Abstract
We report here the generation of recombinant vesicular stomatitis virus (VSV) able to produce the suicide gene product thymidine kinase (TK) or cytokine interleukin 4 (IL-4). In vitro cells infected with the engineered viruses expressed remarkably high levels of biologically active TK or IL-4 and showed no defects in replication compared to the wild-type virus. Recombinant viruses retained their ability to induce potent apoptosis in a variety of cancer cells, while normal cells were evidently more resistant to infection and were completely protected by interferon. Significantly, following direct intratumoral inoculation, VSV expressing either TK or IL-4 exhibited considerably more oncolytic activity against syngeneic breast or melanoma tumors in murine models than did the wild-type virus or control recombinant viruses expressing green fluorescent protein (GFP). Complete regression of a number of tumors was achieved, and increased granulocyte-infiltrating activity with concomitant, antitumor cytotoxic T-cell responses was observed. Aside from discovering greater oncolytic activity following direct intratumoral inoculation, however, we also established that VSV expressing IL-4 or TK, but not GFP, was able to exert enhanced antitumor activity against metastatic disease. Following intravenous administration of the recombinant viruses, immunocompetent BALB/c mice inoculated with mammary adenocarcinoma exhibited prolonged survival against lethal lung metastasis. Our data demonstrate the validity of developing novel types of engineered VSV for recombinant protein production and as a gene therapy vector for the treatment of malignant and other disease.
Vesicular stomatitis virus (VSV) is a negative-stranded virus, comprising only five genes, that preferentially replicates in immortalized and malignant cells, eventually inducing apoptosis (2, 3, 27). The ability of VSV to reproduce in tumor cells has been reported to occur, in part, to a defective interferon (IFN) system (2, 25). Since the IFN system is functional in normal cells, efficient replication of VSV, which is an IFN-sensitive virus, is prevented. Based on in vitro and in vivo observations, it has been demonstrated that VSV effectively replicates in and lyses infected cancer cells while leaving normal cells relatively unaffected (2, 3, 25). Indeed, the use of VSV as an oncolytic agent has several advantages over other virus delivery systems presently used in tumor therapy, such as adenoviruses and retroviruses (24). Foremost, VSV has no known transforming abilities (27). Second, VSV is not gene attenuated, which affects replication and therefore oncolytic antitumor activity (10). Third, the envelope glycoprotein of VSV is highly tropic for a number of cell types and should be effective at targeting a variety of tissues in vivo. As such, the VSV envelope glycoprotein has been adopted in the creation of a number of psuedotyped viruses with the aim of increasing the transduction efficiency of the resultant agents (11). Fourth, VSV appears to be able to replicate in a wide variety of tumorigenic cells and not, for example, in cells only defective in selective tumor suppressor genes such as p53 (3, 10). In this regard, recent in vivo studies have indicated that VSV is able to potently exert its oncolytic activity in tumors harboring defects in the Ras, Myc, and p53 pathways, cellular aberrations that occur in over 90% of all tumors (3). Finally, the ability to modify VSV through genetic engineering affords the opportunity of developing new generations of custom-made VSV vectors that contain immunomodulatory and/or suicide cassettes designed to increase their antitumor activity. To date, however, the generation and evaluation of recombinant VSVs for use in cancer therapy have not been reported. Therefore, to begin to evaluate whether genetically engineered VSV carrying immunomodulatory/suicide genes can be created and, secondly, whether such viruses are more efficacious in tumor therapy than the wild-type VSV, we attempted to develop VSV vectors carrying the herpesvirus thymidine kinase (TK) suicide cassette or the cytokine interleukin 4 (IL-4) gene. It is known that the mechanism of TK action involves the TK protein phosphorylating the nontoxic prodrug ganciclovir (GCV), which becomes incorporated into cellular DNA during replication, leading to chain termination and cell death (17). However, the TK/GCV suicide approach has been reported to have additional benefits in that modified TK can be directly passed from the transduced cell to adjacent cells, thereby increasing tumor killing, a phenomenon known as the “bystander effect.” The activities of IL-4, in contrast, involve influencing the development of effector cells, such as eosinophils and antigen-presenting cells (APCs) (8). IL-4 is also known to regulate T-helper (Th) cell development into Th2 cells and assist in the stimulation of a humoral response (1). High levels of IL-4 have been reported to be critical for the rejection of tumors in the initial phases of tumor development, and implanted, engineered, IL-4-secreting cells, as well as viral vectors transducing IL-4, have been shown to mediate the regression of a number of malignancies, including melanoma, glioma, and colon carcinoma (8, 15, 19, 26). Our results here demonstrate that VSV manipulated to express TK or IL-4 is viable and generates high levels of heterologous protein following infection of the cell. Further, both recombinant viruses, but not control recombinant viruses carrying green fluorescent protein (GFP), exhibited greater oncolytic activity in tumor therapy studies, including metastatic disease, than did the wild-type virus and involved the stimulation of antitumor-specific, cytotoxic T-cell responses. Our data indicate the validity of generating novel forms of engineered VSV for the treatment of disease.
MATERIALS AND METHODS
Cell lines.
BHK-21 and B16(F10) melanoma cells were obtained from the American Type Culture Collection (12) (Manassas, Va.). Mammary adenocarcinoma (TS/A) cells were a gift from A. Rakmilevich (20) (University of Wisconsin, Madison). BHK cells were grown in Dulbecco’s modified essential medium containing 10% fetal bovine serum (HyClone Laboratories Inc., Logan, Utah). B16(F10) cells were propagated in similar medium, except that it contained low-sodium-bicarbonate-concentration (1.5 g/liter) D1-DMBA3 breast tumor. DA-3 cells derived from the tumor were a kind gift from D. Lopez (23) (University of Miami, Miami, Fla.). Human primary cells (human microvascular endothelial cells [HMVECs]) were obtained from Clonetics Corp. (San Diego, Calif.) and were grown according to the manufacturer’s specification.
Construction of recombinant viruses.
The IL-4, TK, and GFP inserts were amplified from pGexp, pKO-TK, and pLEGFP-C1 (Clontech Laboratories, Palo Alto, Calif.) plasmids, respectively, by PCR. For IL-4, the primers 5′ GGCACTCGAGATGGGTCTCAACCCCCAGCTAGTTG and 5′ GCCGTCTAGACTACGAGTAATCCATTTGCATGATGC were used. For the GFP gene, the primers used were 5′ GGCACTCGAGATGGTGAGCAAGGGCGAGGAG and 5′ GCTTGAGCTCTAGATCTGAGTCCCTACTTGTACAGC. The TK gene was amplified using the following primers: 5′ CTTGTAGACTCGAGTATGGCTTCGTACCCCGGCCATCAG and 5′ GTATTGTCTGCTAGCGTGTTTCAGTTAGCCTCCCCCATC (foreign gene is in bold).
