Abstract
Cytotoxic T-lymphocyte (CTL) responses are thought to control human immunodeficiency virus replication during the acute phase of infection. Understanding the CD8+ T-cell immune responses early after infection may, therefore, be important to vaccine design. Analyzing these responses in humans is difficult since few patients are diagnosed during early infection. Additionally, patients are infected by a variety of viral subtypes, making it hard to design reagents to measure their acute-phase immune responses. Given the complexities in evaluating acute-phase CD8+ responses in humans, we analyzed these important immune responses in rhesus macaques expressing a common rhesus macaque major histocompatibility complex class I molecule (Mamu-A*01) for which we had developed a variety of immunological assays. We infected eight Mamu-A*01-positive macaques and five Mamu-A*01-negative macaques with the molecularly cloned virus SIVmac239 and determined all of the simian immunodeficiency virus-specific CD8+ T-cell responses against overlapping peptides spanning the entire virus. We also monitored the evolution of particular CD8+ T-cell responses by tetramer staining of peripheral lymphocytes as well as lymph node cells in situ. In this first analysis of the entire CD8+ immune response to autologous virus we show that between 2 and 12 responses are detected during the acute phase in each animal. CTL against the early proteins (Tat, Rev, and Nef) and against regulatory proteins Vif and Vpr dominated the acute phase. Interestingly, CD8+ responses against Mamu-A*01-restricted epitopes Tat28-35SL8 and Gag181-189CM9 were immunodominant in the acute phase. After the acute phase, however, this pattern of reactivity changed, and the Mamu-A*01-restricted response against the Gag181-189CM9 epitope became dominant. In most of the Mamu-A*01-positive macaques tested, CTL responses against epitopes bound by Mamu-A*01 dominated the CD8+ cellular immune response.
Developing an effective vaccine for human immunodeficiency virus (HIV) will prevent considerable suffering in Africa, where over 30 million individuals are infected (Working Group on Global HIV/AIDS and STD Surveillance, HIV/AIDS: the global epidemic [1997], http://hivinsite.ucsf.edu/social/un/2098.371d.html#estimates). While many vaccine regimens have reduced viral loads in macaques challenged with simian immunodeficiency virus (SIV) or simian/human immunodeficiency virus (SHIV) (4–6, 11, 13, 14, 19, 31–33, 37), the correlates of this “protection” are still unknown. Several lines of evidence suggest that cytotoxic T lymphocytes (CTL) are largely responsible for controlling viral replication. The emergence of a CTL response is coincident with a decline in acute-phase viral RNA concentration (7, 24, 40). Furthermore, depletion of CD8+ lymphocytes in SIV-infected macaques results in increased viral loads during both the acute and chronic phases of infection, implicating antigen-specific CTL in the control of viral replication (20, 26, 38). Additionally, it is now clear that CTL exert significant selective pressure on HIV and SIV (2, 8, 15, 17, 35). Traditional approaches to vaccine design for HIV have sought to elicit antibody responses but have yielded disappointing results. It is unlikely that we will be able to induce neutralizing antibodies against field strains of HIV, given their resistance to neutralization (30). Thus, we will likely have to rely on a vaccine that primarily or initially induces cellular immune responses that might reduce initial viral replication, shifting the balance in favor of the host and preventing the spawning of new variant viruses.
Humans and macaques expressing certain major histocompatibility complex class I (MHC-I) molecules control HIV and SIV replication better than other individuals that do not express these particular MHC-I molecules. In humans, HLA-B*27 and -B*57 are associated with resistance to disease progression (18, 21, 27, 28), whereas the presence of HLA-B*35 and -Cw*04 are associated with the rapid development of AIDS-defining conditions (12). In macaques, the common MHC-I molecule Mamu-A*01 is associated with control of viral replication in the setting of mucosal infection with the SIVmac251 biological isolate (34).
To understand the role that particular MHC-I molecules play during infection, analyzing the breadth and targets of the early CD8+ immune responses to HIV may be important. Additionally, this information is fundamental to the design of an effective vaccine that induces strong CTL responses, since it is during this phase that viral replication is first controlled. Robust and efficient CTL responses are generated during the acute phase, as evidenced by the decline in plasma viremia from its acute-phase peak (7, 24, 40). The CTL repertoire in the acute phase may also be different from that seen in the chronic phase (16). Unfortunately, few HIV-infected patients are identified in the acute phase of infection. On the other hand, rhesus macaques infected with cloned SIV provide an ideal model for analyzing acute-phase responses to lentiviruses.
In this study we utilized a variety of assays to determine the entire CD8+ immune response to SIV during the acute phase of infection in rhesus macaques expressing the common MHC-I molecule Mamu-A*01. We infected Mamu-A*01-positive and Mamu-A*01-negative animals with molecularly cloned SIVmac239. We performed the intracellular cytokine staining (ICS) assay for gamma interferon (IFN-γ) using a complete set of overlapping peptides for SIVmac239 to assess the entire CD8+ T-cell immune response to SIV. CD8+ lymphocyte reactivity against the early proteins (Tat, Rev, and Nef) and Vif and Vpr dominated the acute phase. Rather unexpectedly, we found that the immune response to SIV during the acute phase of infection in Mamu-A*01-positive animals was dominated by CD8+ responses to epitopes bound by Mamu-A*01. These responses were directed against two epitopes, Gag181-189CM9 and Tat28-35SL8. However, analysis with the Gag181-189CM9 and Tat28-35SL8 tetramers in the chronic phase of infection revealed that the Gag181-189CM9-specific response predominated. This is the first analysis of the entire repertoire of CD8+ responses to autologous virus in the acute phase of SIV infection.
MATERIALS AND METHODS
Intracellular IFN-γ cytokine staining.
