope NP263–271 after stimulation with influenza A virus (Resvir-9).
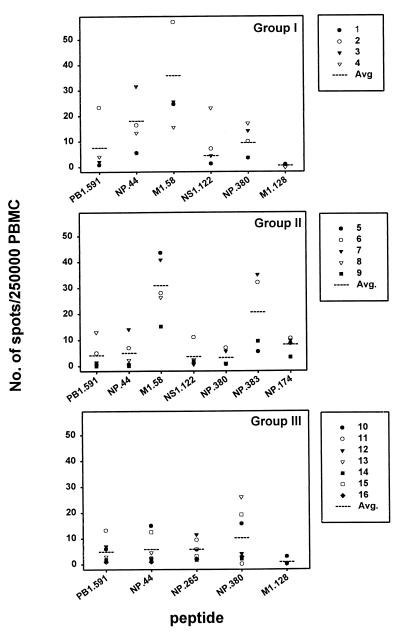
Frequencies of T cells specific for individual influenza virus epitopes in HLA-defined donors.
The highest CTLp frequency in donors 1 and 2 was found to be specific for M158–66 (ranging from 24.6 to 57 per 2.5 × 105 PBMC, respectively) as determined by ELISPOT assay. Donor 3 had the highest frequency of CTLp specific for NP44–52 (31 per 2.5 × 105 PBMC), and donor 4 had the highest frequency of CTLp specific for NS1122–130 (23 per 2.5 × 105 PBMC) (Fig. 4). In donors of group II a dominance of the M158–66 epitope was found in four out of five donors, ranging from 15 spots per 2.5 × 105 PBMC in donor 9 to 43 spots per 2.5 × 105 PBMC in donor 5. Donor 6 showed a higher frequency of NP383–391-specific CTL (32 per 2.5 × 105 PBMC). Five out of seven donors from group III showed a dominant response to the HLA-B8-restricted epitope NP380–388 (2 to 26 per 2.5 × 105 PBMC), while the remaining two donors showed the highest responses to the PB1591–599 epitope (13 spots per 2.5 × 105 PBMC in donor 11) and the NP265–273 epitope (12 spots per 2.5 × 105 PBMC in donor 12), respectively. In donor 17 the dominant response was directed to NP174–184 (88 spots per 2.5 × 105 PBMC), while donor 18 had the highest number of spots after stimulation with M158–66 (13 spots per 2.5 × 105 PBMC) (data not shown).
FIG. 4.
Frequency of CTLp specific for individual influenza virus epitopes. PBMC from HLA-A and -B identical donors were tested for the frequency of influenza A virus epitope-specific CTLp ex vivo by using an ELISPOT assay. The graphs represent the different groups of donors: group I (HLA-A1, -A2, -B8, -B35), group II (HLA-A1, -A2, -B8, -B27), and group III (HLA-A1, -A3, -B8, -B35). The number of spots per 2.5 × 105 PBMC specific for each epitope is given for each donor individually, and the average number of spots is given for the entire group (dotted line). The data are given as the average numbers of specific spots derived from two independent experiments in quadruplicate wells, counted by two individuals. Data from donor 8 (HLA-B*2702+) and donors 15 and 16 (HLA-B*3503+) were excluded for NP383–391 and M1128–135, respectively.
Comparison of the frequencies of CTLp specific for two epitopes presented by the same HLA class I molecule showed that the HLA-A2-restricted epitope M158–66 dominated over NS1122–130 in 10 out of 11 donors (Fig. 4). Within HLA-B27, a higher CTLp frequency was found specific for NP383–391 than for NP174–184 in three out of five donors (Fig. 4 and data not shown). Finally, for the HLA-A1 molecule the NP44–52 epitope was dominantly recognized in 8 out of 16 donors and the PB1591–599 epitope in 4 donors, and the remaining 4 donors had a similar response to both epitopes or no response at all (donor 9).