The IL-4 and GFP PCR products were digested with XhoI and XbaI, while the TK PCR product was digested with XhoI and NheI and ligated to pVSV-XN2 (a kind gift from John Rose, Yale University), which had been digested with XhoI and NheI (compatible with XbaI). The plasmid pVSV-XN2 contains the entire VSV genome and has unique XhoI and NheI sites flanked by VSV transcription start and stop signals. The procedure for recovering infectious recombinant VSV (rVSV) was similar to that described previously (18, 22, 28).
In vitro cell-killing assay.
Murine B16 and DA-3 cells and HMVECs were seeded in 12-well plates at approximately 2 × 105 cells/well. Cells were pretreated with IFN (human alpha-2a IFN [IFN-α-2a] [Hoffman-La Roche, Nutley N.J.] for HMVECs and mouse IFN-α/β [Sigma, St. Louis, Mo.] for B16 and DA-3 cells) at 1,000 U/ml for 24 h. The cells were then infected with wild-type VSV, VSV-TK, VSV-IL-4, or VSV-GFP at a multiplicity of infection (MOI) of 0.1 for 18 h, trypsinized, and counted by trypan blue exclusion analysis.
ELISA for IL-4 production.
Levels of IL-4 in the rVSV-IL-4 supernatants obtained 24 h following infection of BHK cells with rVSV-IL-4 were determined by using an IL-4 enzyme-linked immunosorbent assay (ELISA) kit obtained from Pharmingen (San Diego, Calif.) and by following the manufacturer’s protocol.
TK enzyme assay.
Phosphorylation of GCV was used to measure functional levels of HSV-TK in extracts of VSV-TK-infected cells in vitro or in vivo as follows (29). For the in vitro assay, BHK cells were infected with wild-type or recombinant viruses for 8 h. For the in vivo assay, B16(F10) tumor cells injected with wild-type VSV or VSV-TK were harvested 24 h following virus inoculation. BHK or B16(F10) cells were resuspended in a buffer containing 0.5% NP-40, 50 mM Tris HCl (pH 7.5), 20% glycerol, 5 mM benzamidine, 2 mM dithiothreitol, and 0.5 mM phenylmethylsulfonyl fluoride. They were subjected to four cycles of freeze-thawing followed by centrifugation of the lysates at 13,000 × g for 5 min at 4°C. The enzyme assay was carried out using 60 μg of protein in a reaction mix containing 50 mM Tris HCl, 5 mM magnesium chloride, 5 mM ATP, 10 mM sodium fluoride, and 2 mM dithiothreitol in a 37°C water bath. Twenty-microliter aliquots were taken at 0, 30, and 60 min following the addition of [3H]GCV (45 μM) and spotted on DE-81 (Whatman) paper. The filter papers were washed twice in 1 mM ammonium formate, extracted with 0.1 M KCl-0.1N HCl, and counted in a scintillation counter.
Tumor inoculation in mice.
Female C57BL/6 or BALB/c mice (8 weeks old) from Jackson Laboratories were inoculated subcutaneously with 5 × 105 B16(F10) melanoma cells (left flank) or 1.5 × 106 D1-DMBA3 breast tumor cells (mammary pad). Mice were divided into four groups of five each according to the type of virus administered: heat-inactivated VSV, wild-type VSV, VSV-TK, or VSV-IL-4. As additional controls, rVSV carrying GFP was also used. After the development of palpable tumors, the mice received 2 × 107 PFU of the wild-type VSV or rVSV intratumorally, followed by a second injection 3 days later. Mice that received VSV-TK were also administered GCV (100 mg/kg of body weight) intraperitoneally 1 day following the initial injection, followed by daily injections for 10 days thereafter. Tumors were measured three times weekly. Mice were sacrificed once tumors reached greater than 1.8 cm in any diameter. Mean tumor volumes in the four groups were compared using one-way analysis of variance. Histopathology was carried out as described previously (3).
For metastatic disease studies, BALB/c mice (n = 10 per group) were injected via the tail vein with 5 × 104 TS/A cells. Mice were randomly divided into four groups of 10 each and received tail vein injections of either heat-inactivated VSV, VSV-GFP, VSV-TK, or VSV-IL-4 (5 × 106 PFU) 4 days later. Animals that were administered VSV-TK were also injected with GCV (100 mg/kg) intraperitoneally. The mice were monitored daily following virus injection, and results were statistically analyzed using the Mann-Whitney test.
Cytotoxic assays.
Single-cell spleen suspensions were cultured in 25-mm-diameter upright flasks at a 20:1 ratio with mitomycin C-treated B16(F10) cells for 3 days in the presence of 400 pg of IL-2 (Calbiochem, San Diego, Calif.)/ml at 37°C in a humidified 5% CO2 atmosphere. The cytotoxic activity of spleen cells was determined by performing a standard chromium release assay using 0.1 mCi of Na251CrO4 (Amersham, Arlington Heights, Ill.) at 37°C for 2 h. After a 4-h incubation of effector and target cells, the supernatants were harvested using a SKATRON cell harvester and the amount of 51Cr release was determined in a gamma counter (Beckman, Palo Alto, Calif.). The percent specific lysis was calculated by the following equation for counts per minute (cpm): experimental release cpm − spontaneous release cpm/maximum release cpm − spontaneous release cpm × 100. Maximum release was the cpm obtained by incubating target cells with 2% sodium dodecyl sulfate, and spontaneous release was determined by incubation with growth medium alone. Spontaneous release of 51Cr was always less than 15% of the total release in these assays.
RESULTS
Generation of rVSV expressing TK or IL-4.
