Abstract
Recent studies have suggested that the latency-associated transcript (LAT) region of herpes simplex virus type 1 (HSV-1) is effective at blocking virus-induced apoptosis both in vitro and in the trigeminal ganglia of acutely infected rabbits (Inman et al., J. Virol. 75:3636–3646, 2001; Perng et al., Science 287:1500–1503, 2000). By transfecting cells with a construct expressing the Pst-Mlu segment of the LAT, encompassing the LAT exon 1, the stable 2.0-kb intron, and 5′ part of exon 2, we confirmed that this region was able to diminish the onset of programmed cell death initiated by anti-Fas and camptothecin treatment. In addition, caspase 8-induced apoptosis was specifically inhibited in cells expressing the Pst-Mlu LAT fragment. To further delineate the minimal region of LAT that is necessary for this antiapoptotic function, LAT mutants were used in our cotransfection assays. In HeLa cells, the plasmids lacking exon sequences were the least effective at blocking apoptosis. However, similar to previous work (Inman et al., op. cit.), our data also indicated that the 5′ end of the stable 2.0-kb LAT intron appeared to contribute to the promotion of cell survival. Furthermore, cells productively infected with the 17N/H LAT mutant virus, a virus deleted in the LAT promoter, exon 1, and about half of the intron, exhibited a greater degree of DNA fragmentation than cells infected with wild-type HSV-1. These data support the finding that the exon 1 and 2.0-kb intron region of the LAT transcription unit display an antiapoptotic function both in transfected cells and in the context of the virus infection in vitro. In trigeminal ganglia of mice acutely infected with the wild-type virus, 17, and 17ΔSty, a virus lacking most of exon 1, apoptosis was not detected in cells that were positive for virus particles. However, dual staining was observed in cells from mice infected with 17N/H virus, indicating that the LAT antiapoptotic function demonstrated in cells transfected by LAT-expressing constructs may also play a role in protecting cells from virus-induced apoptosis during acute viral infection in vivo.
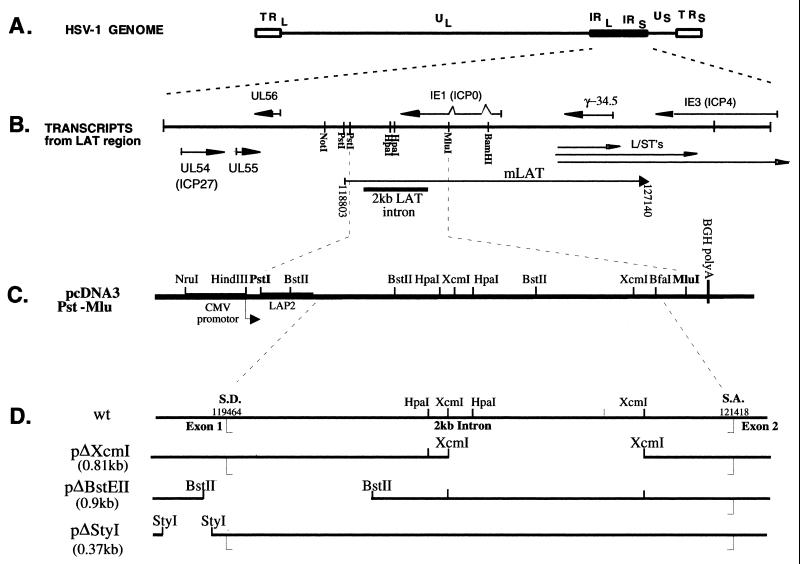
Herpes simplex virus type 1 (HSV-1) is a pathogenic human alphaherpesvirus that causes life-long latent infection in sensory neurons of the peripheral nervous system, with intermittent periods of reactivation and recurrence of disease. During latency of HSV, transcription of the genome is restricted to the latency-associated transcripts (LATs) (for reviews, see references 12, 41, and 49). The LATs map to the long terminal repeat (LTR) regions of the viral genome (Fig. 1A and B). The primary LAT (mLAT or minor LAT), which has been detected by in situ hybridization, is an 8.3-kb transcript that maps from nucleotide 118803.to a polyadenylation site at nucleotide 127140 and beyond on the viral genome (9, 31). However, the most abundant LAT species from this region is a 2.0-kb stable intron (2.0-kb LAT; Fig. 1B). Studies have shown that the 2.0-kb LAT intron is also detected during productive infections (11, 39, 46, 50).
FIG. 1.
HSV-1 LATs. (A) Linear map of the HSV-1 genome with its unique long (UL) and unique short (US) regions flanked by inverted repeat (IR) elements. (B) LAT region of the HSV-1 genome. The LAT region is enlarged to show the different LAT transcripts that map to this area, as well as the other RNAs (L/ST’s, ICP0, ICP4, ICP34.5, UL54, UL55, and UL56). The minor LAT (mLAT), the putative 8.5-kb primary transcript, and the potential spliced exons are shown (including 2.0-kb LAT intron). In addition, a linear diagram of the pcDNA3.Pst-Mlu plasmid expressing the 2.0-kb LAT intron and the exon 1 and 2 regions is shown. (C) LAT deletion mutants in the pcDNA3.Pst-Mlu background.
Since the LATs are transcribed during latent infections, their role in establishment, maintenance, and reactivation from latency has been examined in detail. Early studies hypothesized that the 2.0-kb LAT intron was involved in an antisense suppression mechanism because it overlaps the 3′ end of ICP0 mRNA (50). Other groups have shown that several LAT region deletion viruses exhibit a delayed reactivation phenotype in various animal models (6, 18, 25, 48, 52). Furthermore, studies have suggested that LATs play a role in promoting efficient establishment of latency in trigeminal ganglia of both mice (44) and rabbits (37).
It has been proposed that LATs may facilitate the establishment of latency by reducing productive viral gene expression (15, 29). However, it is as yet uncertain whether LATs play a direct role in each of these effects. Most recently, experiments have suggested that the LAT region promotes neuronal survival after HSV infection by reducing apoptosis in infected cells (19, 36). These results are consistent with the hypothesis that the antiapoptotic phenotype of the LAT region may allow protection of latently infected cells and/or reactivating cells, thereby ensuring a large pool of latently infected cells, which would contribute to the efficient reactivation of virus under conditions of stress.
