Abstract
In contrast to the results of previous in vitro studies, experimental infection of calves with noncytopathic bovine viral diarrhea virus (ncpBVDV) was found to induce strong alpha/beta and gamma interferon responses in gnotobiotic animals. These responses were associated with depressed levels of transforming growth factor β (TGF-β) in serum. The results of this study indicate that the immunosuppression caused by ncpBVDV is not associated with low interferon responses or elevated levels of TGF-β.
Bovine viral diarrhea virus (BVDV) is a positive-sense single-stranded RNA virus belonging to the genus Pestivirus. Two biotypes of the virus, cytopathic and noncytopathic, are identifiable based on their lytic activities in in vitro cultures. The high prevalence of cattle herds infected with BVDV in many countries throughout the world is believed to be a consequence of the ability of noncytopathic BVDV (ncpBVDV) to establish lifelong infections after in utero infection in early pregnancy and thus to generate a reservoir of persistently infected animals. Although acute infections with ncpBVDV are often asymptomatic or produce only mild clinical symptoms, there is evidence that they result in immunosuppression. This supposition is based on experimental studies in which acute infection of calves with BVDV was found to enhance susceptibility to infection with bovine herpesvirus 1 as well as on field observations indicating increased susceptibility to intercurrent infections after acute infection with BVDV. The mechanism of immunosuppression induced by BVDV has not been determined, although there has been considerable speculation, based largely on in vitro observations (reviewed in reference 17). ncpBVDV isolates have been shown not to induce alpha/beta interferon (IFN-α/β) in vitro (2, 8, 16) and to block the induction of IFN by double-stranded RNA or by infection with other viruses (19, 21).
Given the antiviral properties of IFN-α/β and recent evidence that it has a role in promoting Th1 T-cell responses (9), inhibition of IFN-α/β induction by ncpBVDV clearly has the potential to alter immune responses and hence to contribute to increased susceptibility to intercurrent infections. The principal aim of the present study was to determine whether or not ncpBVDV induces IFN-α/β responses in vivo. Gnotobiotic calves (7) were used to provide animals with minimal background cytokine responses and to ensure that any production of IFN was not due to the presence of bacterial endotoxins. Feces samples from each calf were examined each week after birth, and all samples were sterile. In contrast to the in vitro findings with virus-infected cells, ncpBVDV was found to stimulate potent IFN-α/β responses in vivo.
Primary infection with ncpBVDV results in transient suppression of responses to BCG.
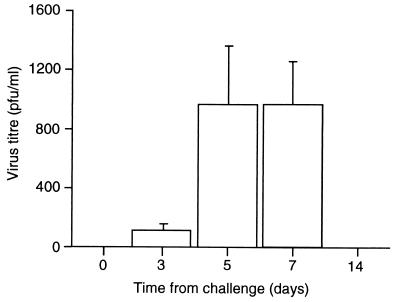
Seven gnotobiotic calves were used in this study. Six of the calves were infected at 30 days of age by intranasal inoculation with 5 × 106 PFU of ncpBVDV 11249, an isolate that has been used extensively in previous experimental studies and that gives reproducible infection profiles (22). The remaining calf was mock infected with virus-free cell culture supernatant instilled intranasally. In order to examine the effect of infection with ncpBVDV on T-cell responses to an unrelated antigen, two of the BVDV-infected calves and the control calf had been vaccinated subcutaneously with 106 CFU of the Pasteur strain of Mycobacterium bovis bacillus Calmette-Guérin (BCG) 28 days prior to infection. Viremia was detected as described previously (11) in the BVDV-challenged animals on days 3, 5, and 7 postinfection (p.i.) (Fig. 1) but not on day 14 or 21 p.i. or in any samples from the mock-infected animal. The virus titers detected after acute infection in this study were consistent with those found in previous studies (11). Blood from these animals was monitored for IFN-γ production and proliferative responses to purified proteins derived from M. bovis (purified protein derivative B [PPD-B]) as described previously (13) prior to and following infection with BVDV. Cell proliferation and IFN-γ production in the in vitro restimulation cultures are shown in Fig. 2.