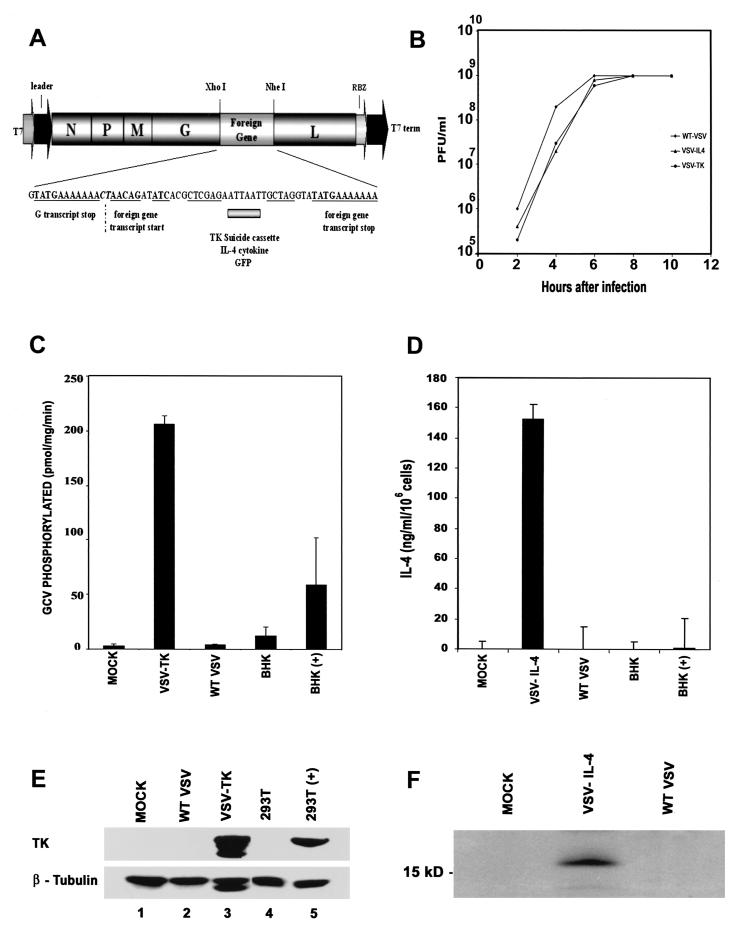
To evaluate whether VSV could be generated to express potential anticancer genes, the VSV-TK, mouse IL-4, or control GFP was cloned into the plasmid pVSV-XN2 (18) as additional transcription units between the VSV glycoprotein and polymerase protein genes (Fig. 1A). Recombinant VSVs expressing either TK, IL-4, or GFP from the modified transcription unit were recovered in cells expressing the full-length antigenomic VSV RNA containing the additional gene as well as the VSV nucleocapsid, phosphoprotein, and polymerase proteins. Preliminary analysis indicated that viable recombinant viruses could be obtained in all cases, and examination of virus production per cell, by one-step growth curve studies, indicated no aberrant variation in replication abilities compared to what was found in wild-type VSV (Fig. 1B). Indeed, all viruses grew to exceptionally high titers of approximately 109 PFU per ml. We next determined whether the recovered VSVs expressed TK, IL-4, or GFP. Accordingly, BHK cells were infected with VSV-TK for 24 h at an MOI of 1 and infected cells were examined for TK protein expression by immunoblot analysis using an anti-TK monoclonal antibody. Our results indicated that the TK protein was being synthesized to extremely high levels in cells infected with VSV-TK, while in contrast we were unable to detect any TK being expressed in cells infected with other types of VSV (Fig. 1E, lanes 1 to 3). Confirmation of TK expression was demonstrated using immunofluorescence microscopy of VSV-TK-infected cells (data not shown). In addition, BHK cells infected with VSV-GFP also expressed high levels of GFP as determined by fluorescence microscopy (data not shown). To ascertain whether the TK was functional, GCV phosphorylation levels were measured in cells infected at an MOI of 1 with VSV-TK or wild-type virus, 8 h postinfection. Our results indicated that TK phosphorylated GCV at high levels, on average approaching 200 pmol/mg/min, which we estimated to be nearly 60-fold greater than in wild-type VSV-infected cells (Fig. 1C). Our data thus indicate that VSV can be generated to express high levels of functional TK, without any adverse effects upon virus replication.
FIG. 1.
Generation of rVSV expressing TK, IL-4, or GFP. (A) cDNA representing the VSV genome (pVSV-XN2), flanked by the T7 RNA polymerase leader and T7 terminator as well as hepatitis virus delta ribozyme (RBZ), was used to create recombinant viruses. IL-4, TK, or GFP was inserted between the glycoprotein and polymerase protein genes of VSV. (B) Growth curves of recombinant viruses. BHK cells were infected with wild-type VSV, VSV-IL-4, and VSV-TK at an MOI of 10. Supernatants from infected cells were harvested at the indicated times postinfection, and viral titers were determined by plaque assay. (C) GCV is phosphorylated in cells infected with VSV-TK. BHK cells were mock infected or infected with VSV-TK or wild-type (WT) VSV (MOI = 1) for 8 h, and cell lysates were assayed for GCV phosphorylation in vitro. BHK cells transiently transfected with CMV promoter-driven HSV-TK [BHK(+)] or empty vector (BHK) were used as controls. (D) High-level expression of IL-4 in cells infected with VSV-IL-4. Culture medium from BHK cells infected with wild-type VSV or VSV-IL-4 was measured for functional IL-4 using capture ELISA. As a further control, IL-4 was measured in culture medium from BHK cells transiently transfected with an empty vector or with CMV promoter-driven IL-4 cDNA. (E) Expression of HSV-TK in VSV-TK-infected cells. BHK cells were mock infected (lane 1) or infected with wild-type VSV (lane 2) or VSV-TK (lane 3) at an MOI of 1 for 24 h, and cell extracts were analyzed for TK expression using an anti-TK monoclonal antibody. 293T cells transiently transfected with an empty vector (lane 4) or with CMV promoter-driven HSV-TK (lane 5) were used as a positive control. (F) Immunoprecipitation of IL-4 from supernatants of VSV-IL-4-infected cells. Extracts from [35S]methionine-labeled cells mock infected (lane 1) or infected with VSV-IL-4 (lane 2) or wild-type VSV (lane 3) were immunoprecipitated with an IL-4 antibody.
We similarly analyzed whether VSV could express functional IL-4. Since IL-4 is rapidly secreted from the cell following translation, preliminary immunoblot analysis of VSV-IL-4-infected cell extracts using an anti-murine IL-4 monoclonal antibody indicated very little IL-4 present in the cytoplasm of infected cells, as expected. However, capture ELISA using a monoclonal antibody that binds functional IL-4 indicated that the cytokine was being secreted into the culture medium at very high levels from VSV-IL-4-infected cells (Fig. 1D). Indeed, VSV-IL-4 generated about 150 ng of IL-4/ml per 106 cells, over 100-fold greater than in the same number of BHK cells transfected with IL-4 cDNA under control of the cytomegalovirus (CMV) promoter. Confirmation of IL-4 expression, was obtained by immunoprecipitating secreted IL-4 from the culture medium of 35S-labeled VSV-IL-4-infected cells using an anti-IL-4 monoclonal antibody (Fig. 1F). Thus, similar to our observations characterizing VSV-TK, we demonstrate that VSV can also be engineered to express high levels of the cytokine IL-4, which is biologically active.
rVSV expressing TK or IL-4 retains oncolytic activity.
