Abstract
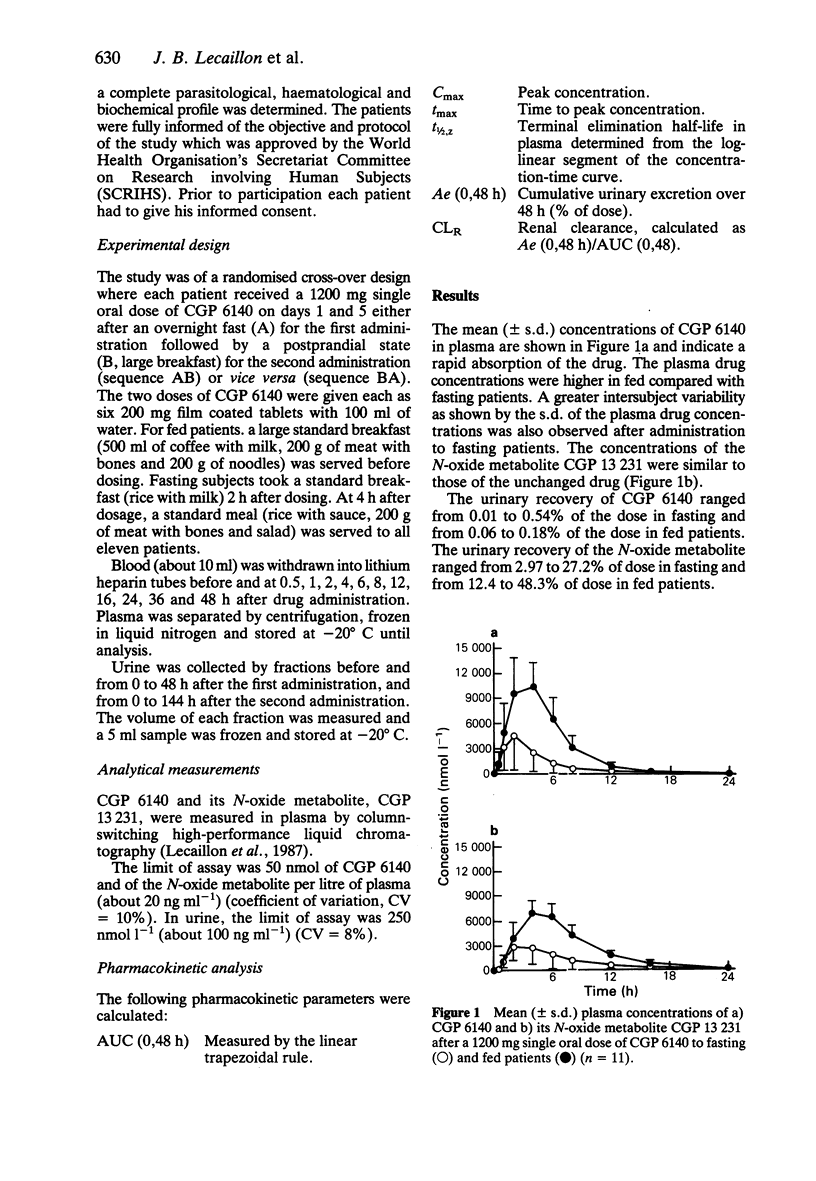
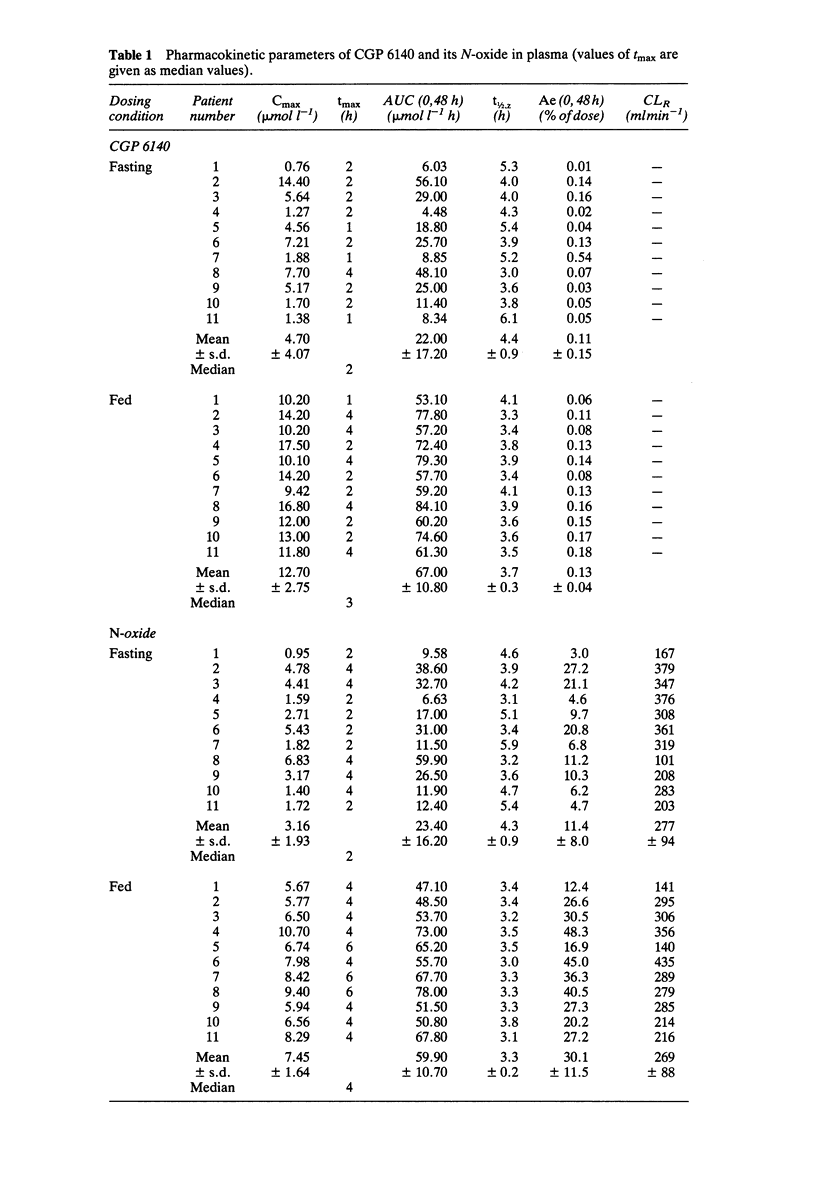
Eleven male patients from Mali with Onchocerca volvulus infections received in random order a 1200 mg single oral dose of CGP 6140 after an overnight fast and after food intake. The concentrations of CGP 6140 and of its N-oxide metabolite, CGP 13231, were measured in plasma and urine. Mean (+/- s.d.) AUC CGP 6140 values were 67.0 +/- 10.8 mumol l-1 h in fed and 22.0 +/- 17.2 mumol l-1 h in fasting patients. The mean maximum concentrations (Cmax) in plasma +/- s.d. were 12.7 +/- 2.8 mumol l-1 in fed and 4.7 +/- 4.1 mumol l-1 in fasting patients. The median time to Cmax was 3 h in fed and 2 h in fasting patients. Mean (+/- s.d.) AUC of the N-oxide metabolite was 59.9 +/- 10.7 mumol l-1 h in fed and 23.4 +/- 16.2 mumol l-1 h in fasting patients. The urinary recovery was less than 0.5% of dose for CGP 6140 in both fed and fasting conditions. It was 30.1 +/- 11.5 and 11.4 +/- 8.0% of the dose for the N-oxide metabolite in fed and fasting conditions, respectively. Variability in plasma concentrations and urinary recovery of CGP 6140 and of the N-oxide metabolite was greater in fasted patients. The low solubility of CGP 6140 in aqueous solutions at neutral pH and its higher solubility at acidic pH might explain the increase in bioavailability after food intake. The administration of CGP 6140 after food intake is therefore recommended for an optimal systemic effect.