Inhibition of apoptosis appears to be a mechanism used by several viruses to prevent the premature death of infected cells in order to maximize production of infectious virions. For example, cowpox virus and baculovirus contain antiapoptotic factors that inhibit proteases (caspases) involved in the induction of apoptosis (7, 51). Epstein-Barr virus (17), herpesvirus saimiri (8), and African swine fever viruses (2) encode proteins that are homologues of the cellular antiapoptotic protein Bcl-2. In addition, the polyomavirus large T antigen (40) and the murine gammaherpesvirus M11 gene product (42) have also been shown to inhibit programmed cell death.
Recent studies indicate that HSV-1 also prevents apoptosis of infected cells and that it is able to protect cells against apoptosis by various inducers (3, 4, 13, 14, 22, 54). Several other viral genes have been proposed to play antiapoptotic roles during HSV-1 infection. Studies indicate that apoptosis is inhibited at early times postinfection in Jurkat cells by the action of two immediate-early genes, Us5 and Us3 (20). Furthermore, cultured human epithelial cells infected with an ICP27 deletion virus exhibited the characteristic signs of apoptosis (3). Finally, it has been suggested that the viral glycoproteins gD (Us6) and gJ (Us5) are involved in blocking the apoptotic pathway during productive infections in neuron-like SK-N-SH cells (55). These studies suggest that several HSV-1 factors may have antiapoptotic functions that contribute to maintaining the viability of the virus during its life cycle.
Furthermore, in cells infected with several mutant viruses, HSV-1 can activate apoptosis (4, 14, 21). However, the specific mechanisms by which HSV activates or suppresses apoptotic pathways and the relative importance or contribution of these pathways to the outcome of the infection are still unclear.
In agreement with these recent findings (19), we report that the region of LAT that includes the 2.0-kb LAT intron exhibits an antiapoptotic function following transfection into tissue culture cells. Using an assay in which cells were transfected with a construct expressing the 2.0-kb LAT as well as several LAT deletion constructs, we demonstrate that the 5′ region of the 2.0-kb LAT intron and the exon 1 region of LAT are important in protection from apoptosis in our in vitro transfection system. Furthermore, HeLa cells productively infected with the 17N/H virus (6), containing a LAT deletion spanning all of exon 1 and the 5′ half of the 2.0-kb intron, exhibited a greater degree of DNA fragmentation than the wild-type virus. However, ΔSty, the virus containing a deletion of most of LAT exon 1, was as effective as wild-type HSV-1 at preventing cell death by anti-Fas-induced apoptosis, indicating that in the context of the lytic virus infection, exon 1 does not play an antiapoptotic role. Similar results were observed in mice acutely infected with the ΔSty and N/H viruses, demonstrating that the NotI-HpaI LAT region of HSV-1 contributes to the prevention of apoptosis in neuronal cells in vivo.
MATERIALS AND METHODS
Cells and viruses.
HeLa cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% calf serum. Human neuroblastoma SY5Y cells were grown in RPMI medium with 7% calf serum. HSV-1 strain 17 and the LAT deletion viruses 17N/H and ΔSty (6, 30) were used in the experiments.
Plasmid constructs.
The 2.8-kb PstI-MluI restriction fragment encompassing the LAT gene of HSV-1 F was subcloned into the EcoRI and HindIII sites of pcDNA3 (Invitrogen) as described previously (53). The plasmid resulting from this subcloning, pcDNA3.Pst-Mlu, was used to transfect HeLa cells and generate a transcript from which the stable 2.0-kb LAT intron is processed. This plasmid was subsequently used for production of the LAT mutants used in this study (23, 53). The mutant pΔXcm was generated by removal of the appropriate restriction fragment and self-ligated, while the consensus branch point mutant pCons was produced with PCR primers (23).
For the pΔBstEII plasmid, the 1.0-kb and 0.9-kb BstE fragments were digested with BstEII, and the 1.0-kb fragment was reinserted into the resulting plasmid. The pΔSty plasmid was generated by inserting the ΔSty mutation from pSty (30) into pcDNA3.Pst-Mlu using the restriction enzymes PstI and BsmI. For pΔCMV(Pst-Mlu), the cytomegalovirus (CMV) promoter was deleted from our original construct and the plasmid was religated.
Two different green fluorescent protein (GFP)-expressing plasmids were used. The vector, pEGFP-C1 (Clontech), expresses non-membrane-bound GFP and was used for transfection experiments where the survival of GFP-positive cells was measured by counting adherent GFP-positive cells after induction of apoptosis. pCG239.GFP (45) expresses membrane-bound GFP and was used in transfection experiments where the survival of cells was measured by determining the sub-G1 population of GFP-positive cells by flow cytometry.
The plasmid expressing caspase 8, pC8, and the plasmid expressing the baculovirus antiapoptotic p35 protein, pCIp35, were generously provided by Jeffrey Cohen (National Institutes of Health).
Transfections.
A total of 5 to 6 μg of plasmid DNA (4 μg of LAT-expressing plasmid, 1 μg of GFP construct, and 1 μg of pC8) per 35-mm dish was transfected into subconfluent HeLa or SY5Y cells with 8 μg of Lipofectamine 2000 reagent (Gibco-BRL) according to the manufacturer’s protocol. After 16 h at 37°C, HeLa cells were washed and fed with DMEM (10% calf serum) and incubated for a total of 48 h. SY5Y cells were washed after 6 h and fed with RPMI-7% calf serum, also for a total incubation time of 48 h. At 48 h posttransfection, cells were either harvested for RNA extraction or exposed to apoptotic stimuli.
Induction of apoptosis.
HeLa cells were exposed to 1 μg of anti-Fas monoclonal antibody CH-11 (Tanvera, Madison, Wis.) per ml of medium, or SY5Y cells were incubated with 4 μg of camptothecin (Sigma) per ml of medium for various time points at 37°C and harvested. In experiments where caspase 8 was responsible for inducing apoptosis, cells were transfected with 1 μg of pC8 plasmid expressing the active form of caspase 8.