To evaluate whether the recombinant viruses expressing IL-4 or TK retained their ability to preferentially replicate in malignant cells, thus eventually inducing cell death, a number of transformed cells were examined in infection assays. An important objective was also to compare the effects of VSV and rVSV on normal cells. To start to appraise this, we selected HMVECs, since they would be likeliest to be exposed to VSV infection after subcutaneous or intravenous administration in tumor therapy. HMVECs (106) were therefore infected at an MOI of 0.1 for 18 h with wild-type VSV or VSV-IL-4, VSV-TK, or VSV-GFP. Cell viability was measured using trypan blue exclusion analysis and revealed that approximately 20% of the HMVECs underwent cell death, an effect that could be essentially eliminated following pretreatment with IFN-α (Fig. 2A and D). In contrast, a similarly infected murine breast tumor cell line (23), DA-3, derived from a D1-DMBA3 tumor, as well as the melanoma cell line B16(F10) (12), underwent dramatic cytolysis (80 to 90%) following infection with either the wild-type virus, VSV-TK, VSV-IL-4, or VSV-GFP (Fig. 2B and E). Indeed, VSV-TK induced potent cytolysis of cells even in the absence of GCV. Interestingly, pretreatment of B16(F10) cells with IFN (1,000 U/ml for 24 h) reduced the amount of virus-mediated cell death observed following infection, regardless of the virus used (Fig. 2B and E). It remained plausible that the mechanisms of IFN production may be predominantly defective in B16(F10) cells, since IFN signaling to induce antiviral protection appeared partially intact. However, subsequent analysis of viral production in IFN-pretreated B16(F10) cells revealed relatively high virus replication (105/ml), suggesting incomplete protection and antiviral activity (Table 1). In contrast, trypan blue exclusion analysis indicated that IFN did not afford any significant protection of breast tumor-derived DA-3 cells, suggesting that IFN function may be defective at multiple levels in these cells (Fig. 2C and F). These data were confirmed by determining that virus replication in IFN-treated DA-3 cells was exceedingly high (107/ml), compared to that in control HMVECs, in which virus production was almost completely ablated (Table 1). To evaluate the mechanisms of virus-mediated cell lysis, infected B16(F10) and DA-3 cells were evaluated for apoptotic activity 18 h postinfection. Similar to what was found in previous studies (2-4), we found that the mechanisms of cytolysis invoked by recombinant VSV-IL-4 or TK as well as the wild-type virus involved the induction of apoptosis, since levels of active caspases 8 and 9 were threefold higher than in untreated cells (data not shown). Thus, rVSV expressing IL-4 or TK does not appear to lose its effectiveness at inducing programmed cell death in infected cells, compared to wild-type VSV.
FIG. 2.
In vitro effects of wild-type (WT) VSV and rVSV on primary or transformed cells. (A to C) rVSVs efficiently kill transformed cells. HMVECs or B16(F10) or DA-3 cells were infected with wild-type VSV, VSV-TK, or VSV-IL-4 with (solid columns) or without (empty columns) prior treatment with IFN-α. Cell viability was assayed by trypan blue exclusion 18 h after infection. (D to F) Efficient replication of VSV-GFP in transformed cells. HMVECs or B16(F10) or DA-3 cells were infected with VSV-GFP with or without prior treatment of IFN-α (1,000 U/ml). Top panels show cells under bright field microscopy (magnification, ×20), and lower panels show the same field by immunofluorescence.
TABLE 1.
Viral titers in IFN-treated transformed and primary cells
| Cell line | Virus | PFU/ml |
|---|---|---|
| B16(F10) | Wild-type VSV | 7.0 × 105 |
| VSV | ||
| VSV-TK | 1.0 × 105 | |
| VSV-IL-4 | 6.2 × 104 | |
| VSV-GFP | 4.0 × 105 | |
| DA-3 | Wild-type VSV | 2.9 × 107 |
| VSV | ||
| VSV-TK | 3.1 × 107 | |
| VSV-IL-4 | 2.7 × 107 | |
| VSV-GFP | 3.0 × 107 | |
| HMVEC | Wild-type VSV | <100 |
| VSV | ||
| VSV-TK | <100 | |
| VSV-IL-4 | <100 | |
| VSV-GFP | <100 |
Oncolytic effect of rVSV expressing TK or IL-4 following intratumoral inoculation.
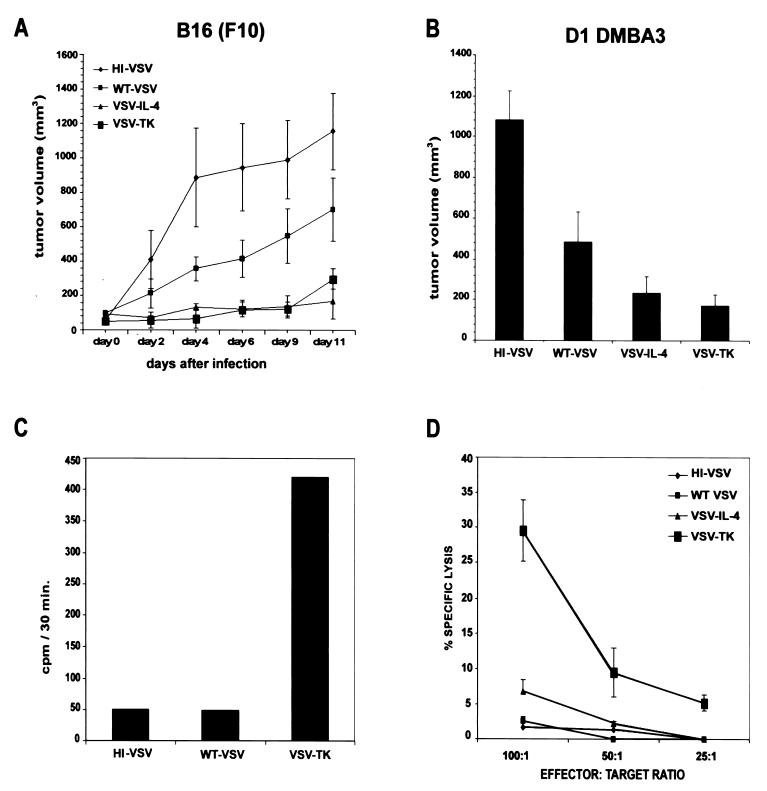
To evaluate the oncolytic activity of the recombinant viruses, immunocompetent mice were subcutaneously implanted with 106 cells of the syngeneic B16 melanoma (C57BL/6) or poorly immunogenic, mammary tumor-derived D1-DMBA3 (BALB/c), both aggressive tumors. Following the formation of palpable tumors, 2 × 107 wild-type VSV or VSV-IL-4 or VSV-TK were introduced intratumorally. As controls, an equivalent amount of heat-inactivated VSV was used as well as recombinant VSV expressing GFP (VSV-GFP). GCV was administered (100 mg/kg) intraperitoneally, daily in animals receiving VSV-TK, although as further controls, GCV was injected alone or VSV-TK was used to intratumorally inoculate animals in the absence of GCV. Virus therapy was repeated once more 3 days after the first injection, and tumor growth was monitored three times weekly. In the case of VSV-TK-treated B16(F10) tumors, in vivo expression of functional TK was confirmed by measuring GCV phosphorylation in retrieved malignant tissue (Fig. 3C). Resultant data demonstrated that wild-type VSV partially inhibited the growth of both the melanoma and breast tumors, compared