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Grieve A. P. The two-period changeover design in clinical trials. Biometrics. 1982 Jun;38(2):517–517. [PubMed] [Google Scholar]
- Lange H., Eggers R., Bircher J. Increased systemic availability of albendazole when taken with a fatty meal. Eur J Clin Pharmacol. 1988;34(3):315–317. doi: 10.1007/BF00540964. [DOI] [PubMed] [Google Scholar]
- Lecaillon J. B., Dubois J. P., Awadzi K., Poltera A. A., Ginger C. D. Pharmacokinetics of CGP 6140 (amocarzine) after oral administration of single 100-1600 mg doses to patients with onchocerciasis. Br J Clin Pharmacol. 1990 Oct;30(4):625–628. doi: 10.1111/j.1365-2125.1990.tb03824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münst G. J., Karlaganis G., Bircher J. Plasma concentrations of mebendazole during treatment of echinococcosis: preliminary results. Eur J Clin Pharmacol. 1980 May;17(5):375–378. doi: 10.1007/BF00558451. [DOI] [PubMed] [Google Scholar]
- Toothaker R. D., Welling P. G. The effect of food on drug bioavailability. Annu Rev Pharmacol Toxicol. 1980;20:173–199. doi: 10.1146/annurev.pa.20.040180.001133. [DOI] [PubMed] [Google Scholar]