FIG. 1.
Mean viral titers in the sera of six gnotobiotic calves challenged intranasally with 5 × 106 PFU of ncpBVDV 11249. The bars show the mean titers (PFU per milliliter) ± standard errors of the means (SEM).
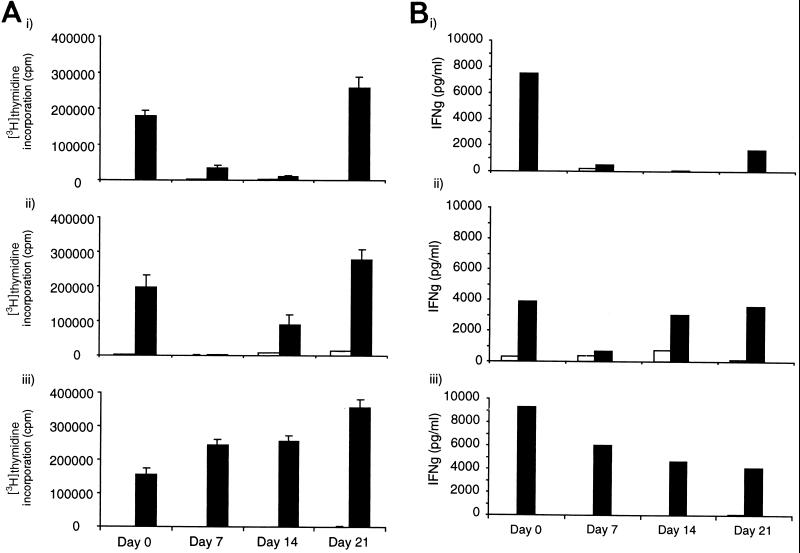
FIG. 2.
Cell proliferation (A) and IFN-γ production (B) in response to PPD-B in blood from gnotobiotic calves. Calves were infected with either BVDV and BCG (i and ii) or BCG alone (iii). BVDV challenge was performed on day 0; BCG challenge had been performed 28 days previously. Cell proliferation in each sample was analyzed in triplicate, and mean values ± standard deviations (error bars) are shown. IFN-γ production in each sample was analyzed in duplicate, and mean values are shown.
At the time of challenge with BVDV, all three BCG-vaccinated calves exhibited strong proliferative responses to PPD-B. Previous studies have shown that CD4+ T cells make up a large portion of the responding T cells in this assay (13). In the calf that was not infected with BVDV, the response to PPD-B continued to increase over the next three weeks. By contrast, the response to PPD-B was almost completely eliminated in the two BVDV-infected calves 7 days after infection. One calf still exhibited complete suppression of the proliferative response and the other exhibited partial suppression on day 14 after challenge. After 21 days, the proliferative response was recovered in both calves challenged with BVDV. Duplicate cultures were used to examine IFN-γ production. A similarly profound suppression of the IFN-γ response to PPD-B was observed following infection with BVDV, with almost complete suppression at 7 days in both animals and also at 14 days in one of the animals.
To determine whether the presence of infectious virus in the cultures had a direct effect on the proliferative response, ncpBVDV was added at titers ranging from 10 to 104 PFU per well to assays with blood collected on day 21 from the animal vaccinated with BCG alone. The added virus had no effect on the proliferative response to the antigen (data not shown), indicating that the suppression of proliferation was not merely due to the presence of virus in the assay.
Primary infection with ncpBVDV results in production of IFN-α/β.
IFN production in response to infection with BVDV was assessed by measuring the levels of biologically active IFN-α/β in duplicate serum samples from the BVDV-infected and mock-infected animals with an IFN-inducible Mx gene promoter reporter assay as previously described (12). Mx protein has been used extensively as a sensitive indicator of IFN-α/β bioactivity (15).