to tumors treated with control heat-inactivated virus (Fig. 3A and B). However, in independent sets of experiments, more potent inhibition of tumor growth [both B16(F10) and D1-DMBA3] was observed in animals treated with either VSV-IL-4 or VSV-TK with GCV (Fig. 3A and B). In contrast, VSV-GFP or VSV-TK (without GCV) exhibited oncolytic activity similar to that of the wild-type virus, with differences in the tumor volume sizes of animals treated with either wild-type VSV, VSV-GFP, VSV-TK (no GCV), or GCV alone being statistically insignificant (P > 0.05 and data not shown). In contrast, the differences in the tumor volume between the heat-inactivated VSV and animals treated with either VSV-TK or VSV-IL-4 was observed to be statistically relevant, at P < 0.01 for animals implanted with either B16(F10) or D1-DMBA3. Since VSV-GFP and other controls did not mediate the same degree of antitumor activity, these data indicate that the additional oncolytic effects are almost certainly mediated by the effects of IL-4 or TK in conjunction with GCV. Moreover, since each virus stock was grown from single plaques and a number of stocks was examined for replicative ability and oncolytic activity, the possibility of the observed effects being largely influenced by defective, interfering VSV particles seems unlikely. In some instances, complete regression of tumors was observed in animals implanted with either B16(F10) (three of five mice) or D1-DMBA3 (two of five mice), following treatment with VSV-TK (data not shown). However, a number of mice implanted with either tumor and infected with heat-inactivated VSV had to be sacrificed 4 days posttreatment because of the excessive tumor size. These data indicate that VSV expressing either IL-4 or TK exhibits potent oncolytic activity, superior to that of VSV alone.
FIG. 3.
rVSV expressing TK and IL-4 inhibits the growth of syngeneic breast and melanoma tumors in immunocompetent mice. (A) C57BL/6 mice were implanted subcutaneously with 5 × 105 B16(F10) melanoma cells. After palpable tumors had formed, animals were treated intratumorally with 2 × 107 PFU of wild-type VSV, VSV-IL-4, or VSV-TK. Injections of virus were repeated after 3 days. Tumor volumes were calculated and are shown as a mean ± standard error of the mean (n = 5). Two mice that received heat-inactivated virus were sacrificed at day 4 due to the large size of tumors. (B) BALB/c mice were implanted subcutaneously with 1.5 × 106 D1-DMBA3 tumor cells. After palpable tumors had formed, animals were intratumorally injected with 2 × 107 PFU of heat-inactivated (HI) virus, wild-type VSV, VSV-IL-4, or VSV-TK. Virus treatment was repeated after 3 days. Tumor volumes at day 21 postimplantation (7 days after the last virus treatment) are shown. Results are presented as a mean ± standard error of the mean (n = 5). Comparable results were obtained in three independent sets of experiments. (C) GCV phosphorylation in vivo in B16(F10) tumor cells following VSV-TK administration. B16(F10) tumors were harvested from C57BL/6 mice 1 day following intratumoral injection with 2 × 107 PFU of heat-inactivated VSV, wild-type VSV, or VSV-TK. The amount of GCV phosphorylated was measured in extracts (60 μg of total protein) from tumor cells 30 min following incubation in a reaction mixture containing [3H]GCV (45 μM). (D) Induction of CTL response against B16(F10) tumors in animals receiving VSV-TK-GCV treatment. C57BL/6 tumor-bearing mice were injected intratumorally with wild-type VSV or rVSV. A second injection was administered 3 days later. Ten days after the first virus injection, spleen cells were isolated and cocultured with B16(F10) cells. Spleen cells were incubated at the indicated effector-to-target ratios with 51Cr-labeled B16(F10) target cells. CTL activity was determined by 51Cr release.
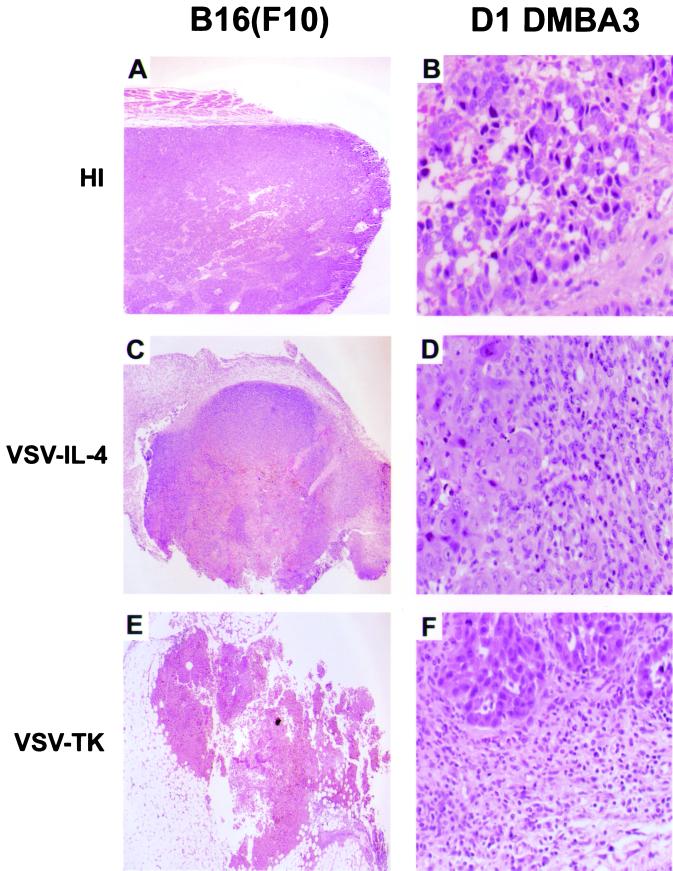
To examine the effects of virus therapy in vivo, tumors inoculated with the various viruses were excised and sections were examined histologically following hematoxylin and eosin staining. As expected, tumors infected with control heat-inactivated VSV exhibited very little morphological abnormality that could be associated with virus-induced oncolytic activity (<30% necrosis [Fig. 4A]). However, a greater proportion of cell death, as exhibited by pyknotic nuclei, was observed in B16(F10) tumors treated with wild-type VSV (∼50%) or with VSV-IL-4 (75%) or VSV-TK (∼95%), indicative of an increase in oncolytic activity (Fig. 4C and E). Significant inflammatory infiltration was also evident in tumors treated with the recombinant viruses, especially in tumors treated with VSV-IL-4, which showed major infiltration of polymorphonuclear cells, including neutrophils and eosinophils (compare Fig. 4B and D). Analysis of viral replication in the brain, liver, and tumors retrieved from mice implanted with B16(F10) or D1-DMBA3 cells and treated with wild-type or recombinant viruses (two were analyzed from each virus-treated group) did not reveal evidence of infectious virus 7 days after the last virus treatment (1 week after the primary inoculation). Thus, following intratumoral inoculation, all VSV types appear to be rapidly cleared from the animals, presumably through the generation of neutralizing antibody (27).