Peripheral blood mononuclear cells (PBMC; 5 × 105) were incubated at 37°C for 1.5 h with anti-CD28 and anti-CD49d antibodies (0.5 μg of each antibody; BD Pharmingen, San Diego, Calif.) and either staphylococcal enterotoxin B (10 μg/ml; Sigma, St. Louis, Mo.), pooled peptides (1 μg of each peptide/sample), Gag181-189CM9 peptide, Tat28-35SL8 peptide, or a negative-control influenza virus peptide (SNEGSYFF). Pools were made with 10 peptides each (Chiron, Emeryville, Calif.); peptides were 15 amino acids in length, overlapping by 11 amino acids, and spanned the Gag, Pol, Vif, Vpx, Vpr, Rev, Tat, and Nef protein sequences of SIVmac239; the Env pools, which were 20 amino acids in length, overlapping by 10 amino acids, also corresponded to the SIVmac239 sequence. An additional set of Pol 20-mer peptides corresponded to the sequence of SIVmac251. This Pol 20-mer set was used to test lymphocytes from Mamu-A*01-positive animals 80025, 87108, 1937, 92077, and 95096 until we obtained the set corresponding to the SIVmac239 sequence. Cells were then treated with 10 μg of brefeldin A (10 μg/ml; Sigma)/ml to inhibit protein trafficking and incubated a further 5 h at 37°C. Cells were then washed with fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline [PBS], 2% fetal calf serum [FCS]) and resuspended in 100 μl of FACS buffer. Cells were surface stained with antibodies specific for CD8α-peridinin chlorophyll protein (PerCP) and CD4-allophycocyanin (APC) (BD Pharmingen) for 40 min at room temperature. Cells were then washed twice with FACS buffer and fixed with 2% paraformaldehyde (PFA) (PBS, 2% PFA [Sigma]). Cells were placed at 4°C overnight. Cells were then washed once with FACS buffer and twice with permeabilization buffer (0.1% saponin [Sigma] in FACS buffer). Cells were incubated in the dark for 50 min and stained with antibodies specific for IFN-γ-fluorescein isothiocyanate (FITC) and for CD69-phycoerythrin (PE) (BD Pharmingen) at room temperature. Cells were then washed two times with 0.1% saponin-buffer. Finally, a 100-μl cell suspension was fixed with 250 μl of 2% PFA. Acquisition was performed on a FACSCaliber flow cytometer collecting 100,000 to 200,000 lymphocyte-gated events per sample.
Peripheral blood tetramer staining.
Fresh unstimulated PBMC (106) were washed two times in FACS buffer (PBS [Gibco] with 2% FCS [BioCell, Rancho Dominguez, Calif.]) in a 96-well U-bottom plate. In a 100-μl volume, cells were stained in the dark for 40 min at room temperature with the tetramer (1 μg/ml for in vitro cultures, 5 μg/ml for fresh PBMC), an anti-rhesus CD3-FITC monoclonal antibody (10 μl; BioSource), and an anti-CD8α-PerCP antibody (3 μl; Becton Dickinson Immunocytometry Systems, San Jose, Calif.). The cells were then washed four times with FACS buffer and fixed by adding 450 μl of 2% PFA. A Gag181-189CM9-specific CTL clone was stained in parallel with isotype controls (mouse immunoglobulin G1 [IgG1]-FITC [BioSource]; mouse IgG2α-PE [Immunotech]; mouse IgG1-PerCP [BD Pharmingen]; mouse IgG1-APC [Immunotech]), anti-CD3-FITC, anti-CD8β-PE (5 μl; Immunotech), anti-CD8α-PerCP, or CD8α-APC (1 μl; Immunotech) to establish compensation parameters. Sample data were acquired on a Becton Dickinson FACSCalibur instrument and analyzed using CellQuest software (Becton Dickinson). Background tetramer staining of fresh, unstimulated PBMC from naive Mamu-A*01-positive animals was routinely less than 0.08%.
In situ tetramer staining of lymph node tissues.
Biotinylated Mamu-A*01/β2m/peptide molecules were produced with either Gag181-189CM9, Tat181-189SL8, or irrelevant (FLPSDYFPSV) peptides at the National Institute of Allergy and Infectious Diseases tetramer facility. Tetramers were generated by adding six aliquots of FITC-labeled ExtraAvidin (Sigma) to biotinylated Mamu-A*01/β2m/peptide monomers over the course of 8 h to a final molar ratio of 4.5:1.
Fresh lymph nodes were processed essentially as described previously (39). Tissues were shipped overnight in PBS on ice. The following day, tissues were cut into 0.5 by 0.5-cm pieces and embedded in 4% low-melt agarose and the agarose was patted dry and secured to vibratome blocks with Loctite vibratome tissue adhesive. After the glue was allowed to set for at least 3 min, the blocks were placed in a vibratome bath containing 0°C PBS. Vibratome sections, 200 μm thick, were generated with a dead-slow speed and maximum amplification using a standard double-edged razor blade set at an angle of 27°.
Fresh sections were stained free floating in a 1-ml solution with four sections per well in 24-well tissue culture plates. Incubations were carried out at 4°C on a rocking platform. Tetramers were added at a concentration of 0.5 μg/ml with 2% normal goat serum (NGS) and 0.5 μg of mouse anti-human CD8 antibodies (clone DK25; [DAKO])/ml and incubated overnight. Sections were washed with PBS and then fixed with PBS-buffered 2% formaldehyde for 30 min at room temperature. Sections were again washed in PBS and then incubated with rabbit anti-FITC antibodies (Biodesign or Zymed) diluted 1:10,000 in PBS with 2% NGS and incubated overnight. Sections were washed three times with PBS for at least 20 min and then incubated with Cy3-conjugated goat anti-rabbit antibodies and Cy5-conjugated goat anti-rat antibodies (Jackson ImmunoResearch), both diluted 1:1,000 in PBS with 2% NGS overnight. Finally, sections were washed three times for at least 20 min and then mounted on slides with warmed glycerol gelatin (Sigma) containing 4 mg of n-propyl galate/ml. Stained sections were analyzed using a Bio-Rad 1000 confocal microscope.
IFN-γ enzyme-linked immunospot (ELISPOT) assay.
Ninety-six-well flat-bottom plates (U-Cytech BV, Utrecht, The Netherlands) were coated with 5 μg of an anti-IFN-γ monoclonal antibody MD-1 (U-Cytech BV) overnight at 4°C. The plates were then washed 10 times with PBST (PBS [Gibco-BRL, Grand Island, N.Y.] containing 0.05% Tween 20 [Sigma Chemical]), and then the plates were blocked with 2% PBSA (PBS containing 2% bovine serum albumin [Sigma Chemical]) for 1 h at 37°C. PBSA (2%) was discarded from the plates, and freshly isolated PBMC were added. Cells were resuspended in RPMI 1640 (Mediatech) supplemented with penicillin, streptomycin, and 5% fetal bovine serum (BioCell; R05). The R05 also contained either 10 μg of concanavalin A (Sigma Chemical)/ml, 0.1 to 100 μg of either the Mamu-A*01-bound Gag181-185CM9 or Tat28-35SL8 peptides/ml, 10 μg of a negative-control influenza virus peptide (SNEGSYFF)/ml, or no peptide. Input cell numbers were 1.0 × 105 peripheral blood lymphocytes in 100 μl/well, in triplicate wells.