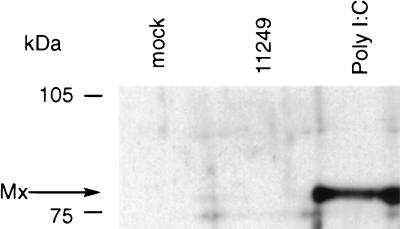
As observed with other isolates of ncpBVDV, isolate 11249 failed to induce either IFN-α/β or Mx protein (Fig. 3) in vitro in a number of bovine cell types, including isolated blood monocytes. By contrast, IFN was readily detected in sera from infected animals; serum IFN was elevated from day 1 until day 7 p.i. (Fig. 4). These IFN responses in BVDV-infected calves that had been vaccinated with BCG were similar to those in the unvaccinated calves. IFN was not detected in the serum of the mock-infected gnotobiotic animal or in those of five age-matched conventional calves after mock infection (data not shown).
FIG. 3.
Mx expression in bovine monocytes infected either with mock antigen or with ncpBVDV 11249 or stimulated with poly(I-C) (1 μg/ml). Mx protein was detected only in monocytes treated with poly(I-C).
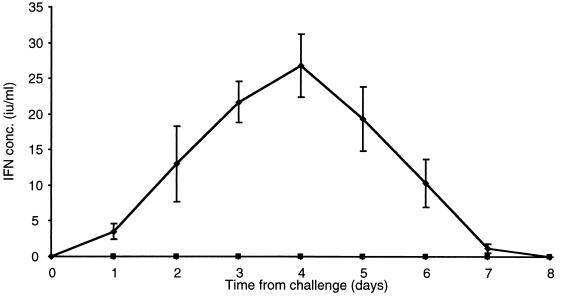
FIG. 4.
Kinetics of IFN-α/β production in the sera of calves after intranasal challenge infection with 5 × 106 PFU of ncpBVDV isolate 11249 (⧫). Individual samples were analyzed in duplicate, and the mean values were determined. Titers are expressed as the means of values for six animals ± SEM. Titers for the mock-infected animal are shown separately (▪).
The kinetics and quantity of IFN-α/β induced following BVDV challenge are consistent with those seen during acute viral infections in other species (3) and indicate that IFN is generated as part of the innate immune response to the virus. The induction of IFN-α/β in vivo by ncpBVDV contrasts with in vitro observations (2, 8, 16) indicating that the virus is able to inhibit endogenous induction of IFN-α/β in virus-infected cells.
Further studies are required to determine which cells produce IFN in vivo, to determine whether or not the IFN-producing cells are infected with BVDV, and to explain the discrepancy between the in vivo and in vitro findings for the induction of IFN-α/β by ncpBVDV.
ncpBVDV stimulates IFN-γ responses in vivo.
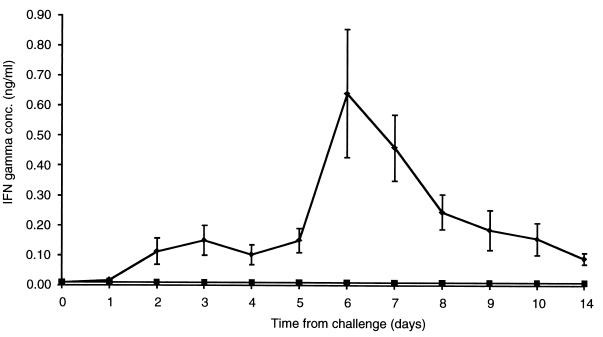
In light of the recent evidence that early IFN-α/β production can promote Th1 T-cell responses in humans (9), the animals were also examined for evidence of IFN-γ production. IFN-γ in serum was quantified using a commercially produced enzyme-linked immunosorbent assay (Bovigam; CSL Limited, Parkville, Australia). Gnotobiotic calves infected with ncpBVDV were found to produce detectable levels of IFN-γ in serum. IFN-γ production appeared to be biphasic, with the first peak occurring after 3 days (although the values on days 2, 3, 4, and 5 were not significantly different [t test, P > 0.05]) and with the second peak occurring after 6 days (Fig. 5). IFN-γ was not detected in the sera of the mock-infected animals. Based on observations of viral infections in mice, it is likely that the first peak of IFN-γ is produced by natural killer (NK) cells and that the second peak is produced by activated T cells (3).