FIG. 4.
Histopathological analysis of tumors. Tumors from C57BL/6 and BALB/c mice were removed 7 days after receiving intratumoral injections of heat-inactivated VSV (A), VSV-IL-4 (B), or VSV-TK (C). The left panels indicate large areas of cell death in B16(F10) tumors treated with VSV-TK and VSV-IL-4. The right panels emphasize increased infiltration of eosinophils in D1-DMBA3 tumors injected with VSV-IL-4.
Although T lymphocytes have been reported to be important for the antitumor activity of IL-4, cellular immune responses have also been reported to play a role in the local and systemic antitumor activity of TK/GCV (29). Since IL-4- and TK/GCV-treated tumors exhibited pronounced host cell infiltration, we examined whether lymphocytes generated by the animals exhibited specific cytotoxic activity to tumor-associated antigens in the implanted, syngeneic, transformed cells. Accordingly, animals harboring B16(F10)-derived tumors were intratumorally inoculated twice with wild-type VSV, heat-inactivated VSV, VSV-IL-4, or VSV-TK. Fourteen days after inoculation, spleens were removed, mononuclear cells were isolated, and chromium release assays were carried out using B16(F10) target cells. Our results indicated that animals generated a robust cytotoxic T-lymphocyte (CTL) response only against tumors receiving VSV-TK and not with VSV-IL-4, the wild-type virus, or controls (Fig. 3D). As discussed further below, this may reflect the preferred role of IL-4 in modulating Th2 responses rather than cytotoxicity-related Th1 responses (additionally underscored by the increased presence of eosinophils and neutrophils in VSV-IL-4-treated tumors [1, 26]). Conversely, VSV-TK-mediated oncolysis may result in greater amounts of tumor antigen being released from the infected cells, as indicated from our histology analysis. This may facilitate tumor antigen uptake by professional APCs, which are responsible for stimulating CTL responses (7, 9). Collectively, our data potently demonstrate that aside from harboring direct oncolytic activity, VSV can be genetically manipulated to efficiently target and express antitumor genes in malignant tissue and thus may be useful in the intratumoral treatment of neoplastic disease.
Inhibition of breast cancer metastases by rVSV expressing TK or IL-4.
Our data indicated that rVSV expressing selected cytokines or suicide cassettes such as TK could exert potent oncolytic activity against subcutaneous tumor growth following direct intratumoral inoculation. To determine whether systematically administered rVSV can improve survival outcome against metastatic disasese, we employed a mammary adenocarcinoma model (TS/A) that gives rise to lung metastases in immunocompetent BALB/c mice following intravenous introduction of the tumor cells (20). Prior to examining the effects of systemic administration of the rVSVs, in vivo, however, we confirmed that rVSV expressing TK, IL-4, or GFP could induce the rapid cytolysis of TS/A cells in vitro (Fig. 5A and C). As expected, TS/A cells were exquisitely sensitive to all types of rVSV replication and cytolysis. Similar to DA-3 cells, however, IFN pretreatment of TS/A cells did not afford significant protection against VSV infection, indicating a serious defect(s) in the IFN antiviral pathway (Fig. 5A). To assess the potential of rVSV as a systematic gene transfer vector and oncolytic agent in anticancer therapy, mice were inoculated via the tail vein with 5 × 104 TS/A cells. Four days later, animals (randomized into treatment groups) were administered a single injection of 5 × 106 rVSV PFU expressing either TK, IL-4, or GFP via the tail vein. Heat-inactivated VSV was also independently administered as an additional control. As can be seen in Fig. 5B, all animals receiving heat-inactivated VSV died by day 45 postimplantation. The cause of death was not through the virus treatment, since animals receiving heat-inactivated VSV or VSV-GFP alone, in the absence of tumor challenge, did not exhibit any overt anomalies and remain viable, nearly 3 months after inoculation (data not shown). Examination of animals succumbing to TS/A implantation revealed advanced lung metastases, with TS/A cells invading fibroadipose tissue and epidermis and forming large tumor masses, hallmarks emblematic of this disease model (20; data not shown). Animals receiving VSV-GFP also succumbed to TS/A-related disease essentially within 45 days (save for 1 out of 10, which died on day 65) and did not appear to exert much more survival effect in this particular model than the heat-inactivated control. Possibly, insertion of the GFP gene caused the resultant virus to become more attenuated and lose oncolytic activity. Certainly, foreign gene insertions or gene rearrangment has been indicated to result in more attenuated viruses (5, 13, 21). In contrast, mice receiving a single injection of VSV-TK or IL-4 exhibited greater survival of TS/A-mediated metastatic disease, with two-tailed P values of animals treated with VSV-IL-4 or VSV-TK being statistically significant at P >0.03, when compared against animals receiving control viruses. While all control animals have died, approximately 50% of animals receiving VSV-TK or VSV IL-4 remain viable nearly 80 days postimplantation. Randomly retrieved control or rVSV-treated animals that succumbed to TS/A-mediated metastasis did not exhibit any residual virus in organs, as examined by plaque titration, and no overt loss in the weight of animals was apparent through the single virus treatment (data not shown). Collectively, our studies provide data demonstrating for the first time that, following systemic administration, rVSV expressing TK or IL-4 is able to prolong the survival of animals harboring metastatic disease.
