Abstract
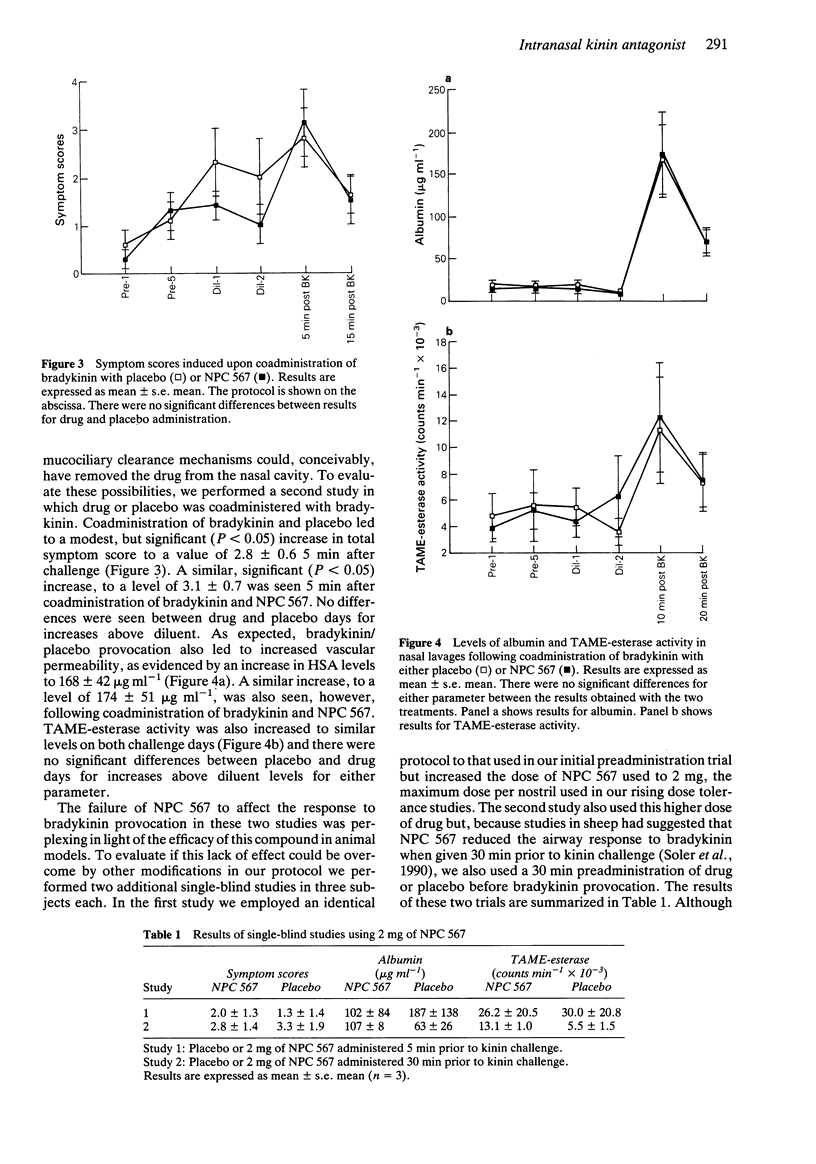
1. In two double-blind, placebo controlled studies, we tested the effects of intranasal administration of 500 micrograms of a competitive kinin receptor antagonist, [DArg0, Hyp3, DPhe7]-bradykinin (NPC 567), on the response to nasal provocation with 20 micrograms of bradykinin. Nasal lavage was performed before and after provocation, and subjects recorded symptom scores. Lavages were assayed for albumin and TAME-esterase activity (indicators of vascular permeability). 2. In our initial study, 12 subjects received NPC 567 or placebo 5 min before bradykinin. After placebo, bradykinin challenge resulted in values (mean +/- s.e. mean) for albumin, TAME-esterase activity and total symptom scores of 275 +/- 51 micrograms ml-1, 32.1 +/- 7.2 counts min-1 x 10(-3), and 1.8 +/- 0.5, respectively. After NPC 567, bradykinin challenge resulted in values of 317 +/- 99 micrograms ml-1, 31.4 +/- 6.9 counts min-1 x 10(-3), and 2.6 +/- 0.4 for these parameters. No significant difference was observed between placebo and drug treatment for any parameter. 3. To evaluate if the lack of drug effect was due to its enzymatic degradation prior to bradykinin administration, a second study was performed in which NPC 567 was coadministered with bradykinin (n = 8). After placebo-bradykinin challenge, values of 168 +/- 42 micrograms ml-1, 11.3 +/- 4.0 counts min-1 x 10(-3), and 2.8 +/- 0.6 were recorded for albumin, TAME-esterase activity, and symptom scores, respectively, while following NPC 567-bradykinin challenge, these values were 174 +/- 51 micrograms ml-1, 12.3 +/- 4.1 counts min-1 x 10(-3), and 3.1 +/- 0.7.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgarten C. R., Nichols R. C., Naclerio R. M., Lichtenstein L. M., Norman P. S., Proud D. Plasma kallikrein during experimentally induced allergic rhinitis: role in kinin formation and contribution to TAME-esterase activity in nasal secretions. J Immunol. 1986 Aug 1;137(3):977–982. [PubMed] [Google Scholar]
- Baumgarten C. R., Togias A. G., Naclerio R. M., Lichtenstein L. M., Norman P. S., Proud D. Influx of kininogens into nasal secretions after antigen challenge of allergic individuals. J Clin Invest. 1985 Jul;76(1):191–197. doi: 10.1172/JCI111945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen S. C., Proud D., Cochrane C. G. Detection of tissue kallikrein in the bronchoalveolar lavage fluid of asthmatic subjects. J Clin Invest. 1987 Jan;79(1):188–197. doi: 10.1172/JCI112782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer S. G., Burch R. M., Meeker S. A., Wilkins D. E. Evidence for a pulmonary B3 bradykinin receptor. Mol Pharmacol. 1989 Jul;36(1):1–8. [PubMed] [Google Scholar]