The plates were incubated with the cells overnight (16 h) at 37°C in 5% CO2. The cells were then removed by shaking them off the plates and 200 μl of ice-cold deionized water/well was added to lyse the remaining PBMC. The plates were incubated on ice for 15 min, after which they were washed 20 times with PBST. Next, 1 μg of rabbit polyclonal biotinylated detector antibody solution (U-Cytech-BV)/well was added, and the plates were incubated for 1 h at 37°C. The plates were washed 10 times with PBST, after which 50 μl of a gold-labeled anti-biotin IgG solution (U-Cytech BV)/well was added. The plates were once again incubated for 1 h at 37°C and washed 10 times with PBST. Activator mixture (30 μl/well; U-Cytech BV) was then added, and the plates were developed for about 30 min. The activator mixture consisted of a silver salt solution that precipitates at the sites of gold clusters (from the gold-labeled antibiotin solution), thereby visualizing the sites where the IFN-γ was secreted. Once these sites or black spots could be seen in the wells under an inverted microscope, the wells were washed with distilled water to stop development. The plates were then air dried.
Wells were imaged with IP Lab Spectrum software, version 3.23, using a Hamamatsu C4880 series camera attached to a Nikon TE 300 inverted microscope. Spots were counted manually. A spot-forming cell (SFC) was defined as a large black spot with a fuzzy border (22). To determine significance levels, a baseline for each peptide was established using the average and standard deviation of the number of SFCs for each peptide. A threshold significance value corresponding to this average plus two standard deviations was then determined. A response was considered positive if the number of SFCs exceeded the threshold significance level of the sample with no peptide.
Animals, viruses, and infections.
Rhesus macaques used in this study were identified as Mamu-A*01 positive by PCR sequence-specific primers (SSP) and direct sequencing as previously described (23). Macaques were infected intrarectally with molecularly cloned virus SIVmac239. For macaques 80025 (simian T-cell leukemia virus type 1 positive [STLV+] simian retrovirus positive [STLV+]), 87108 (SRV+), 1937 (SRV+), and 95096 and 92077 (the last two previously vaccinated with a lipopeptide containing the incorrect Mamu-A*01-restricted TPYDINQM epitope; no Gag181-189CM9 responses were detected), this SIVmac239 stock was amplified on CEMx174 cells only. For other animals (control animals from other vaccine studies), a titered stock of SIVmac239 was used (25, 36). Five animals were infected with the SIVmac251 biological isolate as previously described (34). SIV-infected animals at the University of Wisconsin were cared for according to an experimental protocol approved by the University of Wisconsin Research Animal Resource Committee.
RESULTS
Mamu-A*01-restricted CD8+ T-cell responses are immunodominant during the acute phase.
It is during the acute phase that the setpoint is established, and thus analysis of the CD8+ immune responses in animals that express MHC-I molecules associated with control of viral replication may allow us to design a rational vaccine approach. It is difficult to determine the entire repertoire of acute CD8+ cellular immune responses in HIV infection since patients are infected with heterogeneous inocula that are often inaccessible to the investigator. This makes it almost impossible to design appropriate reagents for the study of the entire repertoire of CD8+ immune responses to HIV. Our overlapping set of peptides corresponded exactly to the sequence of the molecularly cloned SIVmac239 that we used for infection, except for one set of Pol peptides, which was 99% identical to that of the infecting strain. After infection of eight Mamu-A*01-positive macaques with SIVmac239 by the mucosal route, we defined every CD8+ response to SIV during the acute phase using ICS for IFN-γ after stimulation with peptides representing all the SIVmac239 viral proteins (Fig. 1). Mamu-A*01-positive animals made between six and nine CD8+ responses to SIVmac239 pooled peptides (see Fig. 3). The pool containing theTat28-35SL8-specific response was immunodominant over the pool containing the Gag181-189CM9-specific response in the acute phase in the majority of the macaques. This immunodominance was confirmed using ELISPOT assays with fresh PBMC at 2 weeks postinfection (data not shown). ICS performed with lymphocytes from uninfected animals showed no responses to our peptide pools (data not shown).
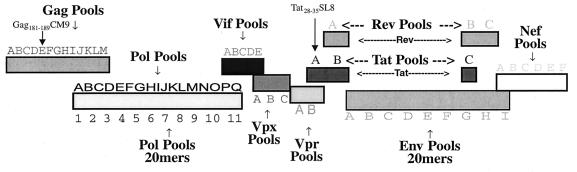
FIG. 1.
Pools of peptides spanning all the proteins from SIVmac239. Our peptide set corresponds exactly to the sequence of the molecularly cloned SIVmac239 that we used for infection (except for one set of Pol 20-mer peptides, which corresponds to the sequence of SIVmac251 and is 99% identical to that of SIVmac239). Pools of peptides are used for intracellular cytokine staining for IFN-γ to determine the entire repertoire of CD8 antigen-specific responses.
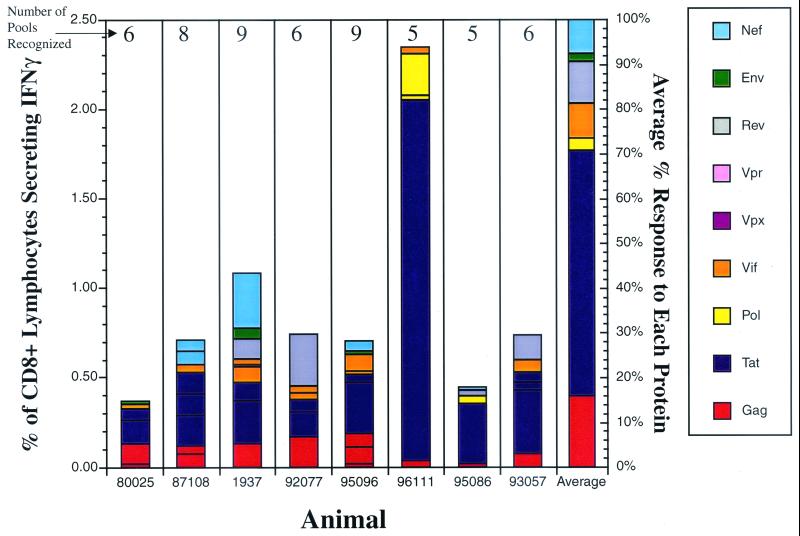
FIG. 3.