FIG. 5.
Intravenous treatment with VSV-TK and VSV-IL-4 increases the survival of mice in a metastatic tumor model. (A) Effects of rVSV on TS/A cells in vitro. TS/A cells were treated with or without murine IFN (1,000 U/ml) for 24 h and were infected with rVSV at an MOI of 0.1 for 18 h. Cell viability in response to virus infection was measured using trypan blue. (B) Effects of rVSVs against metastatic disease. BALB/c mice (n = 10 per group) were injected via the tail vein with 5 × 104 TS/A cells, followed 4 days later by intravenous administration of 5 × 106 PFU of either heat-inactivated (HI) VSV, VSV-GFP, VSV-TK, or VSV-IL-4. The survival rate of mice following virus injection is shown. (C) Morphological examination of TS/A cells following rVSV infection in vitro.
DISCUSSION
We report here the construction of rVSV carrying the well-characterized suicide cassette TK or the cytokine IL-4. Both recombinant viruses were examined in antitumor studies and exhibited greater therapeutic oncolytic activity than did the wild-type virus alone or control viruses expressing GFP. Indeed, intratumorally inoculated viruses expressing TK, but not IL-4, were found to stimulate antitumor cytotoxic T-cell activity that may be critical in helping eradicate tumor occurrence. Our data are consistent with previous findings that TK/GCV-mediated destruction of tumor cells can facilitate antigen uptake by professional APCs (29). Possibly, the process of cellular destruction involves apoptosis through accumulation of p53 and the upregulation of Fas (CD95), which then through aggregation stimulates FADD-dependent cell death in a Fas ligand-independent manner (7). It is therefore plausible that VSV expressing TK exerts a greater oncolytic effect through TK/GCV-mediated apoptosis and enhanced bystander effect, as well as through the generation of specific antitumor CTL responses (14, 29). However, recent studies have indicated that pathological or necrotic cell death, rather than apoptosis, results in the release of antigenic components, including heat shock proteins, that are responsible for APC activation. Thus, it is possible that direct virus lysis of infected cells may also be occurring within the treated tumor and be predominantly responsible for stimulating antitumor CTL activity (6, 9). Our findings would also be in agreement with the fact that, while IL-4 can influence the development of Th cells, IL-4 in this tumor model did not strongly influence the development of tumor-specific cytotoxic T cells, as judged by CTL assays. Nevertheless, VSV expressing IL-4 did exert a greater oncolytic effect that was statistically significant than did wild-type VSV. Although IL-4 has been incorporated into a number of live-virus vectors for either gene therapy or anticancer strategies or to increase an immune response to candidate viral vaccines, the overall positive effects of the cytokine’s contribution vary (8, 19). Our data here indicate that tumors treated with VSV expressing IL-4 may exert a more potent oncolytic effect than VSV alone, possibly due to the increased presence of infiltrating eosinophils and neutrophils, which have been reported to directly have antitumor activity (26). However, the full mechanisms of IL-4-mediated antitumor activity undoubtedly remain to be clarified.
Our data also indicate that the systemic delivery of rVSV may also be useful as a therapy against metastatic disease. While VSV-GFP, VSV-TK, and VSV-IL-4 all effectively induce the cytolysis of tumor cells in vitro, including TS/A, only viruses expressing TK (with GCV) or IL-4 exhibited potent oncolytic activity in vivo. It is known that VSV can cause lethality in certain strains of mice when administered at relatively high doses (27). However, our data indicate that the recombinant viruses containing IL-4, TK, or GFP appear more attenuated than wild-type versions (Indiana strain) or recombinant viruses generated without a foreign gene (data not shown). Possibly, such alterations effect virus pathogenesis, as elegantly emphasized by others (5, 13, 21). This may explain the relatively weak oncolytic effect of VSV-GFP in vivo while underscoring the effective antitumor effects specifically contributed by the TK or IL-4 genes. That attenuation may be a common incidence during the development of such viruses may be advantageous, when considering the suitability of rVSV for the treatment of human disease.
Finally, it is noteworthy that a recent report indicated that expression of IL-4 by ectromelia virus suppressed antiviral cell-mediated immune responses and was associated with high mortality in mice usually resistant to the wild-type virus (16). While these data raise concerns about developing novel viruses that contain immunomodulatory genes, our data would be consistent with studies where retroviruses or adenovirus expressing IL-4 produced no adverse effects in vivo (10, 28). In safety trials we did not note any increase in toxicity in animals inoculated, by various routes, with VSV-IL-4 from the toxicity in animals inoculated with wild-type VSV (data not shown). Aside from our inability to detect infectious VSV in organs or tumors from mice receiving VSV treatment, 7 days after the last tumor inoculation, animals receiving VSV-IL-4 or VSV-TK at 2 × 107 PFU (intraperitoneal) or 2 × 106 PFU (intravenous) presently remain viable 16 weeks after infection.
In summary, our data indicate that VSV can be utilized to transiently express high levels of biologically active recombinant proteins. Essentially, following virus infection, cellular transcription and translation are prevented and cytoplasmic resources are focused on unbridled expression of the virus genes and any accompanying foreign cassettes, even potentially toxic cellular or viral genes. We therefore demonstrate that, aside from directly exerting virus-mediated oncolytic activity, VSV can be modified to deliver key genes, such as suicide and immunomodulatory cassettes, that can greatly increase tumor killing. In addition, VSV possesses high target specificity and proficient transfection efficacy. Thus, the generation of novel, engineered VSVs able to efficiently transport and express genes capable of stimulating host-mediated immune responses, considered critical in helping eradicate tumor presence as well as in protecting against further tumor development, could be considered a valid therapeutic strategy.
Acknowledgments
We thank John Rose for VSV plasmids, Vladimir Vincek for assistance with histopathology, William Summers for anti-TK antibody, Heather Ezelle and Mario Moreno for technical assistance, and Diana Lopez and Lynn Herbert for breast tumor lines.