- Fuller R. W., Dixon C. M., Cuss F. M., Barnes P. J. Bradykinin-induced bronchoconstriction in humans. Mode of action. Am Rev Respir Dis. 1987 Jan;135(1):176–180. doi: 10.1164/arrd.1987.135.1.176. [DOI] [PubMed] [Google Scholar]
- Holmberg K., Bake B., Pipkorn U. Vascular effects of topically applied bradykinin on the human nasal mucosa. Eur J Pharmacol. 1990 Jan 3;175(1):35–41. doi: 10.1016/0014-2999(90)90149-z. [DOI] [PubMed] [Google Scholar]
- Marceau F., Lussier A., Regoli D., Giroud J. P. Pharmacology of kinins: their relevance to tissue injury and inflammation. Gen Pharmacol. 1983;14(2):209–229. doi: 10.1016/0306-3623(83)90001-0. [DOI] [PubMed] [Google Scholar]
- Naclerio R. M., Proud D., Lichtenstein L. M., Kagey-Sobotka A., Hendley J. O., Sorrentino J., Gwaltney J. M. Kinins are generated during experimental rhinovirus colds. J Infect Dis. 1988 Jan;157(1):133–142. doi: 10.1093/infdis/157.1.133. [DOI] [PubMed] [Google Scholar]
- Naclerio R. M., Proud D., Togias A. G., Adkinson N. F., Jr, Meyers D. A., Kagey-Sobotka A., Plaut M., Norman P. S., Lichtenstein L. M. Inflammatory mediators in late antigen-induced rhinitis. N Engl J Med. 1985 Jul 11;313(2):65–70. doi: 10.1056/NEJM198507113130201. [DOI] [PubMed] [Google Scholar]
- Proud D., Baumgarten C. R., Naclerio R. M., Ward P. E. Kinin metabolism in human nasal secretions during experimentally induced allergic rhinitis. J Immunol. 1987 Jan 15;138(2):428–434. [PubMed] [Google Scholar]
- Proud D., Kaplan A. P. Kinin formation: mechanisms and role in inflammatory disorders. Annu Rev Immunol. 1988;6:49–83. doi: 10.1146/annurev.iy.06.040188.000405. [DOI] [PubMed] [Google Scholar]
- Proud D., Naclerio R. M., Gwaltney J. M., Hendley J. O. Kinins are generated in nasal secretions during natural rhinovirus colds. J Infect Dis. 1990 Jan;161(1):120–123. doi: 10.1093/infdis/161.1.120. [DOI] [PubMed] [Google Scholar]
- Proud D., Reynolds C. J., Lacapra S., Kagey-Sobotka A., Lichtenstein L. M., Naclerio R. M. Nasal provocation with bradykinin induces symptoms of rhinitis and a sore throat. Am Rev Respir Dis. 1988 Mar;137(3):613–616. doi: 10.1164/ajrccm/137.3.613. [DOI] [PubMed] [Google Scholar]