When the CTLp frequency against the different epitopes was compared between the three groups, a threefold lower NP380–388-specific CTLp frequency was found for the HLA-B8+, HLA-B27+ donors (P = 0.09, group II) compared to that of HLA-B8-positive, HLA-B27-negative donors (Fig. 4). Furthermore, the NP44–52-specific CTLp frequency in donors from group I was three times higher than the CTLp frequency in donors from groups II and III (P = 0.013). No difference in the CTLp frequency specific for M158–66, PB1591–599, and NS1122–130 was observed between the different groups. Finally, it was noted that the response to the HLA-B35-restricted epitope M1128–135 was virtually absent in all donors.
Frequencies of influenza virus-specific T cells in HLA-defined donors.
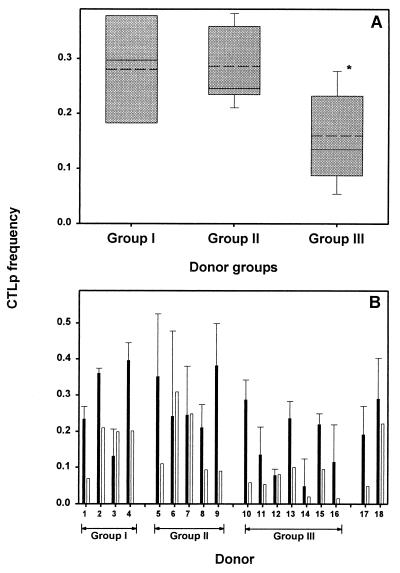
The sum of frequencies of CTLp specific for synthetic peptides representing known CTL epitopes was lower in HLA-A2-negative donors (group III) than in HLA-A2+ donors (groups I and II) (P < 0.05). However, since the whole repertoire of HLA class I-restricted epitopes for influenza A virus is not known, it is possible that in HLA-A2-negative donors a dominant response was elicited against unknown epitopes. To test whether this was the case or that, indeed, in HLA-A2-negative donors the CTL response towards influenza virus is less, the number of influenza A virus-specific CD8+ CTL was enumerated in all donors. In Fig. 5A the average percentage of CTLp frequency is plotted for each of the three groups. In HLA-A1-, HLA-A2-, HLA-B8-, HLA-B35-positive donors (group I) 0.28% of the CD8+ T cells were influenza A virus specific, and in HLA-A1-, HLA-A2-, HLA-B8-, HLA-B27-positive donors (group II) 0.29% were found to be specific for influenza virus. Donors in group III (HLA-A1, -A3, -B8, -B35) had a significantly lower (P = 0.028) influenza virus-specific CTLp frequency (0.16%) compared to that of group II (Fig. 5A) but not compared to that of group I (P = 0.09). Comparison of all HLA-A2+ donors (including donors 17 and 18) with all HLA-A2-negative donors found a significantly higher frequency of CTLp specific for influenza virus for HLA-A2+ donors (P = 0.011).
FIG. 5.
Lower influenza virus-specific CTLp frequencies in HLA-A2-negative donors. (A) The influenza A virus-specific CTLp frequency (presented as the percentage of virus-specific cells of CD8+ T cells), determined in ELISPOT assay after stimulation with influenza virus-infected autologous BLCL and with uninfected autologous BLCL, is plotted for each group of donors: group I (HLA-A1, -A2, -B8, -B35), group II (HLA-A1, -A2, -B8, -B27), and group III (HLA-A1, -A3, -B8, -B35). A significant difference (*, P = 0.028) was found between CTLp frequency specific for influenza virus in HLA-A2+, -B27+ donors (group II) compared to that with HLA-A2-negative donors (group III). The box comprises the 25th to 75th percentiles, while the error bars represent the 10th and 90th percentiles from the average CTLp frequency (solid line). Median CTLp frequency is represented by a dotted line. (B) Representation of the contribution of CTLp frequency specific for individual peptides (open bars) to the total influenza virus-specific CTLp frequency (black bars). The percentage of epitope-specific CTLp was calculated for each donor by dividing the sum of the specific spots found for all epitopes by the number of CD8+ T cells within the PBMC (determined by flow cytometry). Results are given as the average percentages of virus-specific CTLp frequency (black bars) calculated from 2 to 3 independently repeated experiments ± SD, whereas the epitope-specific CTLp frequency (open bars) was determined in two independently repeated experiments. Donors 11 and 13 were stimulated with HLA-matched BLCL from donor 10.