Acute-phase CD8+ T-cell responses in Mamu-A*01-positive macaques. Intracellular cytokine staining was performed on lymphocytes using pools spanning all the SIV proteins during the acute phase of SIVmac239 infection (3 to 4 weeks postinfection) in eight Mamu-A*01-positive animals. Bars, percentages of CD8+ IFN-γ+ responses for every SIV protein in each animal; last bar on the right, average percent contribution of each protein to the overall CD8+ response. Responses to the Gag and Tat proteins predominate in the acute phase of infection in most Mamu-A*01-positive animals. Number above each bar, number of responses detected in the animal.
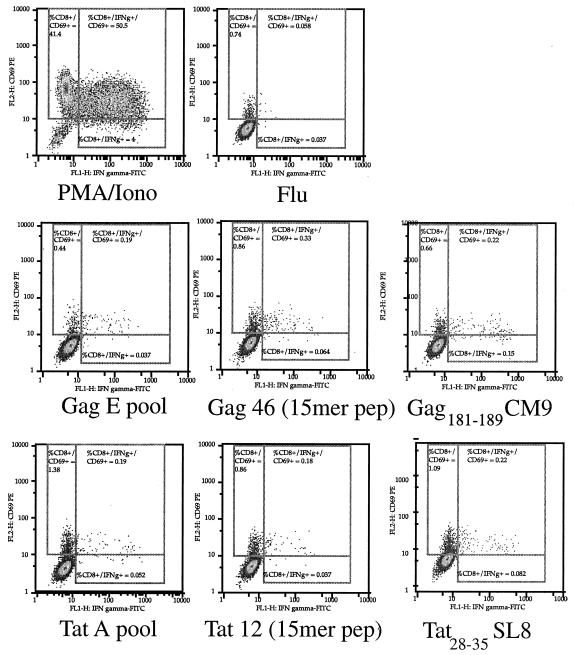
To determine whether the responses to the Tat A and Gag E pools could be attributed to the Tat28-35SL8 and Gag181-189CM9 epitopes, we compared the CD8+ T-cell responses to the pools, the individual 15-mers, and the minimal epitopes (Fig. 2).
FIG. 2.
Detection of IFN-γ production using the intracellular cytokine staining assay. PBMC from Mamu-A*01-positive SIV-infected animal 95096 were tested with Gag E and Tat A pools, the Gag 46 and Tat 12 15-mer peptides (pep), and the Gag181-189CM9 and Tat28-35SL8 peptides. Results depict the production of CD69 and IFN-γ in response to each pool of peptides and peptides for CD8+ lymphocytes. PMA, phorbol myristate acetate; Iono, ionomycin.
The Tat28-35SL8- and Gag181-189CM9-specific responses were similar to the responses obtained using the pools and the 15-mer peptides containing those epitopes.
Remarkably, analysis of responses to all SIV proteins revealed that the Tat-specific CD8+ responses averaged 55% of the total CD8+ responses to the virus in these eight Mamu-A*01-positive animals (Fig. 3). The pools containing the Gag181-189CM9- and the Tat28-35SL8-Mamu-A*01-restricted responses dominated the acute phase (Fig. 4), contributing, on average, about 56% of the total SIV-specific response.
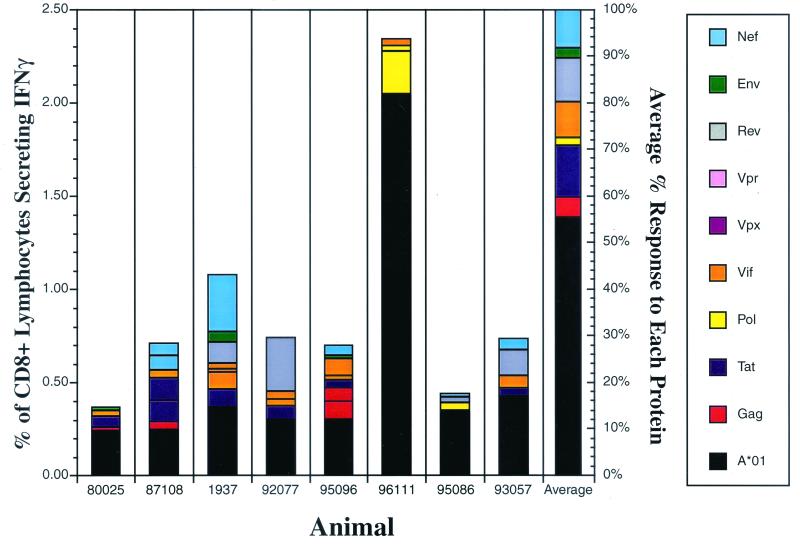
FIG. 4.
Contribution of CD8+ responses directed against pools containing Mamu-A*01-bound peptides to acute-phase responses. Intracellular cytokine staining was performed on lymphocytes using pools spanning all the SIV proteins and Mamu-A*01 epitopes during the acute phase of SIVmac239 infection (3 to 4 weeks postinfection) in eight Mamu-A*01-positive animals. Bars, percentages of CD8+ IFN-γ+ responses for each SIV protein (except the Tat A and the Gag E pools) and the pools containing the Mamu-A*01-restricted epitopes, the Gag181-189CM9 and Tat28-35SL8 epitopes, in each animal. Responses to the pools containing the Mamu-A*01 epitopes predominate in the acute phase of infection in most Mamu-A*01-positive animals.
Tat-specific Mamu-A*01-restricted CTL responses dominate in the acute phase of both SIVmac239 and SIVmac251 infection.