FIG. 5.
Kinetics of IFN-γ production (nanograms per milliliter) in the sera of gnotobiotic calves after intranasal challenge with 5 × 106 PFU of ncpBVDV isolate 11249 (⧫). Individual samples were analyzed in duplicate, and the mean values were determined. Titers are expressed as the means of values for six animals ± SEM. Titers for the mock-infected animal are shown separately (▪).
The quantity of circulating TGF-β is reduced during acute ncpBVDV infection.
IFN-γ has been shown to inhibit the production of latent transforming growth factor β1 (TGF-β1) by mouse inflammatory macrophages (20). Hence, we investigated whether there are changes in the quantity of circulating TGF-β which correlate with the period of IFN-γ production.
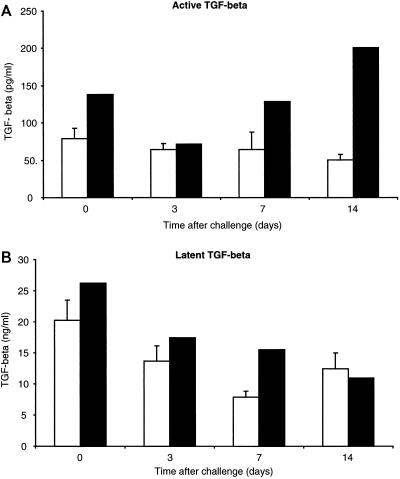
Infection with ncpBVDV failed to induce either active or latent TGF-β1 protein (Quantikine human TGF-β1 assay; R & D Systems, Abingdon, United Kingdom) in the sera of gnotobiotic calves (Fig. 6). The concentrations of circulating latent TGF-β1 protein were consistent with values found in studies of infection with Epstein-Barr virus in humans; however, the quantity of circulating active TGF-β1 protein was approximately 100-fold lower than that found in the same study (23) and was probably not biologically significant. Also, there was no significant difference among the quantities of active TGF-β1 in the sera of the infected calves at each time point studied (t test, P > 0.05). However, the quantities of active TGF-β1 in the sera of the infected calves were significantly lower than that of the control animal on days 7 and 14 postchallenge (t test, P < 0.05). In conclusion, acute infection with ncpBVDV does not induce either latent or active TGF-β1 in the sera of gnotobiotic calves.
FIG. 6.
Profiles of TGF-β in sera from gnotobiotic calves challenged intranasally with 5 × 106 PFU of ncpBVDV isolate 11249. Sera were added to the TGF-β1 assay either directly (A) or after activation by acidification (B). Individual samples were analyzed in duplicate, and the mean values were determined. Open bars show the mean values for six gnotobiotic calves ± SEM. Solid bars show the values for the mock-infected animal.
Relevance of findings to BVDV-associated immunosuppression.