FACS analysis.
HeLa cells in 60-mm dishes were transfected with 1 μg of pC8 together with 6 μg of pcDNA3, pcDNA3.Pst-Mlu, or pcDNA3 and 2 μg of pCG239.GFP with Lipofectamine 2000 (Gibco-BRL). After transfecting for a total of 24 or 48 h, cells were trypsinized and fixed in 1% paraformaldehyde for 5 min. Cells were then washed with phosphate-buffered saline (PBS) and resuspended in 75% ethanol (in PBS) for 30 min on ice or overnight at 4°C. The ethanol was aspirated and cells were washed in PBS. They were then diluted to 106 cells per 300 μl of PBS and processed for propidium iodide staining in the presence of RNase A. Fluorescence-activated cell-sorting (FACS) analysis of sub-G1 peaks of GFP-positive cells after induction of apoptosis was carried out by the flow cytometry facility at the Wistar Institute on the EPICS XL flow cytometer (Beckman/Coulter Corp.)
RNA extraction and Northern blot analysis.
Total RNA was extracted by Trizol reagent (Gibco) according to the manufacturer’s protocol, and the absorbance at 260 nm was quantitated by spectrophotometry. Northern blot analysis was carried out as previously described (52). Basically, a 5-μg aliquot of each RNA sample was treated with glyoxal and run on a 1.2% agarose gel. RNA was transferred onto GeneScreen Plus (NEN) membranes and cross-linked. The filters were prehybridized for 2 h at 50°C in 50% formamide-10× dextran sulfate-1× Denhardts’ solution-1% sodium dodecyl sulfate (SDS)-5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-1 mM EDTA-0.1% denatured salmon sperm DNA. A 32P-labeled probe specific for the LAT region was synthesized by nick translation, heat denatured, and added to the prehybridization solution. The membranes were incubated with probe overnight at 50°C. The blots were washed twice in decreasing concentrations of SSPE (1×, 0.5×, and 0.1×)-1% SDS at 65°C for 20 min and exposed for autoradiography.
DNA fragmentation assay.
Detection of low-molecular-weight DNA after induction of apoptosis was carried out (55). Briefly, infected or mock-infected cells were washed twice in PBS, lysed in buffer containing 10 mM Tris-HCl (pH 8.0), 10 mM EDTA, and 0.5% Triton X-100, and incubated for 1 h at 37°C with RNase A (0.1 mg/ml). The lysate was centrifuged at 12,000 rpm for 30 min to separate chromosomal DNA from the low-molecular-weight DNA in the supernatant. Proteinase K (1 mg/ml) was added to the supernatant and incubated at 50°C for 1 h in the presence of 1% SDS. DNA was extracted with phenol-chloroform, precipitated in ethanol, and subjected to electrophoresis on a 1.5% agarose gel.
Infection of mice, immunohistochemistry, and apoptosis detection in trigeminal ganglia.
Four- to six-week-old female BALB/c BYJ mice (Jackson Laboratory) were anesthetized with intraperitoneal injection of ketamine (87 mg/kg)-xylazine (13 mg/kg) and inoculated after corneal scarification with 5 × 104 PFU per eye of HSV-1 17, 17N/H, and ΔSty viruses. At 3 and 6 days postinfection, mice were sacrificed by cervical dislocation and trigeminal ganglia were removed. Trigeminal ganglia were incubated for 2 to 4 h at 4°C in 5% sucrose solution (in 1× PBS) and then incubated overnight in 20% sucrose solution (in 1× PBS). Ganglia were washed in OCT mounting medium (O.C.T. 4583 Compound; Tissue Tek) and frozen in OCT on dry ice. Serial sections were cut and processed for immunohistochemistry and apoptotic cell detection.
Apoptotic cells were detected using the DeadEnd colorimetric apoptosis detection system (Promega) with DAB as a chromogen, followed by immunohistochemistry for detection of replicating virus. Immunohistochemistry was carried out using anti-HSV-1 rabbit polyclonal antiserum (American Qualex, San Clemente, Calif.) with Vecta Red as a chromogen, as described elsewhere (38).
RESULTS
Transcription from the Pst-Mlu fragment of LAT protects HeLa cells from anti-Fas-induced apoptosis and SY5Y cells from apoptosis induced by camptothecin.
Published data suggest that LAT is an antiapoptotic factor that may be essential for maintaining healthy neurons during latency to ultimately allow efficient reactivation under conditions of stress (19, 36). These data examined the effect of LAT from the McKrae and KOS viral strains on programmed cell death in tissue culture cells as well as the effect of LAT from the McKrae strain in the rabbit model.
In similarly designed experiments in which we cotransfected tissue culture cells with the plasmid pcDNA3.Pst-Mlu expressing the Pst-Mlu fragment of LAT (see Fig. 1) together with a GFP-expressing plasmid to mark transfected cells, we observed similar results. The stable 2.0-kb LAT intron as well as exon 1 and a 5′ fragment of the exon 2 region of the LAT are expressed from this pcDNA3.Pst-Mlu construct (53). Plasmid DNA (pcDNA3) alone was used as a negative control in these experiments, while the construct expressing the baculovirus antiapoptotic protein, p35, was used as a positive control. At 48 h posttransfection, HeLa cells were treated with the anti-Fas antibody and SY5Y cells were treated with the chemotherapeutic drug camptothecin to induce apoptosis in these cells. At several time points posttreatment, GFP-positive cells that remained adherent on the plates were counted, and the data were expressed as the percentage of GFP-positive cells that survived treatment.