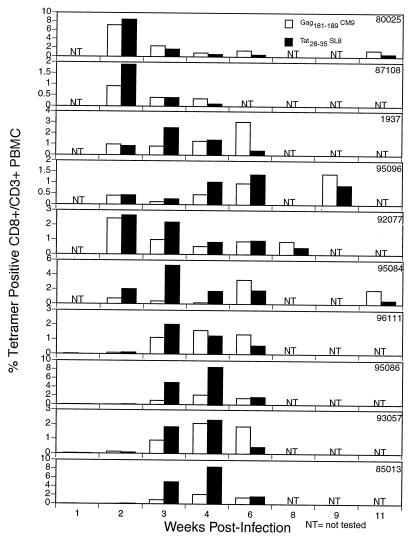
We then explored the possibility that this Tat28-35SL8-specific response represents the strongest cellular immune response to SIV during the acute phase in Mamu-A*01-positive animals. Previously, it was suggested that the immunodominant response to SIV in Mamu-A*01-positive animals was directed against the single epitope, Gag181-189CM9 (29). Recently, we described a strong acute-phase immune response to the Tat28-35SL8 epitope, from which the virus escapes in the first 4 weeks of infection (2). In this previous study, we analyzed PBMC from animals that were immunized with the Gag181-189CM9 CTL epitope, and thus these animals had large anamnestic CTL responses to Gag181-189CM9 (2). It was, therefore, difficult to compare immune responses to the Gag181-189CM9 and Tat28-35SL8 epitopes in the acute phase. We, therefore, undertook a tetramer analysis of the acute and chronic phases of infection in 10 naive Mamu-A*01-positive animals infected with molecular clone SIVmac239 and five naive Mamu-A*01-positive animals infected with biological isolate SIVmac251 by the mucosal route. Surprisingly, in all but one of these animals, the Tat28-35SL8-specific response was immunodominant over the Gag181-189CM9-specific response (P = 0.004 by paired t test; 3 weeks postinfection) (Fig. 5 and Table 1). In the chronic phase of infection, however, the Gag181-189CM9-specific response predominated.
FIG. 5.
Tat28-35SL8 responses predominate in Mamu-A*01-positive rhesus macaques in the acute phase of SIV infection as visualized by tetramer staining of fresh PBMC. Ten Mamu-A*01-positive rhesus macaques were infected with cloned SIVmac239. Fresh PBMC were stained for CD3 and CD8 and the specific tetramer at room temperature. CD8+ responses to the two predominant Mamu-A*01-restricted epitopes, Gag181-189CM9 and Tat28-35SL8, were monitored using tetramers specific for each epitope. In the first few weeks postinfection the Tat28-35SL8-specific response predominates.
TABLE 1.
Tat28-35SL8-specific responses predominate during the acute phase in macaques mucosally infected with SIVmac251 (3 weeks postinfection)
| Animal | Response to:
|
|
|---|---|---|
| Gag181-189CM9 | Tat28-35SL8 | |
| 408 | 1.94 | 8.61 |
| 427 | 1.12 | 8.38 |
| 444 | 0.34 | 0.14 |
| 575 | 1.20 | 11.50 |
| 460 | 0.26 | 2.67 |
In situ tetramer staining of lymph node tissues during the acute phase reveals dominance of Tat28-35SL8- and Gag181-189CM9-specific lymphocytes, whereas chronic phase staining shows that the Gag181-189CM9-specific lymphocytes are dominant.
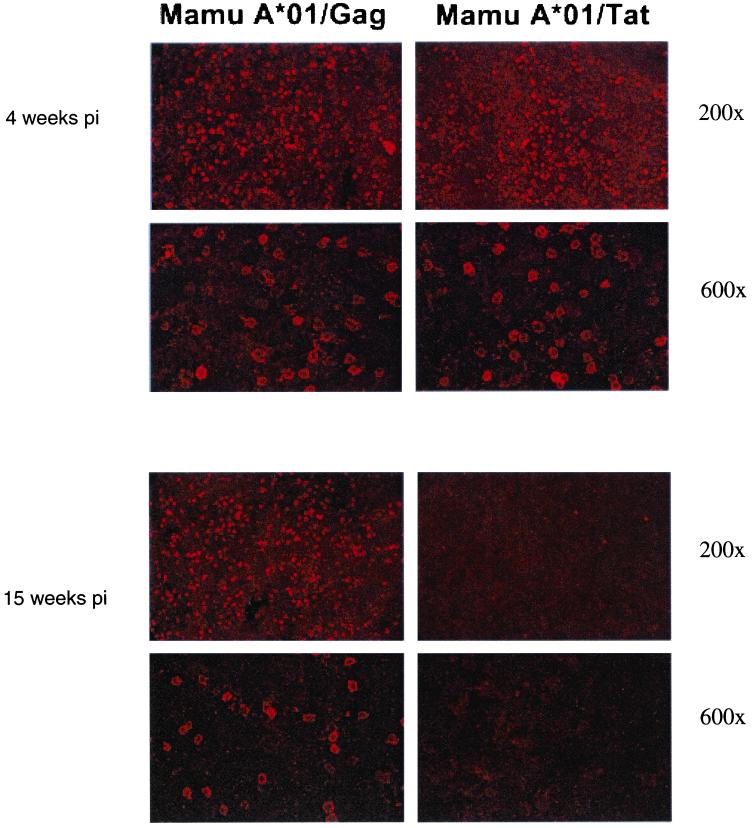
To determine whether the immunodominance seen in the PBMC is also present in the lymph nodes, we performed in situ tetramer staining on samples from infected Mamu-A*01-positive macaques during both acute and chronic stages of infection. We found similar quantities of cells specific for the Gag181-189CM9 and Tat28-35SL8 epitopes during acute infection (Fig. 6). We detected abundant Gag181-189CM9-specific cells but not Tat28-35SL8-specific cells in lymph node tissues from chronically infected Mamu-A*01-positive macaques. These findings demonstrate that the loss of Tat28-35SL8-reactive T cells and maintenance of relatively large quantities of Gag181-189CM9-specific T cells occur in both the periphery and lymph nodes.
FIG. 6.
Gag- and Tat-specific T cells in lymph nodes from acute and chronic SIV infection by in situ tetramer staining. Images show lymph node sections from acutely and chronically SIV-infected macaques stained with Mamu-A*01-Gag181-189CM9- or Mamu-A*01-Tat28-35SL8-specific tetramers. Note the loss of detection of Tat-specific T cells in the sections from a chronically infected animal. Sections were counterstained with CD8+ antibodies (not shown). Each ×200 image is a projection of nine confocal Z-scans collected at 2-μm intervals. Each of the ×600 images was made by projecting 14 confocal Z-scans collected at 1-μm intervals.
CD8+ responses in Mamu-A*01-negative animals.