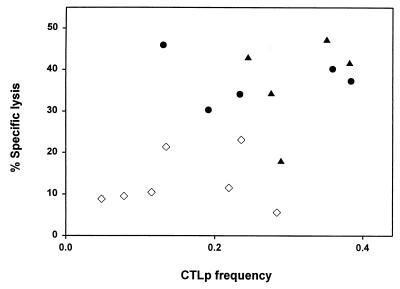
Comparison of the number of spots found after stimulation with whole virus with that found after stimulation with individual peptides revealed that the response against the individual peptides accounted for 12 to 100% of the total response against influenza virus (Fig. 5B). Four donors had a response against the epitopes that was equal to the entire virus-specific response. Overall a correlation (r = 0.8; with the exception of two outliers, donors 3 and 10) was observed between frequencies of virus-specific CTLp and CTL activity (Fig. 6). Figure 6 also shows the difference in CTLp frequency and CTL activity between HLA-A2-positive and HLA-A2-negative donors.
FIG. 6.
Correlation between CTL activity and influenza virus-specific CTLp frequency. Virus-specific CTL activity was plotted, using influenza virus-infected HLA-matched BLCL at an E:T ratio of 20:1, against the frequency of virus-specific CTLp (presented as the percentage of virus-specific cells of CD8+ T cells) obtained from the ELISPOT assays using influenza virus-infected autologous BLCL. All 18 donors were divided into groups: HLA-A2-negative donors (⋄), HLA-A2-positive, HLA-B27-negative donors (•), and HLA-A2-positive, HLA-B27-positive donors (▴). Two HLA-A2-negative donors (11 and 13) were stimulated with HLA-matched BLCL (donor 10). Donor 8 was excluded from the analysis.
DISCUSSION
In this study it was shown that there is a relationship between HLA class I background and the specificity and magnitude of the CTL response specific for influenza A virus and its individual epitopes.
Prior to this study the in vitro stimulation protocol and the phenotype of the resulting effector cells were validated. It was concluded that influenza virus-infected PBMC were able to activate large numbers of cells of a CD8+ T-cell clone (A1/NP) efficiently and quantitatively. In addition, the effector function was solely mediated by CD8+ T cells and the observed CTL activities were not the result of bystander activation, since the response to an influenza B virus epitope (NP263–271) was similar to the response against uninfected control cells.
The virus used for this study was a reassortant virus of A/Puerto Rico/8/34 and A/Nanchang/933/95, which contains the HA, NA, and NP from the A/Nanchang/933/95 virus and all known CTL epitopes for influenza virus. The donors selected for this study were between 35 and 50 years of age, thereby controlling for age-related differences in immune reactivity and reducing the impact of the number of times the donors had been infected with influenza virus. All donors had been infected with influenza virus at least once, as indicated by the presence of virus-specific serum antibodies. Based on the age of the donors it can be expected, however, that they had been infected at least two or three times consecutively, which is important because it has been described that responses against single epitopes differ between primary and secondary infections (10, 11).
The number of virus-specific CTLp ex vivo corresponded to approximately 0.1 to 0.5% of the total number of CD8+ T cells, which is within the normal range of percentages of IFN-γ+ influenza A virus-specific T cells measured by flow cytometry (unpublished observation). Comparison of the percentage of virus-specific CTL between groups revealed a significant difference in CTLp frequency between HLA-A2-positive donors and HLA-A2-negative donors (Fig. 5 and 6), paralleled by a lower in vitro CTL activity against influenza virus-infected target cells in HLA-A2-negative donors. The clinical relevance of a lower frequency of virus-specific CTLp in individuals infected with influenza virus and whether a reduced CTLp frequency in vivo results in a similar reduction in specific CTL activity during virus infection remains unknown. This study is not the first to describe an association between magnitude of CTL response and HLA phenotype in humans. Disease severity in malaria, progression to AIDS in HIV-infected individuals, and human T-cell lymphotropic type I-associated myelopathy were reported to be associated with certain HLA molecules. However, these findings were mainly based on epidemiological data in heterogeneous cohorts of donors (20, 26, 30). In contrast, our study used a very well-defined cohort of HLA identical donors, enabling us to investigate the relationship between HLA phenotype and CTL response in detail.