REFERENCES
- 1.Asnagli, H., and K. M. Murphy. 2001. Stability and commitment in T helper cell development. Curr. Opin. Immunol. 13:242–247. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran, S., and G. N. Barber. 2000. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life 50:135–138. [DOI] [PubMed] [Google Scholar]
- 3.Balachandran, S., M. Porosnicu, and G. N. Barber. 2001. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or Myc function and involves the induction of apoptosis. J. Virol. 75:3474–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balachandran, S., P. C. Roberts, L. E. Brown, H. Truong, A. K. Pattnaik, D. R. Archer, and G. N. Barber. 2000. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13:129–141. [DOI] [PubMed] [Google Scholar]
- 5.Ball, L. A., C. R. Pringle, B. Flanagan, V. P. Perepelitsa, and G. W. Wertz. 1999. Phenotypic consequences of rearranging the P, M, and G genes of vesicular stomatitis virus. J. Virol. 73:4705–4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basu, S., R. J. Binder, R. Suto, K. M. Anderson, and P. K. Srivastava. 2000. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int. Immunol. 12:1539–1546. [DOI] [PubMed] [Google Scholar]
- 7.Beltinger, C., S. Fulda, T. Kammertoens, E. Meyer, W. Uckert, and K. M. Debatin. 1999. Herpes simplex virus thymidine kinase/ganciclovir-induced apoptosis involves ligand-independent death receptor aggregation and activation of caspases. Proc. Natl. Acad. Sci. USA 96:8699–8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benedetti, S., B. Pirola, B. Pollo, L. Magrassi, M. G. Bruzzone, D. Rigamonti, R. Galli, S. Selleri, F. Di Meco, C. De Fraja, A. Vescovi, E. Cattaneo, and G. Finocchiaro. 2000. Gene therapy of experimental brain tumors using neural progenitor cells. Nat. Med. 6:447–450. [DOI] [PubMed] [Google Scholar]
- 9.Berwin, B., R. C. Reed, and C. V. Nicchitta. 2001. Virally induced lytic cell death elicits the release of immunogenic grp94/gp96. J. Biol. Chem. 276:21083–21088. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff, J. R., D. H. Kirn, A. Williams, C. Heise, S. Horn, M. Muna, L. Ng, J. A. Nye, A. Sampson-Johannes, A. Fattaey, and F. McCormick. 1996. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274:373–376. [DOI] [PubMed] [Google Scholar]
- 11.Burns, J. C., T. Friedmann, W. Driever, M. Burrascano, and J. K. Yee. 1993. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. USA 90:8033–8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fidler, I. J. 1975. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 35:218–224. [PubMed] [Google Scholar]
- 13.Flanagan, E. B., J. M. Zamparo, L. A. Ball, L. L. Rodriguez, and G. W. Wertz. 2001. Rearrangement of the genes of vesicular stomatitis virus eliminates clinical disease in the natural host: new strategy for vaccine development. J. Virol. 75:6107–6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman, S. M., K. A. Whartenby, J. L. Freeman, C. N. Abboud, and A. J. Marrogi. 1996. In situ use of suicide genes for cancer therapy. Semin. Oncol. 23:31–45. [PubMed] [Google Scholar]
- 15.Giezeman-Smits, K. M., H. Okada, C. S. Brissette-Storkus, L. A. Villa, J. Attanucci, M. T. Lotze, I. F. Pollack, M. E. Bozik, and W. H. Chambers. 2000. Cytokine gene therapy of gliomas: induction of reactive CD4+ T cells by interleukin-4-transfected 9L gliosarcoma is essential for protective immunity. Cancer Res. 60:2449–2457. [PubMed] [Google Scholar]
- 16.Jackson, R. J., A. J. Ramsay, C. D. Christensen, S. Beaton, D. F. Hall, and I. A. Ramshaw. 2001. Expression of mouse interleukin-4 by a recombinant ectromelia virus suppresses cytolytic lymphocyte responses and overcomes genetic resistance to mousepox. J. Virol. 75:1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lal, S., U. M. Lauer, D. Niethammer, J. F. Beck, and P. G. Schlegel. 2000. Suicide genes: past, present and future perspectives. Immunol. Today 21:48–54. [DOI] [PubMed] [Google Scholar]
- 18.Lawson, N. D., E. A. Stillman, M. A. Whitt, and J. K. Rose. 1995. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. USA 92:4477–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagai, S., and M. Toi. 2000. Interleukin-4 and breast cancer. Breast Cancer 7:181–186. [DOI] [PubMed] [Google Scholar]
- 20.Rakhmilevich, A. L., K. Janssen, Z. Hao, P. M. Sondel, and N. S. Yang. 2000. Interleukin-12 gene therapy of a weakly immunogenic mouse mammary carcinoma results in reduction of spontaneous lung metastases via a T-cell-independent mechanism. Cancer Gene Ther. 7:826–838. [DOI] [PubMed] [Google Scholar]
- 21.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539–549. [DOI] [PubMed] [Google Scholar]
- 22.Schnell, M. J., J. E. Johnson, L. Buonocore, and J. K. Rose. 1997. Construction of a novel virus that targets HIV-1-infected cells and controls HIV-1 infection. Cell 90:849–857. [DOI] [PubMed] [Google Scholar]
- 23.Sotomayor, E. M., Y. X. Fu, M. Lopez-Cepero, L. Herbert, J. J. Jimenez, C. Albarracin, and D. M. Lopez. 1991. Role of tumor-derived cytokines on the immune system of mice bearing a mammary adenocarcinoma. II. Down-regulation of macrophage-mediated cytotoxicity by tumor-derived granulocyte-macrophage colony-stimulating factor. J. Immunol. 147:2816–2823. [PubMed] [Google Scholar]
- 24.Steele, T. A. 2000. Recent developments in the virus therapy of cancer. Proc. Soc. Exp. Biol. Med. 223:118–127. [DOI] [PubMed] [Google Scholar]
- 25.Stojdl, D. F., B. Lichty, S. Knowles, R. Marius, H. Atkins, N. Sonenberg, and J. C. Bell. 2000. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 6:821–825. [DOI] [PubMed] [Google Scholar]
- 26.Tepper, R. I., R. L. Coffman, and P. Leder. 1992. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science 257:548–551. [DOI] [PubMed] [Google Scholar]
- 27.Wagner, R. R., and J. K. Rose. 1996. Rhabdoviridae: the viruses and their replication, p.1121–1135. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 28.Whelan, S. P., L. A. Ball, J. N. Barr, and G. T. Wertz. 1995. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc. Natl. Acad. Sci. USA 92:8388–8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto, S., S. Suzuki, A. Hoshino, M. Akimoto, and T. Shimada. 1997. Herpes simplex virus thymidine kinase/ganciclovir-mediated killing of tumor cell induces tumor-specific cytotoxic T cells in mice. Cancer Gene Ther. 4:91–96. [PubMed] [Google Scholar]