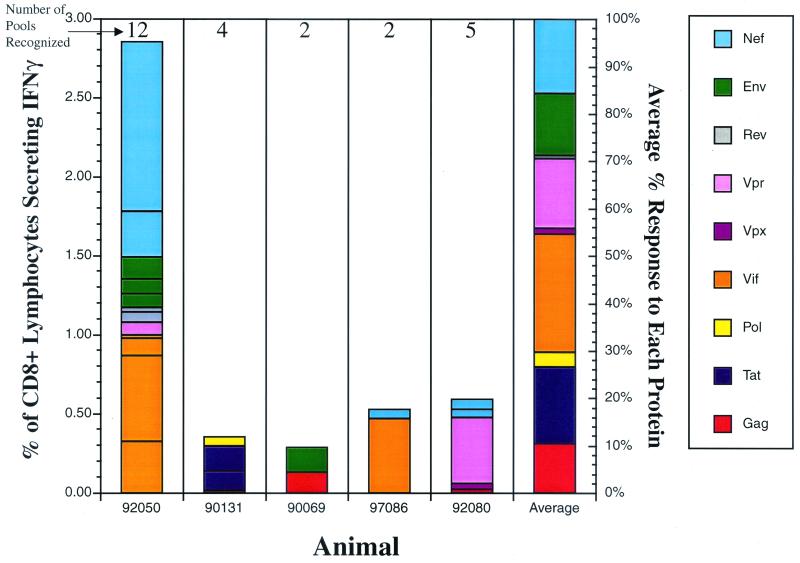
To determine the repertoire of CD8 responses to SIV in animals that do not express Mamu-A*01, we infected five Mamu-A*01-negative macaques with SIVmac239 by the mucosal route. We subsequently defined every CD8+ response to SIV during the acute phase of infection (Fig. 7) using ICS for IFN-γ after stimulation with peptides representing all the SIVmac239 viral proteins. Mamu-A*01-negative animals made between 2 and 12 CD8+ responses to SIVmac239 pooled peptides (Fig. 7). The overall magnitude of SIV-specific CD8+ T-cell responses in Mamu-A*01-negative animals ranged from 0.284 to 2.855% (average of 1.06%), similar to the magnitude obtained in Mamu-A*01-negative animals, which ranged from 0.369 to 2.347% (average of 0.892%). Additionally, only three of the responses detected in the Mamu-A*01-negative animals (92050: Nef D; 97086: VifA/B; 92080: Vpr B) had magnitudes that were comparable to those of the responses to the pools containing either Tat28-35SL8 or Gag181-189CM9 epitopes.
FIG. 7.
Acute-phase CD8+ T-cell responses in Mamu-A*01-negative macaques. Intracellular cytokine staining was performed on lymphocytes using pools spanning all the SIV proteins during the acute phase of SIVmac239 infection (3 weeks postinfection) in five Mamu-A*01-negative animals. Bars, percentages of CD8+ IFN-γ+ responses for every SIV protein in each animal; last bar on the right, average percent contribution of each protein to the overall CD8+ response; number above each bar, number of responses detected in the animal.
Tat, Rev, Nef, Vpr, and Vif are well recognized during the acute phase of infection.
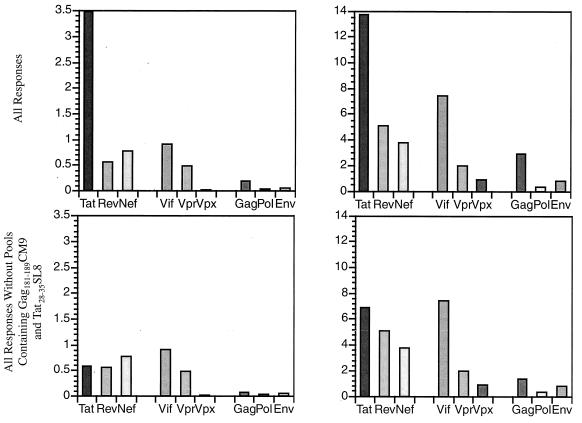
Since it is thought that acute-phase CTL control initial viral replication, definition of the targets of this acute-phase CTL response might be important in vaccine design. As some SIV proteins are larger than others, we divided the strength of all animals’ CTL responses and numbers of pools recognized by the amino acid length of each protein (Fig. 8). Given the dominance of the pools containing the Tat28-35SL8- and Gag181-189CM9-specific responses in Mamu-A*01-positive animals, we also analyzed our data without these responses. Interestingly, the large structural proteins (Gag, Pol, and Env) were poorly recognized. In contrast, the smaller regulatory and accessory proteins (Nef, Rev, Vpr, Vif, and Tat) engendered multiepitopic, strong immune responses during the acute phase of infection.
FIG. 8.
Relative contribution of each protein to CD8 responses in the acute phase in all animals tested using ICS. (A) Total IFN-γ response to each protein per 100 amino acids. The sum of the IFN-γ responses to each protein was calculated and divided by the amino acid length of each protein. This number was then multiplied by 100 to determine the contribution per 100 amino acids. Additionally, we calculated the relative contribution of each protein without the pools containing the Gag181-189CM9 and Tat28-35SL8 responses. (B) Relative number of responses to each protein detected per 100 amino acids. The sum of the number of responses to each protein was calculated and divided by the amino acid length of each protein. This number was then multiplied by 100 to determine the contribution per 100 amino acids. Additionally, we calculated the relative contribution of each protein without the pools containing the Gag181-189CM9 and Tat28-35SL8 responses. Regulatory and accessory proteins Tat, Rev, Nef, and Vif were best recognized. On the other hand, structural proteins Env, Gag, and Pol were poorly recognized.
DISCUSSION
We analyzed the entire acute CD8+ T-cell immune response to SIV in eight Mamu-A*01-positive rhesus macaques and in five Mamu-A*01-negative rhesus macaques. In this first analysis of the entire CD8+ response to autologous virus in the acute phase of immunodeficiency virus infection, we show that between 2 and 12 peptide pools are recognized. We also show that CD8+ responses against pools containing Mamu-A*01-restricted epitopes dominate the immune response in Mamu-A*01-positive macaques. In the acute phase, on average, approximately 60% of the entire SIV-specific CD8+ response is directed against pools of peptides containing Mamu-A*01-restricted epitopes. This is an underestimation of the total contribution that Mamu-A*01-restricted epitopes make to the CD8+ response since we have only taken into account two immunodominant epitopes, Tat28-35SL8 and Gag181-189CM9.
The relative paucity of acute-phase CD8+ responses against the structural proteins Gag, Env, and Pol was somewhat surprising. The robust and frequent responses against Vif made this small protein the most common target of acute-phase CD8+ responses in these animals. In contrast, only one CTL epitope in HIV Vif has been described that is restricted by HLA-A*03 (3, 10). Similarly, there have been only a few HIV Tat-derived CTL epitopes identified to date (1, 3, 10). Analysis of acute-phase HIV Vif- and Tat-specific CD8+ responses against autologous virus should result in the description of several new epitopes. Given the paucity of studies addressing the immunogenetics of other viral infections in rhesus macaques, we cannot speculate as to whether Mamu-A*01 plays a similarly dominant role in different viral infections in the species.