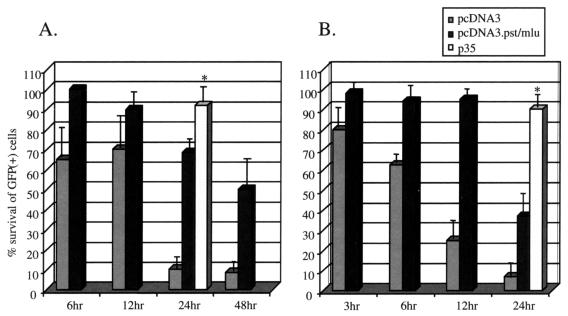
In HeLa cells (Fig. 2A), at 24 h posttreatment with anti-Fas antibody, close to 100% of GFP-positive cells were protected from apoptosis by the p35 protein. Control (anti-Fas antibody treated) cells at this time point are mostly apoptotic, whereas prior to this time, no significant numbers of apoptotic cells are detected using the DeadEnd assay as described in the Materials and Methods (data not shown). Cells transfected with pcDNA3 showed only 10% protection from anti-Fas-induced apoptosis at 24 h, indicating that vector DNA alone is unable to prevent apoptotic cell death. However, close to 70% of cells expressing LAT from the pcDNA3 plasmid were viable. Furthermore, greater than 50% of these cells remained protected up to at least 48 h posttreatment.
FIG. 2.
In vitro inhibition of apoptosis by LAT in HeLa cells (A) and in neuron-like SY5Y cells (B). Cells were transfected with 1 μg of pEGFP-C1 (GFP-expressing construct) and 3 μg of pcDNA3 vector, pcDNA3.Pst-Mlu, or pCIp35 expressing the baculovirus antiapoptotic protein. At 48 h posttransfection, anti-Fas antibody was added to HeLa cells, while camptothecin was added to SY5Y cultures. At various times after treatment, GFP-positive cells were identified under a fluorescent microscope. ✻, positive control. The number of GFP-positive cells in control untreated cells represents 100% survival. Data are averages from five separate experiments.
The human neuroblastoma cell line SY5Y was insensitive to apoptosis induced by anti-Fas antibody but sensitive to camptothecin. By 12 h posttreatment, control SY5Y cells treated with camptothecin showed significant apoptosis (data not shown). At this time, close to 100% of cells were protected from apoptosis in the presence of the LAT-expressing plasmid, whereas cells treated with the vector alone showed approximately 20% survival of GFP-expressing cells (Fig. 2B). By 24 h after treatment, the protection elicited by LAT decreased to approximately 30% but was still greater than the negative control (5%). At this time point, in both cell lines, about 90% of p35-transfected cells survived camptothecin-induced apoptosis.
These results indicate that the LAT-expressing plasmid protects cells against apoptosis induced by exogenous stimuli in both HeLa and SY5Y cells. However, the effect is not as protective as the p35 positive control.
Pst-Mlu LAT fragment blocks caspase 8-induced apoptosis in tissue culture.
To quantitatively determine whether LAT directly protects cells from apoptosis induced by caspase 8 in vitro, we cotransfected HeLa cells with a plasmid expressing an active form of caspase 8 together with the protecting plasmid and the GFP-expressing vector. Caspase 8 is a protease which is normally activated in response to extracellular stimuli by tumor necrosis factor (TNF)-like ligands to cause the formation of apoptotic cell bodies. Therefore, in these experiments, the cells expressing GFP will also be undergoing apoptosis induced by caspase 8. Thus, the protective effect of the cotransfected plasmid of interest on cells receiving apoptotic stimuli can be monitored by scoring survival of GFP-positive cells.
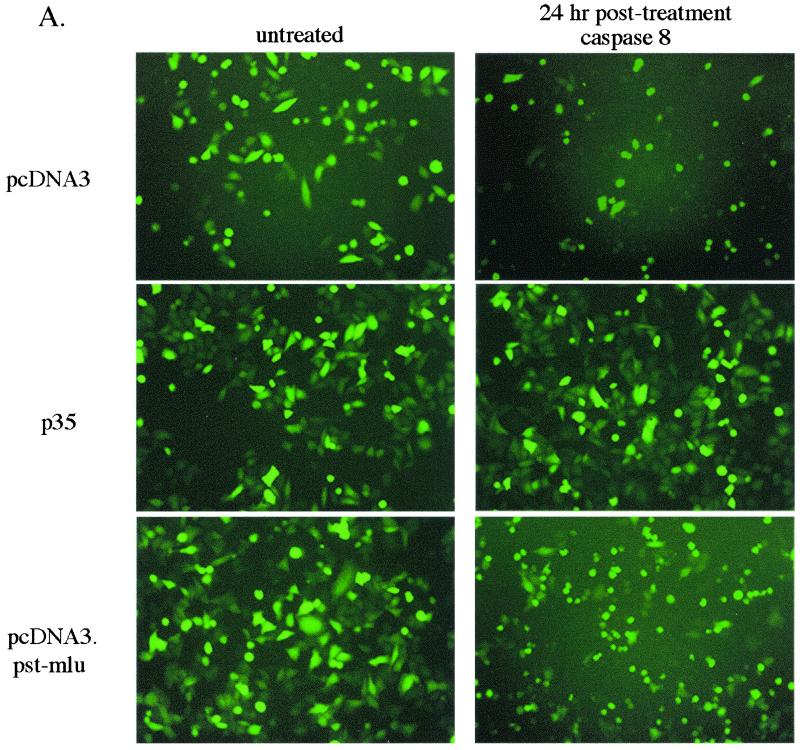
Figure 3A shows pictures of GFP-expressing cells at 24 h posttransfection with pcDNA3, p35, or pcDNA3.Pst-Mlu, with or without the caspase 8-expressing construct. With vector alone (without LAT insert), in the presence of caspase 8, most of the GFP-positive cells were rounded and showed signs characteristic of apoptosis. In fact, by this time, several GFP-positive cells had rounded and lifted from the dishes. However, as expected when p35 was expressed from a cotransfected plasmid, almost all of the cells remained healthy and protected from apoptosis. By 24 h posttreatment, cells expressing LAT were also undergoing apoptosis, but not to the same extent as the negative control (as indicated in Fig. 3A).
FIG. 3.