The findings of this study show that strong IFN-α/β and IFN-γ responses are induced in vivo after acute ncpBVDV infection. Thus, despite the in vitro observation that ncpBVDV is able to inhibit endogenous induction of IFN-α/β, infection does not appear to result in impaired IFN responses in vivo that might account for enhanced susceptibility to other viral infections. There is also no evidence of increased production of TGF-β, which is known to suppress T-cell responses (10) and has been implicated in immunosuppression associated with equine herpesvirus 2 infections (5). Evidence that infection with the isolate of ncpBVDV used in this study results in immunosuppression was obtained by demonstrating a marked suppression in T-cell proliferation and IFN-γ production in response to PPD-B in calves previously vaccinated with BCG. Somewhat surprisingly, the initial detection of IFN-γ suppression coincided with the period when high levels of IFN-γ were detected in the sera of the BVDV-infected calves. Despite this potent IFN-γ response, we have shown previously that T-cell proliferative responses to ncpBVDV are not readily detected during the first 4 to 5 weeks after infection with the virus (6). These findings collectively suggest that ncpBVDV induces an aberrant early T-cell response. Potgieter (17) has reviewed possible mechanisms of immunosuppression, and, more specifically, production of an interleukin-1 (IL-1) inhibitor has been suggested (14).
It is well established that the cytokine profile of T-cell responses is influenced by the cytokine environment during the early stages of T-cell activation (1). Both IL-12 and IFN-γ itself are known to promote differentiation to a Th1 phenotype (4). There is also recent evidence from studies of human cells that IFN-α/β can promote Th1 responses (9). Hence, both the early IFN-α/β response and the IFN-γ produced 2 to 3 days after infection with ncpBVDV are likely to have a role in determining the cytokine profile of the subsequent T-cell response. A previous study of T-cell responses of cattle that had recovered from infection with BVDV, involving analysis of cytokine production by purified T-cell subsets stimulated in vitro with virus-infected cells, showed that CD4+ T cells produced IL-4 biological activity but no IFN-γ but that CD8+ T cells produced IFN-γ but no IL-4 (18). It was suggested that a Th2 CD4+-T-cell response might interfere with protective Th1 responses to other pathogens, such as bovine herpesvirus 1. However, no evidence of a Th2-like response was found during the acute phase of the infection in the present study. Furthermore, detailed studies are required to determine how the cytokine profiles of virus-specific T-cell responses change during and following infection with ncpBVDV.
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787–793. [DOI] [PubMed] [Google Scholar]
- 2.Adler, B., H. Adler, H. Pfister, T. W. Jungi, and E. Peterhans. 1997. Macrophages infected with cytopathic bovine viral diarrhea virus release a factor(s) capable of priming uninfected macrophages for activation-induced apoptosis. J. Virol. 71:3255–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biron, C. A. 1994. Cytokines in the generation of immune responses to, and resolution of, virus infection. Curr. Opin. Immunol. 6:530–538. [DOI] [PubMed] [Google Scholar]
- 4.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-γ. Annu. Rev. Immunol. 15:749–795. [DOI] [PubMed] [Google Scholar]
- 5.Charan, S., K. Palmer, P. Chester, A. R. Mire-Sluis, A. Meager, and N. Edington. 1997. Transforming growth factor-β induced by live or ultraviolet-inactivated equid herpes virus type-1 mediates immunosuppression in the horse. Immunology 90:586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collen, T., and W. I. Morrison. 2000. CD4(+) T-cell responses to bovine viral diarrhoea virus in cattle. Virus Res. 67:67–80. [DOI] [PubMed] [Google Scholar]