- Proud D., Togias A., Naclerio R. M., Crush S. A., Norman P. S., Lichtenstein L. M. Kinins are generated in vivo following nasal airway challenge of allergic individuals with allergen. J Clin Invest. 1983 Nov;72(5):1678–1685. doi: 10.1172/JCI111127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Regoli D., Drapeau G., Rovero P., Dion S., Rhaleb N. E., Barabé J., D'Orléans-Juste P., Ward P. Conversion of kinins and their antagonists into B1 receptor activators and blockers in isolated vessels. Eur J Pharmacol. 1986 Aug 15;127(3):219–224. doi: 10.1016/0014-2999(86)90367-5. [DOI] [PubMed] [Google Scholar]
- Solèr M., Sielczak M., Abraham W. M. A bradykinin-antagonist blocks antigen-induced airway hyperresponsiveness and inflammation in sheep. Pulm Pharmacol. 1990;3(1):9–15. doi: 10.1016/0952-0600(90)90003-2. [DOI] [PubMed] [Google Scholar]
- Steranka L. R., DeHaas C. J., Vavrek R. J., Stewart J. M., Enna S. J., Snyder S. H. Antinociceptive effects of bradykinin antagonists. Eur J Pharmacol. 1987 Apr 14;136(2):261–262. doi: 10.1016/0014-2999(87)90723-0. [DOI] [PubMed] [Google Scholar]
- Steranka L. R., Farmer S. G., Burch R. M. Antagonists of B2 bradykinin receptors. FASEB J. 1989 Jul;3(9):2019–2025. doi: 10.1096/fasebj.3.9.2545496. [DOI] [PubMed] [Google Scholar]
- Steranka L. R., Manning D. C., DeHaas C. J., Ferkany J. W., Borosky S. A., Connor J. R., Vavrek R. J., Stewart J. M., Snyder S. H. Bradykinin as a pain mediator: receptors are localized to sensory neurons, and antagonists have analgesic actions. Proc Natl Acad Sci U S A. 1988 May;85(9):3245–3249. doi: 10.1073/pnas.85.9.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo J., Burch R. M., DeHaas C. J., Connor J. R., Steranka L. R. D-Phe7-substituted peptide bradykinin antagonists are not substrates for kininase II. Peptides. 1989 Jan-Feb;10(1):109–112. doi: 10.1016/0196-9781(89)90085-5. [DOI] [PubMed] [Google Scholar]
- Varonier H. S., Panzani R. The effect of inhalations of bradykinin on healthy and atopic (asthmatic) children. Int Arch Allergy Appl Immunol. 1968;34(3):293–296. doi: 10.1159/000230120. [DOI] [PubMed] [Google Scholar]
- Vavrek R. J., Stewart J. M. Competitive antagonists of bradykinin. Peptides. 1985 Mar-Apr;6(2):161–164. doi: 10.1016/0196-9781(85)90033-6. [DOI] [PubMed] [Google Scholar]