Interestingly, we have shown that the same responses that are present during the acute phase are not necessarily present in the chronic phase of infection. This shifting CD8+ repertoire is reminiscent of the recently described situation in HIV-infected humans (16). The Mamu-A*01-restricted Tat28-35SL8-specific response was immunodominant during the acute phase as measured by ELISPOT and tetramer staining. In the chronic phase, the frequency of CD8+ lymphocytes that recognized the Tat28-35SL8 epitope declined. As we previously showed (2), the virus escapes from the Tat28-35SL8-specific response by 4 weeks postinfection. The Gag181-189CM9-specific response then becomes immunodominant in these Mamu-A*01-positive animals. This is the first example of a shift in the recognition by CTL during SIV infection.
The dominance of the Mamu-A*01-restricted responses is somewhat surprising given that MHC-I haplotype organization in the rhesus macaque is different from that in humans (9). We have never found an animal that expresses less than five MHC-I molecules. It is, therefore, remarkable that a single MHC molecule, Mamu-A*01, should dominate the entire SIV-specific CD8+ response in the acute phase of infection.
In the setting of mucosal SIVmac251 infection, Mamu-A*01-positive animals are more successful than Mamu-A*01 negative animals in controlling viral replication (34). It is tempting to speculate that this preferential ability to withstand SIVmac251 infection is due to the evolution of strong Tat28-35SL8- and Gag181-189CM9-specific CTL responses. These findings may be relevant to HIV infection in which the presence of particular MHC-I molecules is associated with control of viral replication (21, 27, 28).
Acknowledgments
B.R.M. and H.H. contributed equally to this work.
This work was supported by NIH grants A149120, AI45461, AI46366, RR15371, and RR00167 and a Cremer Scholarship from the Department of Pathology, UW-Madison (B.R.M.). David Watkins is an Elizabeth Glaser Scientist.
We thank the Immunology and Virology Core Laboratory at WRPRC for infection and monitoring of rhesus macaques. We thank Ron Desrosiers for supplying SIVmac239.
REFERENCES
- 1.Addo, M. M., M. Altfeld, E. S. Rosenberg, R. L. Eldridge, M. N. Philips, K. Habeeb, A. Khatri, C. Brander, G. K. Robbins, G. P. Mazzara, P. J. Goulder, and B. D. Walker. 2001. The HIV-1 regulatory proteins Tat and Rev are frequently targeted by cytotoxic T lymphocytes derived from HIV-1-infected individuals Proc. Natl. Acad. Sci. USA 98:1781–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, T. M., D. H. O’Connor, P. Jing, J. L. Dzuris, B. R. Mothe, E. Dunphy, M. E. Liebl, T. U. Vogel, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific CTL select for SIV escape variants during resolution of primary viremia. Nature 407:386–390. [DOI] [PubMed] [Google Scholar]
- 3.Altfeld, M. A., A. Trocha, R. L. Eldridge, E. S. Rosenberg, M. N. Phillips, M. M. Addo, R. P. Sekaly, S. A. Kalams, S. A. Burchett, K. McIntosh, B. D. Walker, and P. J. Goulder. 2000. Identification of dominant optimal HLA-B60- and HLA-B61-restricted cytotoxic T-lymphocyte (CTL) epitopes: rapid characterization of CTL responses by enzyme-linked immunospot assay. J. Virol. 74:8541–8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O’Neil, S. J. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69–74. [DOI] [PubMed] [Google Scholar]
- 5.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, and M. G. Lewis. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486–492. [DOI] [PubMed] [Google Scholar]
- 6.Benson, J., C. Chougnet, M. Robert-Guroff, D. Montefiori, P. Markham, G. Shearer, R. C. Gallo, M. Cranage, E. Paoletti, K. Limbach, D. Venzon, J. Tartaglia, and G. Franchini. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J. Virol. 72:4170–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borrow, P., H. Lewicki, B. Hahn, G. Shaw, and M. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrow, P., H. Lewicki, X. P. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. A. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205–211. [DOI] [PubMed] [Google Scholar]
- 9.Boyson, J. E., C. Shufflebotham, L. F. Cadavid, J. A. Urvater, L. A. Knapp, A. L. Hughes, and D. I. Watkins. 1996. The MHC class I genes of the rhesus monkey: different evolutionary histories of MHC class I and II genes in primates. J. Immunol. 156:4656–4665. [PubMed] [Google Scholar]
- 10.Brander, C., and P. J. Goulder. 2001. The evolving field of HIV CTL epitope mapping: new approaches to the identification of novel epitopes, p.IV-1. In B. Korber, C. Brander, B. Haynes, R. A. Koup, C. Kuiken, J. Moore, B. D. Walker, and D. I. Watkins (ed.), HIV molecular immunology database. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 11.Cafaro, A., A. Caputo, C. Fracasso, M. T. Maggiorella, D. Goletti, S. Baroncelli, M. Pace, L. Sernicola, M. L. Koanga-Mogtomo, M. Betti, A. Borsetti, R. Belli, L. Akerblom, F. Corrias, S. Butto, J. Heeney, P. Verani, F. Titti, and B. Ensoli. 1999. Control of SHIV-89.6P infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat. Med. 5:643–650. [DOI] [PubMed] [Google Scholar]
- 12.Carrington, M., G. W. Nelson, M. P. Martin, T. Kissner, D. Vlahov, J. J. Goedert, R. Kaslow, S. Buchbinder, K. Hoots, and S. J. O’Brien. 1999. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science 283:1748–1752. [DOI] [PubMed] [Google Scholar]
- 13.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live-attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938–1941. [DOI] [PubMed] [Google Scholar]
- 14.Desrosiers, R. C., M. S. Wyand, T. Kodama, D. J. Ringler, L. O. Arthur, P. K. Sehgal, N. L. Letvin, and M. D. Daniel. 1989. Vaccine protection against simian immunodeficiency virus infection. Proc. Natl. Acad. Sci. USA 86:6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans, D. T., D. H. O’Connor, P. Jing, T. M. Allen, H. Horton, J. E. Venham, R. A. Rudersdorf, J. daSilva, C. D. Pauza, R. E. Bontrop, R. DeMars, A. L. Hughes, and D. I. Watkins. 1999. Virus-specific CTL responses select for amino acid variation in SIV Env and Nef. Nat. Med. 5:1270–1276. [DOI] [PubMed] [Google Scholar]