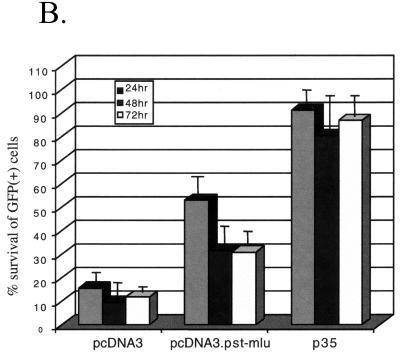
Inhibition of caspase 8-induced apoptosis by LAT in vitro. HeLa cells were transfected with 3 μg of pcDNA3, pcDNA3.Pst-Mlu, or pICp35 together with 1 μg of pEGFP-C1 and 1 μg of the plasmid expressing caspase 8 (pC8). The pC8 plasmid was not transfected into control cells. (A) At 24 h after transfection, GFP-positive cells were visualized under a fluorescent scope and photographed. (B) GFP-positive cells were counted at 24, 48, and 72 h posttransfection, and data are expressed as the percentage of surviving GFP-positive cells over the control cells.
When these results were quantitated by counting GFP-positive cells on transfected tissue culture plates at different times posttreatment (see Materials and Methods), the results indicated that LAT expression allows a level of protection that is intermediate between the negative (vector plasmid only) control and the positive p35 control at all time points (Fig. 3B). Using this assay, LAT was found to be a less effective inhibitor of apoptosis than indicated in the previous experiment using anti-Fas antibody or camptothecin as the apoptotic stimulus (Fig. 2A and B) and that observed previously using other systems (36). Therefore, it seems apparent that this decreased level of protection is the result of the different methods used to induce apoptosis.
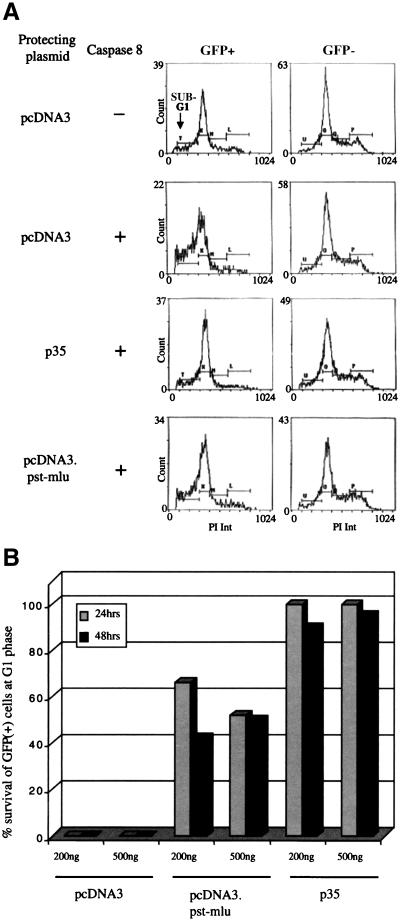
To further verify our results, FACS analysis of GFP-positive cells was carried out in our cotransfection experiments in the presence of propidium iodide (Fig. 4A). To determine the percentage of cells undergoing apoptosis by caspase 8 cotransfection, the sub-G1 peak was analyzed. The data clearly indicate that cells transfected with pcDNA3.Pst-Mlu show a level of apoptosis that is between that of the negative and positive controls. The results are quantitated in Fig. 4B. These data show that expression from the vector alone did not protect cells from apoptosis, whereas approximately 40% of cells expressing LAT were protected from apoptosis induced by caspase 8 at either 24 or 48 h. Again, as demonstrated earlier, the antiapoptotic protein p35 prevented the induction of apoptosis in these cells.
FIG. 4.
FACS analysis to confirm the ability of LAT to protect cells from caspase 8-induced apoptosis. HeLa cells were transfected with 6 μg of pcDNA3, pcDNA3.Pst-Mlu, or pICp35 together with 2 μg of pCG239.GFP (membrane-bound GFP) and 1 μg of pC8. Control cells were transfected without pC8. At 24 and 48 h posttransfection, cells were harvested and processed for propidium iodide (PI) staining. Analysis of sub-G1 peaks of GFP-positive cells after induction of apoptosis was carried out by the flow cytometry facility at the Wistar Institute. (A) Representative FACS data at 24 h after transfection. (B) Percent protection of GFP-positive cells at 24 and 48 h posttransfection.
Mapping of LAT elements required for protection from apoptosis.
As mentioned previously, pcDNA3.Pst-Mlu expresses the 2.0-kb LAT intron in addition to exon 1 and a small portion of exon 2 of the LAT. Therefore, it is possible that either the exon 1 region, the 2.0-kb intron, or the small part of exon 2 plays a role in protection from apoptosis. To determine which region of LAT contains the antiapoptotic function, mutants in the pcDNA3.Pst-Mlu background (Fig. 1C) were used in an experiment similar to that in Fig. 3. These deletion constructs have been described previously (23, 53). Table 1 summarizes the features of the exon 1, 2.0-kb LAT intron, and exon 2 regions expressed by each of the constructs (as indicated by Northern blot analysis in Fig. 5A).
TABLE 1.
Plasmids and viruses used
| Plasmid or virus | Exon 1 | 2-kb LAT
|
Exon 2 (kb) | Apoptosis protectiona | |
|---|---|---|---|---|---|
| Size (kb) | Stability | ||||
| Plasmids | |||||
| pcDNA3 | None | No | |||
| ΔCMV(Pst-Mlu) (promoterless) | Complete | 2.0 | Stable | 0.23 | No |
| pcDNA3.Pst-Mlu | Complete | 2.0 | Stable | 0.23 | +++ |
| pΔXcm | Complete | 1.2 (3 deletion) | Stable | 0.23 | ++ |
| pCons | Complete | 2.0 | Unstable | 0.23 | ++ |
| pΔBstE | 3′ deletion | None (5′ deletion) | 0.23 | No | |
| pΔSty | Middle deletion | 2.0 | Stable | 0.23 | No |
| pΔSty + pΔXcm | Complete | 2.0 | Stable | 0.23 | +++ |
| Viruses | |||||
| 17 | Complete | 2.0 | Stable | ∼7 | +++ |
| 17N/H | None | None (5′ deletion) | ∼7 | No | |
| ΔSty | Middle deletion | 2.0 | Stable | ∼7 | +++ |
++, 20 to 30% protection; +++, 30 to 50% protection.