HLA class I phenotype was found to influence the frequency of CTLp specific for individual influenza virus epitopes. The CTLp frequency specific for the HLA-A1-restricted epitope NP44–52 was significantly higher in HLA-A1-, HLA-A2-, HLA-B8-, HLA-B35-positive donors (group I) than in both groups II and III (Fig. 4), resulting in a higher epitope-specific CTL activity following in vitro stimulation of PBMC with influenza virus. Since the higher CTLp frequency was observed in group I and not in groups II and III, it is difficult to link this difference to one HLA-A or -B molecule. Possibly, HLA-C or HLA class II molecules have influenced the outcome of the CTL response to this epitope. It was also noted that the frequency of CTLp specific for the HLA-B8-restricted epitope NP380–388 was threefold lower in HLA-B8+, B27+ donors (group II). This reduction in frequency of cells specific for the NP380–388 peptide in HLA-B27+ donors can be explained by the presentation of overlapping peptides, as described previously by Tussey et al. (48). However, after in vitro stimulation with influenza virus the difference in CTL activity specific for the NP380–388 epitope between HLA-B27-positive and HLA-B27-negative donors was less pronounced.
From the peptide-specific responses it was concluded that the CTL response against influenza virus is multispecific and directed against a number of different epitopes. On average, 50% (ranging from 12 to 100%) of the virus-specific response is accounted for by known viral epitopes, indicating that in some donors more unknown epitopes are recognized in the response to influenza virus infections. The variability of the peptide-specific responses indicates that these data should be used with caution for the interpretation of the virus-specific CTL response against all epitopes (9). CTL activity in individual donors ranged from specificity towards all known peptides or two peptides, independent of HLA background. The observation that in humans the influenza virus-specific CTL response is directed to multiple epitopes is in agreement with previously described studies (24, 28).
The dominance of the HLA-A2-restricted epitope M158–66 (24) is less strict than previously thought. Although all donors had a response to this epitope, 4 out of 11 HLA-A2+ donors showed a greater response against another epitope. In each of these four cases the dominant epitope was different. This finding adds to a study by Martinon et al., describing a greater response against an HLA-B37-restricted epitope than the HLA-A2 epitope M158–66 (35). It was also noted that in several cases the CTL response restricted to one HLA class I molecule was directed to both epitopes equally, indicating that there was no immunodominant response to one of the two epitopes. A small CTL response to the HLA-B35-restricted epitope M1128–135 was detected in 2 out of 13 HLA-B35+ donors. A possible explanation for the low reactivity against this peptide is the cysteine residue within the epitope enabling the epitope to form dimers and therefore reduce the ability to bind to the HLA molecule (14). However, freshly diluted peptide loaded onto target cells for 1 h did not result in a higher CTL activity. A second possibility is the relatively low binding affinity of the epitope to the HLA molecule (19). Finally, the donors selected for this study can have an undetectable frequency of M1128–135-specific CTL.
To our knowledge this is the first systematic study in which influenza virus-specific CTL responses are compared in groups of donors of well-defined HLA phenotype. The data presented here indicate that the HLA class I background of individuals has a major influence on the magnitude and specificity of the CTL response against influenza A virus. Even in individuals who share a certain HLA allele, the CTL response restricted by this HLA allele may be affected by other nonmatching HLA molecules resulting in different CTL responses. This finding may have implications on vaccination strategies aiming at the induction of CD8+ T-cell responses.
Acknowledgments
Part of this work was supported by the Foundation for Respiratory Virus Infections, Notably Influenza (SRVI), and Numico Research B.V.
