Abstract
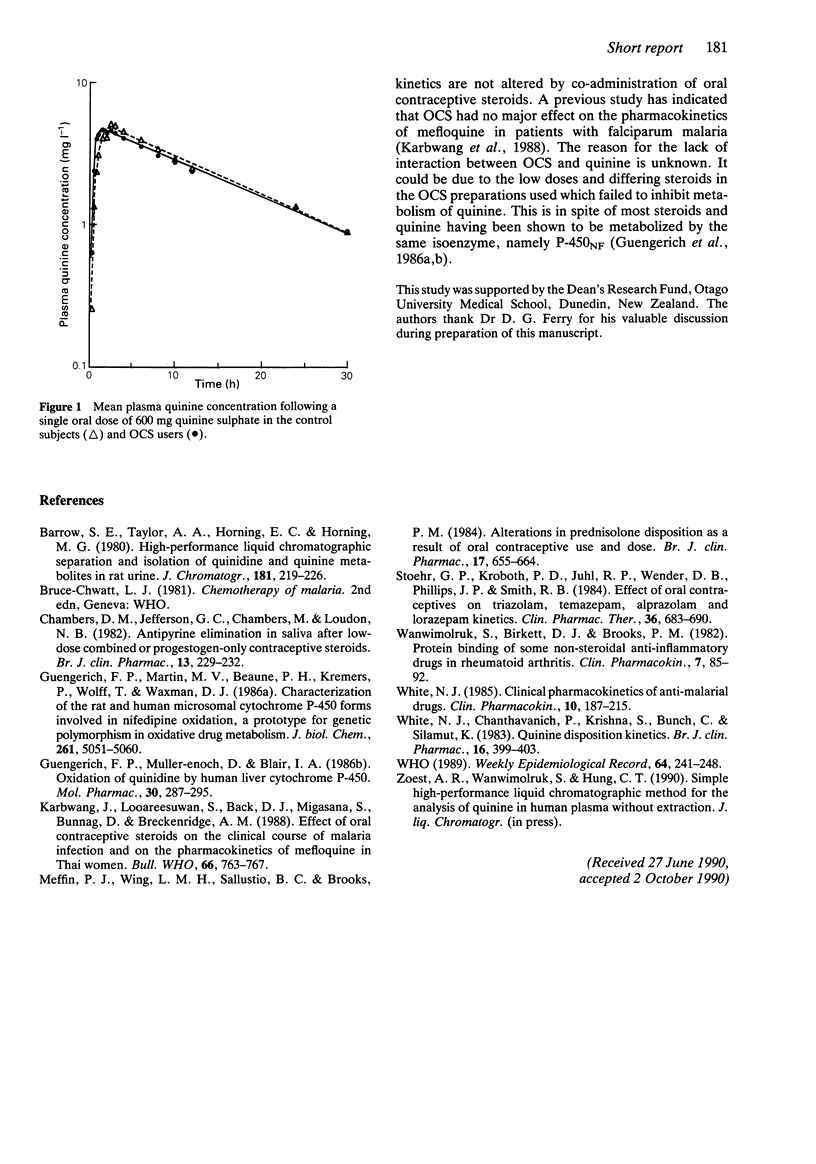
The pharmacokinetics of quinine after a single 600 mg oral dose of quinine sulphate were studied in seven female subjects who used oral contraceptives and in seven age-matched female controls who did not. There were no significant differences (P greater than 0.05) in the maximum plasma concentration (Cmax) and the time of peak concentration (tmax) between the subjects who used oral contraceptives (Cmax = 5.3 +/- 1.0 (s.d.) mg l-1; tmax = 1.4 +/- 0.7 h) and the control subjects (Cmax = 5.6 +/- 0.9 mg l-1; tmax = 2.1 +/- 0.9 h). The mean elimination half-life of quinine in the oral contraceptives user group (12.5 +/- 1.9 h) was similar (P greater than 0.05) to that in the control group (11.8 +/- 2.7 h). The oral clearance of quinine in the oral contraceptive user group was 0.133 +/- 0.055 l h-1 kg-1 (range 0.073-0.233) and was not significantly different (P greater than 0.05) from that observed in the control group (0.125 +/- 0.025 l h-1 kg-1, range 0.075-0.148).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrow S. E., Taylor A. A., Horning E. C., Horning M. G. High-performance liquid chromatographic separation and isolation of quinidine and quinine metabolites in rat urine. J Chromatogr. 1980 Feb 8;181(2):219–226. doi: 10.1016/s0378-4347(00)81607-2. [DOI] [PubMed] [Google Scholar]
- Chambers D. M., Jefferson G. C., Chambers M., Loudon N. B. Antipyrine elimination in saliva after low-dose combined or progestogen-only oral contraceptive steroids. Br J Clin Pharmacol. 1982 Feb;13(2):229–232. doi: 10.1111/j.1365-2125.1982.tb01362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich F. P., Martin M. V., Beaune P. H., Kremers P., Wolff T., Waxman D. J. Characterization of rat and human liver microsomal cytochrome P-450 forms involved in nifedipine oxidation, a prototype for genetic polymorphism in oxidative drug metabolism. J Biol Chem. 1986 Apr 15;261(11):5051–5060. [PubMed] [Google Scholar]
- Guengerich F. P., Müller-Enoch D., Blair I. A. Oxidation of quinidine by human liver cytochrome P-450. Mol Pharmacol. 1986 Sep;30(3):287–295. [PubMed] [Google Scholar]
- Karbwang J., Looareesuwan S., Back D. J., Migasana S., Bunnag D., Breckenridge A. M. Effect of oral contraceptive steroids on the clinical course of malaria infection and on the pharmacokinetics of mefloquine in Thai women. Bull World Health Organ. 1988;66(6):763–767. [PMC free article] [PubMed] [Google Scholar]
- Meffin P. J., Wing L. M., Sallustio B. C., Brooks P. M. Alterations in prednisolone disposition as a result of oral contraceptive use and dose. Br J Clin Pharmacol. 1984 Jun;17(6):655–664. doi: 10.1111/j.1365-2125.1984.tb02400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoehr G. P., Kroboth P. D., Juhl R. P., Wender D. B., Phillips J. P., Smith R. B. Effect of oral contraceptives on triazolam, temazepam, alprazolam, and lorazepam kinetics. Clin Pharmacol Ther. 1984 Nov;36(5):683–690. doi: 10.1038/clpt.1984.240. [DOI] [PubMed] [Google Scholar]
- Wanwimolruk S., Birkett D. J., Brooks P. M. Protein binding of some non-steroidal anti-inflammatory drugs in rheumatoid arthritis. Clin Pharmacokinet. 1982 Jan-Feb;7(1):85–92. doi: 10.2165/00003088-198207010-00005. [DOI] [PubMed] [Google Scholar]
- White N. J., Chanthavanich P., Krishna S., Bunch C., Silamut K. Quinine disposition kinetics. Br J Clin Pharmacol. 1983 Oct;16(4):399–403. doi: 10.1111/j.1365-2125.1983.tb02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N. J. Clinical pharmacokinetics of antimalarial drugs. Clin Pharmacokinet. 1985 May-Jun;10(3):187–215. doi: 10.2165/00003088-198510030-00001. [DOI] [PubMed] [Google Scholar]