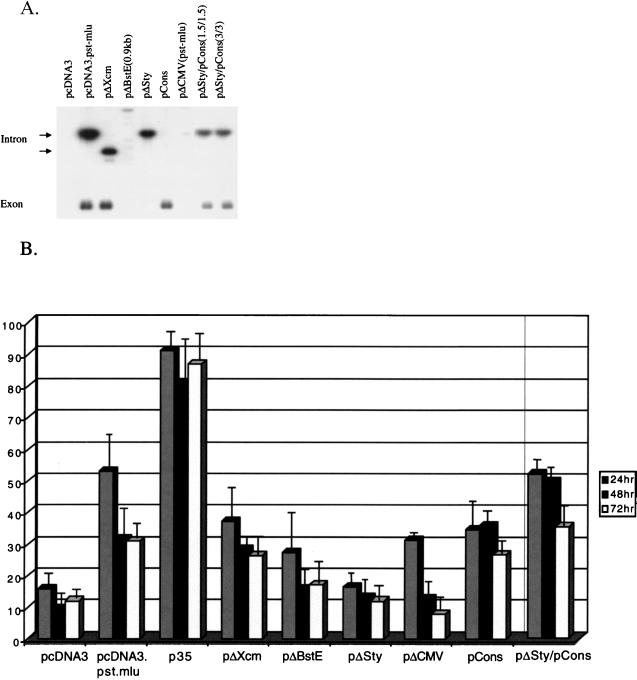
FIG. 5.
Inhibition of apoptosis by LAT mutants expressed from the pcDNA3 vector. (A) Northern blot analysis measuring the expression of LAT mutants. HeLa cells were transfected with 3 μg of pcDNA3, pcDNA3.Pst-Mlu, or the LAT mutant plasmids pΔXcm, pΔBstE, pΔSty, pΔCMV(Pst-Mlu), and pCons (described in Table 1). In cells cotransfected with both pCons and pΔSty, 3 mg of each DNA was used. Total RNA was extracted at 48 h posttransfection, and Northern blot analysis was carried out as previously described (Materials and Methods). The filters were hybridized with 32P-labeled probes specific for the 2-kb LAT and exposed for autoradiography for visualization. (B) Inhibition of apoptosis by LAT mutants. HeLa cells were transfected with 3 μg of each of the indicated plasmids together with 1 μg of pEGFP-C1 and 1 μg of the plasmid expressing caspase 8 (pC8). In the experiment where pΔSty and pCons were cotransfected into cells, 3 μg of each plasmid was added. Control cells were transfected without pC8. GFP-positive cells were counted at 24, 48, and 72 h posttransfection. Data are expressed as a percentage of GFP-positive cells in control dishes. Results represent an average of four independent experiments.
The pΔXcm1 mutant contains a deletion of the 3′ half of the 2.0-kb LAT. When transfected into cells, this mutant expresses a truncated 2.0-kb LAT intron and a wild-type exon. The pΔBstE (0.9 kb) mutant contains a deletion of a portion of exon 1 as well as part of the 5′ end of the 2.0-kb LAT intron and is unable to express either the 2.0-kb LAT intron or the exon. The pΔSty vector contains a deletion of 371 of the 660 nucleotides of the LAT exon 1 region. This mutant expresses a complete 2.0-kb LAT intron but only a truncated exon. Additional mutants used in this study were the pCons and pΔCMV(Pst-Mlu) mutants. The pCons mutant has a consensus splice branch point sequence inserted and thus produces an unstable 2.0-kb LAT intron, while the pΔCMV(Pst-Mlu) mutant contains a deletion of the CMV promoter, thus producing no transcript (unless transcription from the LAP2 promoter is seen) (16) and will allow determination of whether the DNA sequence itself has antiapoptotic activity.
The ability of the mutant LAT plasmids to protect cells from apoptosis induced by caspase 8 was tested and is quantitated in Fig. 5. The pΔCMV(Pst-Mlu) plasmid elicited some protection at 24 h posttreatment, perhaps due to some activity in the LAP2 promoter (Fig. 1) (16). However, by 48 h, the percentage of cells that survived was close to background levels, indicating that expression of the LAT region is necessary for protection against apoptosis. The pΔXcm mutant was less effective than the wild-type 2.0-kb LAT at preventing cells from undergoing apoptosis at 24 h posttransfection. However, at later times posttreatment, this plasmid was as effective as the wild-type sequences at blocking cell death by apoptosis, indicating that the 3′ end of the 2.0-kb intron is not necessary for a protective phenotype. Nevertheless, this truncation was able to slightly modify the kinetics of the effect.
On the other hand, the mutant which did not express a complete exon 1 (pΔSty) was less protective than the wild-type LAT construct at all time points. Similarly, pΔBstE, which contains a deletion covering the 3′-terminal sequence of exon 1 and the 5′-proximal sequence of the 2.0-kb LAT intron, also shows reduced protection against the effects of caspase 8 expression. These results suggest that the exon 1 region and 5′ sequences of the 2.0-kb LAT intron are important in defending the virus against cellular apoptosis. The deletion in pΔBstE also removes the 2.0-kb LAT splice donor site and results in lack of expression of the 2.0-kb LAT intron. Therefore, it is possible that the 2.0-kb LAT intron also contributes to the antiapoptotic effect mediated by the LAT region.
In this regard it is interesting that, at the earlier time point (24 h), the plasmid expressing an unstable LAT but retaining wild-type exon and 5′ 2.0-kb LAT sequences (pCons) was less potent at preventing apoptosis than the pcDNA3.Pst-Mlu plasmid. However, at the later time points (48 and 72 h), this effect was minimal. Interestingly, in cells cotransfected with both the pΔSty mutant and the pCons mutant, the ability to protect cells from caspase 8-induced apoptosis was additive. In fact, protection was close to wild-type levels. Taken together, these results show that expression from the exon 1 region of LAT is important in maintaining the viability of cells under conditions of apoptosis. In addition, the 2.0-kb LAT intron, in particular the 5′-terminal sequences, also appears to mediate an antiapoptotic effect, although the effect is less than that mediated by exon 1.