We also acknowledge Liane van de Kemp for growing and purifying the influenza A viruses. Furthermore, we thank Wilfried Levering for performing the HLA typing, Saskia Sakko for organizing the blood collection of the blood donors (Bloodbank Rotterdam), and Ger van der Water for continuous support.
REFERENCES
- 1.Badovinac, V. P., A. R. Tvinnereim, and J. T. Harty. 2000. Regulation of antigen-specific CD8(+) T cell homeostasis by perforin and interferon-gamma. Science 290:1354–1358. [DOI] [PubMed] [Google Scholar]
- 2.Bednarek, M. A., S. Y. Sauma, M. C. Gammon, G. Porter, S. Tamhankar, A. R. Williamson, and H. J. Zweerink. 1991. The minimum peptide epitope from the influenza virus matrix protein. Extra and intracellular loading of HLA-A2. J. Immunol. 147:4047–4053. [PubMed] [Google Scholar]
- 3.Belz, G. T., J. D. Altman, and P. C. Doherty. 1998. Characteristics of virus-specific CD8(+) T cells in the liver during the control and resolution phases of influenza pneumonia. Proc. Natl. Acad. Sci. USA 95:13812–13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belz, G. T., P. G. Stevenson, and P. C. Doherty. 2000. Contemporary analysis of MHC-related immunodominance hierarchies in the CD8+ T cell response to influenza A viruses. J. Immunol. 165:2404–2409. [DOI] [PubMed] [Google Scholar]
- 5.Belz, G. T., W. Xie, J. D. Altman, and P. C. Doherty. 2000. A previously unrecognized H-2D(b)-restricted peptide prominent in the primary influenza A virus-specific CD8(+) T-cell response is much less apparent following secondary challenge. J. Virol. 74:3486–3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belz, G. T., W. Xie, and P. C. Doherty. 2001. Diversity of epitope and cytokine profiles for primary and secondary influenza A virus-specific CD8+ T cell responses. J. Immunol. 166:4627–4633. [DOI] [PubMed] [Google Scholar]
- 7.Bennink, J. R., and J. W. Yewdell. 1988. Murine cytotoxic T lymphocyte recognition of individual influenza virus proteins.High frequency of nonresponder MHC class I alleles. J. Exp. Med. 168:1935–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berzofsky, J. A. 1988. Immunodominance in T lymphocyte recognition. Immunol. Lett. 18:83–92. [DOI] [PubMed] [Google Scholar]
- 9.Betts, M. R., J. P. Casazza, B. A. Patterson, S. Waldrop, W. Trigona, T. M. Fu, F. Kern, L. J. Picker, and R. A. Koup. 2000. Putative immunodominant human immunodeficiency virus-specific CD8(+) T-cell responses cannot be predicted by major histocompatibility complex class I haplotype. J. Virol. 74:9144–9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blattman, J. N., D. J. D. Sourdive, K. Murali-Krishna, R. Ahmed, and J. D. Altman. 2000. Evolution of the T cell repertoire during primary, memory, and recall responses to viral infection. J. Immunol. 165:6081–6090. [DOI] [PubMed] [Google Scholar]
- 11.Bousso, P., F. Lemaitre, J. Bilsborough, and P. Kourilsky. 2000. Facing two T cell epitopes: a degree of randomness in the primary response is lost upon secondary immunization. J. Immunol. 165:760–767. [DOI] [PubMed] [Google Scholar]
- 12.Chen, W., S. Khilko, J. Fecondo, D. H. Margulies, and J. McCluskey. 1994. Determinant selection of major histocompatibility complex class I-restricted antigenic peptides is explained by class I-peptide affinity and is strongly influenced by nondominant anchor residues. J. Exp. Med. 180:1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, W., C. C. Norbury, Y. Cho, J. W. Yewdell, and J. R. Bennink. 2001. Immunoproteasomes shape immunodominance hierarchies of antiviral CD8(+) T cells at the levels of T cell repertoire and presentation of viral antigens. J. Exp. Med. 193:1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, W., J. W. Yewdell, R. L. Levine, and J. R. Bennink. 