- 7.Dennis, M. J., D. C. Davies, and M. N. Hoare. 1976. A simplified apparatus for the microbiological isolation of calves. Br. Vet. J. 132:642–646. [DOI] [PubMed] [Google Scholar]
- 8.Diderholm, H., and Z. Dinter. 1966. Interference between strains of bovine virus diarrhea virus and their capacity to suppress interferon of a heterologous virus. Proc. Soc. Exp. Biol. Med. 121:976–980. [DOI] [PubMed] [Google Scholar]
- 9.Farrer, J. D., and K. M. Murphy. 2000. Type 1 interferons and T helper development. Immunol. Today 21:484–489. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick, D. R., and H. Bielefeldt-Ohmann. 1999. Transforming growth factor β in infectious disease: always there for the host and the pathogen. Trends Microbiol. 7:232–236. [DOI] [PubMed] [Google Scholar]
- 11.Fray, M. D., G. E. Mann, M. C. Clarke, and B. Charleston. 1999. Bovine viral diarrhea virus: its effects on estradiol, progesterone and prostaglandin secretion in the cow. Theriogenology 51:1533–1546. [DOI] [PubMed] [Google Scholar]
- 12.Fray, M. D., G. E. Mann, and B. Charleston. 2001. Validation of a Mx/CAT reporter gene assay for the quantification of bovine type-I interferon. J. Immunol. Methods 249:235–244. [DOI] [PubMed] [Google Scholar]
- 13.Hope, J. C., L. S. Kwong, P. Sopp, R. A. Collins, and C. J. Howard. 2000. Dendritic cells induce CD4+ and CD8+ T-cell responses to Mycobacterium bovis and M. avium antigens in bacille Calmette Guerin vaccinated and nonvaccinated cattle. Scand. J. Immunol. 52:285–291. [DOI] [PubMed] [Google Scholar]
- 14.Jensen, J., and R. D. Schultz. 1991. Effect of infection by bovine viral diarrhoea virus (BVDV) in vitro on interleukin-1 activity of bovine monocytes. Vet. Immunol. Immunopathol. 29:251–265. [DOI] [PubMed] [Google Scholar]
- 15.Kim, C. H., M. C. Johnson, J. D. Drennan, B. E. Simon, E. Thomann, and J.-A. C. Leong. 2000. DNA vaccines encoding viral glycoproteins induce nonspecific immunity and Mx protein synthesis in fish. J. Virol. 74:7048–7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura, S., T. Shimazaki, K. Sakamoto, A. Fukusho, Y. Inoue, and N. Ogawa. 1995. Enhanced replication of orbiviruses in bovine testicle cells infected with bovine viral diarrhoea virus. J. Vet. Med. Sci. 57:677–681. [DOI] [PubMed] [Google Scholar]
- 17.Potgieter, L. N. D. 1995. Immunology of bovine viral diarrhea virus. Vet. Clin. N. Am. 11:501–520. [DOI] [PubMed] [Google Scholar]
- 18.Rhodes, S. G., J. M. Cocksedge, R. A. Collins, and W. I. Morrison. 1999. Differential cytokine responses of CD4+ and CD8+ T cells in response to bovine viral diarrhoea virus in cattle. J. Gen. Virol. 80:1673–1679. [DOI] [PubMed] [Google Scholar]
- 19.Rossi, C. R., and G. K. Kiesel. 1980. Factors affecting the production of bovine type 1 interferon on bovine embryonic lung cells by polyriboinosinic-polyribocytidylic acid. Am. J. Vet. Res. 41:557–560. [PubMed] [Google Scholar]
- 20.Schindler, H., A. Diefenbach, M. Rollinghoff, and C. Bogdan. 1998. IFN-γ inhibits the production of latent transforming growth factor-β1 by mouse inflammatory macrophages. Eur. J. Immunol. 28:1181–1188. [DOI] [PubMed] [Google Scholar]
- 21.Schweizer, M., and E. Peterhans. 2001. Noncytopathic bovine viral diarrhea virus inhibits double-stranded RNA-induced apoptosis and interferon synthesis. J. Virol. 75:4692–4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stott, E. J., L. H. Thomas, C. J. Howard, and R. N. Gourlay. 1987. Field trials of a quadrivalent vaccine against respiratory disease. Vet. Rec. 121:342–347. [DOI] [PubMed] [Google Scholar]
- 23.Xu, J., A. Ahmad, J. F. Jones, R. Dolcetti, E. Vaccher, U. Prasad, and J. Menezes. 2000. Elevated serum transforming growth factor β1 levels in Epstein-Barr virus-associated diseases and their correlation with virus-specific immunoglobulin A (IgA) and IgM. J. Virol. 74:2443–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]