- 16.Goulder, P. J., M. A. Altfeld, E. S. Rosenberg, T. Nguyen, Y. Tang, R. L. Eldridge, M. M. Addo, S. He, J. S. Mukherjee, M. N. Phillips, M. Bunce, S. A. Kalams, R. P. Sekaly, B. D. Walker, and C. Brander. 2001. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. J. Exp. Med. 193:181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goulder, P. J. R., R. E. Phillips, R. A. Colbert, S. McAdam, G. Ogg, M. A. Nowak, J. P. Giangrande, G. Luzzi, B. Morgan, A. Edwards, A. J. McMichael, and S. Rowland-Jones. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3:212–217. [DOI] [PubMed] [Google Scholar]
- 18.Hill, A. V. 1998. The immunogenetics of human infectious diseases. Annu. Rev. Immunol. 16:593–617. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch, V. M., S. Goldstein, N. A. Hynes, W. R. Elkins, W. T. London, P. M. Zack, D. Montefiori, and P. R. Johnson. 1994. Prolonged clinical latency and survival of macaques given a whole inactivated simian immunodeficiency virus vaccine. J. Infect. Dis. 170:51–59. [DOI] [PubMed] [Google Scholar]
- 20.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaslow, R. A., M. Carrington, R. Apple, L. Park, A. Munoz, A. J. Saah, J. J. Goedert, C. Winkler, S. J. O’Brien, C. Rinaldo, R. Detels, W. Blattner, J. Phair, H. Erlich, and D. L. Mann. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2:405–411. [DOI] [PubMed] [Google Scholar]
- 22.Klinman, D. M. 1994. ELISPOT assay to detect cytokine-secreting murine and human cells. Curr. Prot. Immunol. 6:191–198. [DOI] [PubMed] [Google Scholar]
- 23.Knapp, L. A., E. Lehmann, M. S. Piekarczyk, J. A. Urvater, and D. I. Watkins. 1997. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens 50:657–661. [DOI] [PubMed] [Google Scholar]
- 24.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis, M. G., S. Bellah, K. McKinnon, J. Yalley-Ogunro, P. M. Zack, W. R. Elkins, R. C. Desrosiers, and G. A. Eddy. 1994. Titration and characterization of two rhesus-derived SIVmac challenge stocks. AIDS Res. Hum. Retroviruses 10:213–220. [DOI] [PubMed] [Google Scholar]
- 26.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72:164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNeil, A. J., P. L. Yap, S. M. Gore, R. P. Brettle, M. McColl, R. Wyld, S. Davidson, R. Weightman, A. M. Richardson, and J. R. Robertson. 1996. Association of HLA types A1-B8-DR3 and B27 with rapid and slow progression of HIV disease. Q. J. Med. 89:177–185. [DOI] [PubMed] [Google Scholar]
- 28.Migueles, S. A., M. S. Sabbaghian, W. L. Shupert, M. P. Bettinotti, F. M. Marincola, L. Martino, C. W. Hallahan, S. M. Selig, D. Schwartz, J. Sullivan, and M. Connors. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 97:2709–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, M. D., H. Yamamoto, A. L. Hughes, D. I. Watkins, and N. L. Letvin. 1991. Definition of an epitope and MHC class I molecule recognized by gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys. J. Immunol. 147:320–329. [PubMed] [Google Scholar]
- 30.Moore, J. P., J. A. McKeating, Y. X. Huang, A. Ashkenazi, and D. D. Ho. 1992. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J. Virol. 66:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mossman, S. P., F. Bex, P. Berglund, J. Arthos, S. P. O’Neil, D. Riley, D. H. Maul, C. Bruck, P. Momin, A. Burny, P. N. Fultz, J. I. Mullins, P. Liljestrom, and E. A. Hoover. 1996. Protection against lethal simian immunodeficiency virus SIVsmmPBj14 disease by a recombinant Semliki Forest virus gp160 vaccine and by a gp120 subunit vaccine. J. Virol. 70:1953–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy-Corb, M., L. N. Martin, and B. Davison-Fairburn. 1989. A formalin-inactivated whole SIV vaccine confers protection in macaques. Science 246:1293. [DOI] [PubMed] [Google Scholar]
- 33.Osterhaus, A. D. M. E., C. A. van Baalen, R. A. Gruters, M. Schutten, C. H. J. Siebelink, E. G. J. Hulskotte, E. J. Tijhaar, R. E. R. Randall, G. van Amerongen, A. Fleuchaus, V. Erfle, and G. Sutter. 1999. Vaccination with Rev and Tat against AIDS. Vaccine 17:2713–2714. [DOI] [PubMed] [Google Scholar]
- 34.Pal, R., D. Venzon, N. L. Letvin, S. Santra, D. C. Montefiori, N. R. Miller, E. Tryniszewska, M. G. Lewis, T. C. Vancott, V. Hirsch, R. Woodward, A. Gibson, M. Grace, E. Dobratz, P. D. Markham, Z. Hel, J. Nacsa, M. Klein, J. Tartaglia, and G. Franchini. 2001. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J. Virol. 76:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips, R. E., S. Rowland-Jones, D. F. Nixon, F. M. Gotch, J. P. Edwards, A. O. Ogunlesi, J. G. Elvin, J. A. Rothbard, C. R. Bangham, C. R. Rizza, et al. 1991. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature 354:453–459. [DOI] [PubMed] [Google Scholar]
- 36.Regier, D. A., and R. C. Desrosiers. 1990. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS. Res. Hum. Retroviruses 6:1221–1231. [DOI] [PubMed] [Google Scholar]
- 37.Robinson, H. L., D. C. Montefiori, R. P. Johnson, K. H. Manson, M. L. Kalish, J. D. Lifson, T. A. Rizvi, S. Lu, S.-L. Hu, G. P. Mazzara, S. L. Lydy, M. S. Wyand, and H. M. McClure. 1999. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat. Med. 5:526. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857–860. [DOI] [PubMed] [Google Scholar]
- 39.Skinner, P. J., M. A. Daniels, C. S. Schmidt, S. C. Jameson, and A. T. Haase. 2000. Cutting edge: in situ tetramer staining of antigen-specific T cells in tissues. J. Immunol. 165:613–617. [DOI] [PubMed] [Google Scholar]
- 40.Yasutomi, Y., K. Reimann, C. Lord, M. Miller, and N. Letvin. 1993. Simian immunodeficiency virus-specific CD8+ lymphocyte response in acutely infected rhesus monkeys. J. Virol. 67:1707–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]