1999. Modification of cysteine residues in vitro and in vivo affects the immunogenicity and antigenicity of major histocompatibility complex class I-restricted viral determinants. J. Exp. Med. 189:1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crotzer, V. L., R. E. Christian, J. M. Brooks, J. Shabanowitz, R. E. Settlage, J. A. Marto, F. M. White, A. B. Rickinson, D. F. Hunt, and V. H. Engelhard. 2000. Immunodominance among EBV-derived epitopes restricted by HLA-B27 does not correlate with epitope abundance in EBV-transformed B-lymphoblastoid cell lines. J. Immunol. 164:6120–6129. [DOI] [PubMed] [Google Scholar]
- 16.Daly, K., P. Nguyen, D. L. Woodland, and M. A. Blackman. 1995. Immunodominance of major histocompatibility complex class I-restricted influenza virus epitopes can be influenced by the T-cell receptor repertoire. J. Virol. 69:7416–7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiBrino, M., T. Tsuchida, R. V. Turner, K. C. Parker, J. E. Coligan, and W. E. Biddison. 1993. HLA-A1 and HLA-A3 T cell epitopes derived from influenza virus proteins predicted from peptide binding motifs. J. Immunol. 151:5930–5935. [PubMed] [Google Scholar]
- 18.Doherty, P. C., W. E. Biddison, J. R. Bennink, and B. B. Knowles. 1978. Cytotoxic T-cell responses in mice infected with influenza and vaccinia viruses vary in magnitude with H-2 genotype. J. Exp. Med. 148:534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong, T., D. Boyd, W. Rosenberg, N. Alp, M. Takiguchi, A. McMichael, and S. Rowland-Jones. 1996. An HLA-B35-restricted epitope modified at an anchor residue results in an antagonist peptide. Eur. J. Immunol. 26:335–339. [DOI] [PubMed] [Google Scholar]
- 20.Flores-Villanueva, P. O., E. J. Yunis, J. C. Delgado, E. Vittinghoff, S. Buchbinder, J. Y. Leung, A. M. Uglialoro, O. P. Clavijo, E. S. Rosenberg, S. A. Kalams, J. D. Braun, S. L. Boswell, B. D. Walker, and A. E. Goldfeld. 2001. Control of HIV-1 viremia and protection from AIDS are associated with HLA-Bw4 homozygosity. Proc. Natl. Acad. Sci. USA 98:5140–5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallimore, A., T. Dumrese, H. Hengartner, R. M. Zinkernagel, and H. G. Rammensee. 1998. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J. Exp. Med. 187:1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallimore, A., J. Hombach, T. Dumrese, H. G. Rammensee, R. M. Zinkernagel, and H. Hengartner. 1998. A protective cytotoxic T cell response to a subdominant epitope is influenced by the stability of the MHC class I/peptide complex and the overall spectrum of viral peptides generated within infected cells. Eur. J. Immunol. 28:3301–3311. [DOI] [PubMed] [Google Scholar]
- 23.Gavioli, R., M. G. Kurilla, P. O. de Campos-Lima, L. E. Wallace, R. Dolcetti, R. J. Murray, A. B. Rickinson, and M. G. Masucci. 1993. Multiple HLA A11-restricted cytotoxic T-lymphocyte epitopes of different immunogenicities in the Epstein-Barr virus-encoded nuclear antigen 4. J. Virol. 67:1572–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gianfrani, C., C. Oseroff, J. Sidney, R. W. Chesnut, and A. Sette. 2000. Human memory CTL response specific for influenza A virus is broad and multispecific. Hum. Immunol. 61:438–452. [DOI] [PubMed] [Google Scholar]
- 25.Gotch, F., A. McMichael, G. Smith, and B. Moss. 1987. Identification of viral molecules recognized by influenza-specific human cytotoxic T lymphocytes. J. Exp. Med. 165:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill, A. V., J. Elvin, A. C. Willis, M. Aidoo, C. E. Allsopp, F. M. Gotch, X. M. Gao, M. Takiguchi, B. M. Greenwood, A. R. Townsend, et al. 1992. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature 360:434–439. [DOI] [PubMed] [Google Scholar]