These results (summarized in Table 1), using plasmids with deletions or insertions in the LAT exon 1 or intron region, support the hypothesis that transcription of the LAT exon and the LAT 2.0-kb intron facilitate protection from apoptosis in transfected tissue culture cells. Furthermore, they show that the effects of the exon and the intron are additive and function in trans. To determine whether this effect is seen in the presence of other viral genes during the lytic cycle of virus infection, experiments were performed using virus-infected tissue culture cells.
17N/H deletion virus is less effective at preventing apoptosis induced by anti-Fas than either the wild-type or ΔSty deletion viruses in productively infected HeLa cells.
HSV-1 contains several proteins that have been shown to inhibit apoptosis during the lytic cycle of infection (20, 55). Therefore, to further determine the contribution of LAT as an antiapoptotic factor during the various stages of virus infection, two LAT deletion viruses, 17N/H and ΔSty, were tested for their effectiveness at preventing programmed cell death during infection of tissue culture cells and primary infection of the mouse peripheral nervous system. The ability of these mutant viruses to express exon 1, 2.0-kb LAT, and exon 2 is described in Table 1. The 17N/H virus (see Fig. 1) does not express exon 1 or the 2.0-kb LAT intron due to an extensive deletion of the LAT promoter exon 1 and the 5′ half of the 2.0-kb LAT, but retains the LAT exon 2, whereas the ΔSty virus expresses the stable LAT intron but is deleted of most of exon 1.
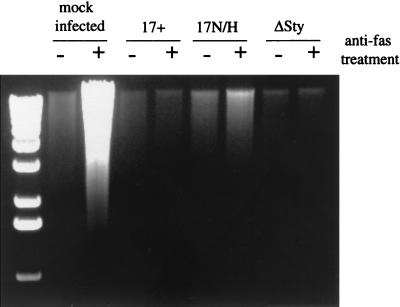
In Figure 6, HeLa cells were infected with wild-type HSV 17, 17N/H virus, or ΔSty virus or mock infected. At 16 h postinfection, cells were either mock treated or treated with anti-Fas antibody for 6 h to induce apoptosis. After cell lysis, DNA was isolated and run on agarose gels. In mock-infected cells, in the presence of anti-Fas, there was a large amount of fragmented DNA, and in HSV 17-infected cells virus was clearly protected against apoptosis induced by anti-Fas, as expected. Furthermore, HSV 17 did not induce apoptosis in untreated cells (Fig. 6).
FIG. 6.
DNA fragmentation in HeLa cells productively infected with LAT deletion viruses. Mock-infected cells or cells infected with strain 17, 17N/H, or ΔSty virus (10 PFU/cell) were lysed in buffer containing 0.5% Triton X-100 and incubated for 1 h at 37°C with RNase A (0.1 mg/ml). The lysates were centrifuged to separate chromosomal DNA from the low-molecular-weight DNA in the supernatant. Proteinase K (1 mg/ml) was added to the supernatant and incubated at 50°C for 1 h in the presence of 1% SDS. DNA was extracted with phenol-chloroform, precipitated in ethanol, and subjected to electrophoresis on a 1.5% agarose gel. The presence of low-molecular-weight DNA in the gel represents DNA fragmentation by apoptosis.
The 17N/H virus was less effective at protecting cells from anti-Fas-mediated apoptosis than HSV 17, its parental virus, indicating that the LAT region affected by the deletion in this virus elicits some protection against apoptosis during acute infection in tissue culture. However, in contrast to the transfection data, the ΔSty virus was as effective as strain 17 virus at preventing the onset of apoptosis. These results indicate that in the context of the lytic virus infection in tissue culture cells, the exon of the LAT is not necessary for protection against apoptosis. However, the 17N/H mutant phenotype indicates that during tissue culture infections, some region of the LAT locus, perhaps the 2.0-kb LAT or the LAT promoter region, does contribute to the inhibition of cellular apoptosis. Interestingly other transcripts have been mapped in the N/H deletion region, 1.8 kb and 1.1 kb (56) (see Fig. 1) that may play a role in the antiapoptotic effect.
LAT region of HSV-1 contributes to the viral antiapoptotic function in the acutely infected mouse peripheral nervous system.
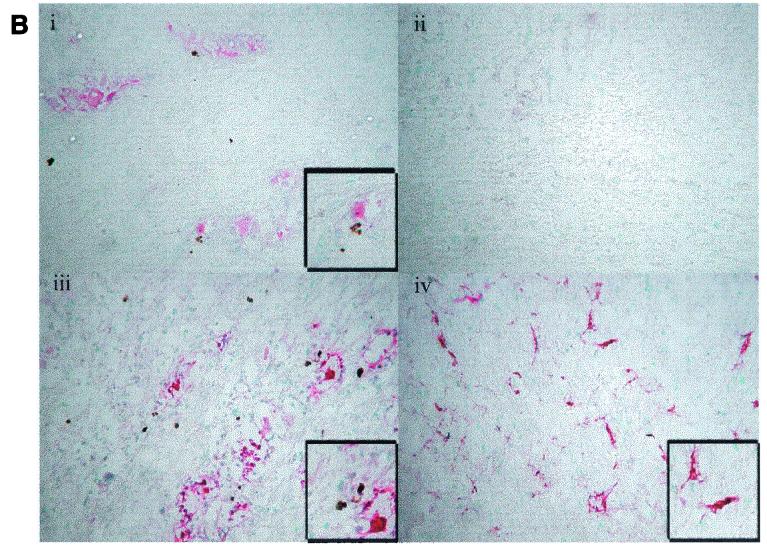
To determine the effects of the LAT deletion viruses on apoptosis in vivo, female BALB/c mice were infected with the strain 17, 17N/H, and ΔSty viruses by corneal scarification. At 3 and 6 days postinfection, mice were sacrificed, and the trigeminal ganglia of each mouse were sectioned and processed for the presence of virus by immunohistochemistry and for apoptotic cells by the DeadEnd colorimetric assay (Promega). Although the amount of viral staining in 17N/H was low compared to that of 17 and 17ΔSty (Fig. 7A and B), the results indicate that each of the viruses replicate in the trigeminal ganglia of acutely infected mice.