- 27.Huet, S., D. F. Nixon, J. B. Rothbard, A. Townsend, S. A. Ellis, and A. J. McMichael. 1990. Structural homologies between two HLA B27-restricted peptides suggest residues important for interaction with HLA B27. Int. Immunol. 2:311–316. [DOI] [PubMed] [Google Scholar]
- 28.Jameson, J., J. Cruz, and F. A. Ennis. 1998. Human cytotoxic T-lymphocyte repertoire to influenza A viruses. J. Virol. 72:8682–8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jameson, J., J. Cruz, M. Terajima, and F. A. Ennis. 1999. Human CD8+ and CD4+ T lymphocyte memory to influenza A viruses of swine and avian species. J. Immunol. 162:7578–7583. [PubMed] [Google Scholar]
- 30.Jeffery, K. J., A. A. Siddiqui, M. Bunce, A. L. Lloyd, A. M. Vine, A. D. Witkover, S. Izumo, K. Usuku, K. I. Welsh, M. Osame, and C. R. Bangham. 2000. The influence of HLA class I alleles and heterozygosity on the outcome of human T cell lymphotropic virus type I infection. J. Immunol. 165:7278–7284. [DOI] [PubMed] [Google Scholar]
- 31.Lalvani, A., R. Brookes, S. Hambleton, W. J. Britton, A. V. Hill, and A. J. McMichael. 1997. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186:859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukacher, A. E., V. L. Braciale, and T. J. Braciale. 1984. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J. Exp. Med. 160:814–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Man, S., M. H. Newberg, V. L. Crotzer, C. J. Luckey, N. S. Williams, Y. Chen, E. L. Huczko, J. P. Ridge, and V. H. Engelhard. 1995. Definition of a human T cell epitope from influenza A non-structural protein 1 using HLA-A2.1 transgenic mice. Int. Immunol. 7:597–605. [DOI] [PubMed] [Google Scholar]
- 34.Mandelboim, O., N. Lieberman, M. Lev, L. Paul, T. I. Arnon, Y. Bushkin, D. M. Davis, J. L. Strominger, J. W. Yewdell, and A. Porgador. 2001. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 409:1055–1060. [DOI] [PubMed] [Google Scholar]
- 35.Martinon, F., E. Gomard, C. Hannoun, and J. P. Levy. 1990. In vitro human cytotoxic T cell responses against influenza A virus can be induced and selected by synthetic peptides. Eur. J. Immunol. 20:2171–2176. [DOI] [PubMed] [Google Scholar]
- 36.Masurel, N., P. Ophof, and P. de Jong. 1981. Antibody response to immunization with influenza A/USSR/77 (H1N1) virus in young individuals primed or unprimed for A/New Jersey/76 (H1N1) virus. J. Hyg. 87:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMichael, A. J., F. M. Gotch, G. R. Noble, and P. A. Beare. 1983. Cytotoxic T-cell immunity to influenza. N. Engl. J. Med. 309:13–17. [DOI] [PubMed] [Google Scholar]
- 38.Mo, A. X., S. F. van Lelyveld, A. Craiu, and K. L. Rock. 2000. Sequences that flank subdominant and cryptic epitopes influence the proteolytic generation of MHC class I-presented peptides. J. Immunol. 164:4003–4010. [DOI] [PubMed] [Google Scholar]
- 39.Morrison, J., J. Elvin, F. Latron, F. Gotch, R. Moots, J. L. Strominger, and A. McMichael. 1992. Identification of the nonamer peptide from influenza A matrix protein and the role of pockets of HLA-A2 in its recognition by cytotoxic T lymphocytes. Eur. J. Immunol. 22:903–907. [DOI] [PubMed] [Google Scholar]
- 40.Mullbacher, A., M. Lobigs, J. W. Yewdell, J. R. Bennink, R. Tha Hla, and R. V. Blanden. 1999. High peptide affinity for MHC class I does not correlate with immunodominance. Scand. J. Immunol. 50:420–426. [DOI] [PubMed] [Google Scholar]
- 41.Palmer, D., W. Dowdle, M. Coleman, and G. Schild. 1975. Advanced laboratory techniques for influenza diagnosis, p.25–62. Procedural guide. U.S. Department of Health, Atlanta, Ga.
- 42.Rimmelzwaan, G. F., M. Baars, E. C. Claas, and A. D. Osterhaus. 1998. Comparison of RNA hybridization, hemagglutination assay, titration of infectious virus and immunofluorescence as methods for monitoring influenza virus replication in vitro. J. Virol Methods 74:57–66. [DOI] [PubMed] [Google Scholar]
- 43.Rimmelzwaan, G. F., N. Nieuwkoop, A. Brandenburg, G. Sutter, W. E. Beyer, D. Maher, J. Bates, and A. D. Osterhaus. 2000. A randomized, double blind study in young healthy adults comparing cell mediated and humoral immune responses induced by influenza ISCOM vaccines and conventional vaccines. Vaccine 19:1180–1187. [DOI] [PubMed] [Google Scholar]
- 44.Robbins, P. A., P. A. Rota, and S. Z. Shapiro. 1997. A broad cytotoxic T lymphocyte response to influenza type B virus presented by multiple HLA molecules. Int. Immunol. 9:815–823. [DOI] [PubMed] [Google Scholar]
- 45.Rowland-Jones, S. L., T. Dong, K. R. Fowke, J. Kimani, P. Krausa, H. Newell, T. Blanchard, K. Ariyoshi, J. Oyugi, E. Ngugi, J. Bwayo, K. S. MacDonald, A. J. McMichael, and F. A. Plummer. 1998. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J. Clin. Investig. 102:1758–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silins, S. L., S. M. Cross, S. L. Elliott, S. J. Pye, S. R. Burrows, J. M. Burrows, D. J. Moss, V. P. Argaet, and I. S. Misko. 1996. Development of Epstein-Barr virus-specific memory T cell receptor clonotypes in acute infectious mononucleosis. J. Exp. Med. 184:1815–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sutton, J., S. Rowland-Jones, W. Rosenberg, D. Nixon, F. Gotch, X. M. Gao, N. Murray, A. Spoonas, P. Driscoll, M. Smith, et al. 1993. A sequence pattern for peptides presented to cytotoxic T lymphocytes by HLA B8 revealed by analysis of epitopes and eluted peptides. Eur. J. Immunol. 23:447–453. [DOI] [PubMed] [Google Scholar]
- 48.Tussey, L. G., S. Rowland-Jones, T. S. Zheng, M. J. Androlewicz, P. Cresswell, J. A. Frelinger, and A. J. McMichael. 1995. Different MHC class I alleles compete for presentation of overlapping viral epitopes. Immunity 3:65–77. [DOI] [PubMed] [Google Scholar]
- 49.Ulmer, J. B., J. J. Donnelly, S. E. Parker, G. H. Rhodes, P. L. Felgner, V. J. Dwarki, S. H. Gromkowski, R. R. Deck, C. M. DeWitt, A. Friedman, et al. 1993. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science 259:1745–1749. [DOI] [PubMed] [Google Scholar]
- 50.van der Most, R. G., A. Sette, C. Oseroff, J. Alexander, K. Murali-Krishna, L. L. Lau, S. Southwood, J. Sidney, R. W. Chesnut, M. Matloubian, and R. Ahmed. 1996. Analysis of cytotoxic T cell responses to dominant and subdominant epitopes during acute and chronic lymphocytic choriomeningitis virus infection. J. Immunol. 157:5543–5554. [PubMed] [Google Scholar]
- 51.Voeten, J. T., T. M. Bestebroer, N. J. Nieuwkoop, R. A. Fouchier, A. D. Osterhaus, and G. F. Rimmelzwaan. 2000. Antigenic drift in the influenza A virus (H3N2) nucleoprotein and escape from recognition by cytotoxic T lymphocytes. J. Virol. 74:6800–6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yewdell, J. W., and J. R. Bennink. 1999. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 17:51–88. [DOI] [PubMed] [Google Scholar]