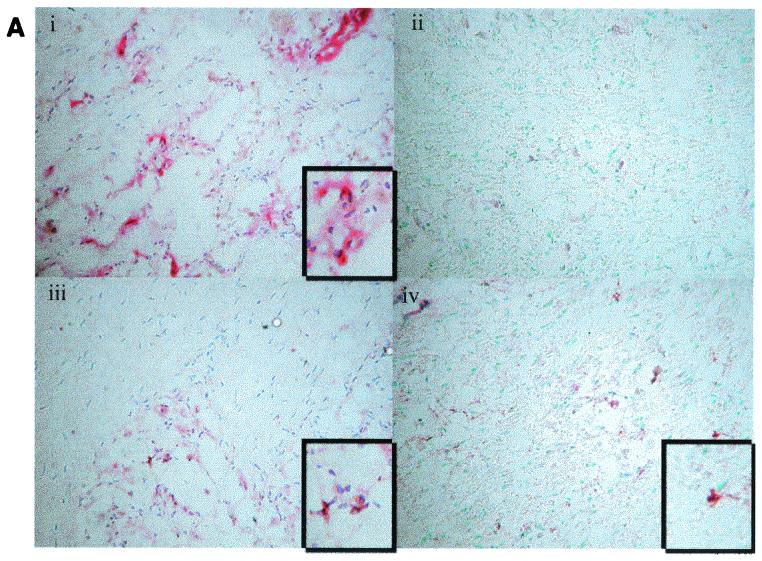
FIG. 7.
Detection of apoptosis in mice trigeminal ganglia. BALB/c mice were ocularly infected in both eyes with HSV 17, 17N/H, or ΔSty viruses at 5 × 104 PFU/eye. At days 3 (A) and 6 (B) postinfection, five mice were sacrificed per group, and trigeminal ganglia were obtained. Trigeminal ganglia were fixed and serial sections were cut and processed for immunohistochemistry to detect replicating virus (pink; described in Materials and Methods). Apoptotic cells were distinguished by the DeadEnd colorimetric apoptosis detection system (brown; Promega). Sections were visualized by light microscopy and photographed. Virus strain: i, 17; ii, none (mock infected); iii, ΔSty; iv, 17N/H.
Interestingly, in 17- and 17ΔSty-infected sections, we saw significant staining for HSV antigens with few apoptosis-positive staining cells associated with these regions (Fig. 7Ai, Aiii, Bi, and Biii). However, in 17N/H sections, we observed cells showing dual staining for HSV antigens and apoptosis (Fig. 7Aiv and Biv, and shown more clearly in the inset of panel Biv). Therefore, consistent with our tissue culture infection data (Fig. 6), these results indicate that during the acute infection in vivo, the exon region of LAT does not exert an antiapoptotic effect. On the other hand, the N/H region of LAT appears to play a role in protecting cells from apoptosis, both in productively infected tissue culture cells and in the trigeminal ganglia of acutely infected mice.
DISCUSSION
Regions of LAT involved in protecting cells from apoptosis in vitro.
The first 1.5 kb of the 8.3-kb primary LAT has been shown to have an antiapoptotic function when transfected into CV-1 cells and neuro-2A cells treated with the apoptosis inducer etoposide or sodium butyrate (19). In this study, we report that transcription from the 2.9-kb Pst-Mlu region of the LAT prolonged cell survival in human HeLa cells after anti-Fas- and caspase 8-induced apoptosis and in neuron-like SY5Y cells after camptothecin-induced apoptosis (Fig. 2 and 3). These results are similar to the previous findings of Perng (36) and Inman et al. (19). Using a FACS analysis method (Fig. 4) to determine the protection from apoptosis, the data showed that transcripts from our truncated LAT gene had a protective effect against apoptosis, although this was not as strong as seen following transfection with a positive control plasmid expressing p35.
To determine which parts of the truncated LAT (Pst-Mlu fragment) transcript are necessary for inhibition of apoptosis, we examined the antiapoptotic effect exerted by several mutants with deletions in this region of the genome. We found that a deletion in the exon 1 region of LAT (pΔSty plasmid) produced a transcript that was less protective than Pst-Mlu (Fig. 5B) from caspase 8-induced apoptosis. In fact, transfection results indicated that the LAT expressed from the pΔSty plasmid was the least effective of all the mutant plasmids tested in our transfection assay at preventing cell death. Thus, this region must play an important role in protection from apoptosis.
Transfection experiments with the pCons plasmid, expressing an unstable 2.0-kb LAT intron and a wild-type exon, showed that it was almost as effective as the pcDNA3.Pst-Mlu construct at blocking apoptosis (Fig. 5B). When the plasmid pΔXcm, which has a deletion in the 3′ end of the 2.0-kb LAT and yet still produces a stable intron, (Fig. 5A) was used, the protection seen was less than with pcDNA3.Pst-Mlu but was similar to that seen with pCons. These results indicate a major role of exon 1 sequences in blocking apoptosis in vitro and, in addition, a more minor role of the 2.0-kb LAT intron, in particular the 5′-terminal sequences. In agreement with this conclusion, we found that when the pCons and pΔSty plasmids were cotransfected into cells, they were complementary in their ability to overcome apoptosis induced by caspase 8 (Fig. 5B), suggesting that although the exon is sufficient to inhibit apoptosis, the 2-kb LAT region is able to contribute to this protective phenotype in trans.
In what context does the LAT region of the genome exert an antiapoptotic effect?
Initially, we hypothesized that the intact stable 2.0-kb LAT intron that was expressed by the pcDNA3.Pst-Mlu plasmid was a likely candidate for eliciting protection against apoptosis. However, our finding that the 5′ end of the LAT intron, expressed by the pΔXcm plasmid, plays a role in inhibiting apoptosis in vitro (Fig. 5B) suggests that much of the intron is dispensable. Inman et al. (20) determined that the 5′-terminal half of the 2.0-kb LAT region was required to protect against apoptosis in vitro and that these results correlated directly with spontaneous reactivation phenotypes in vivo. Northern blot analysis (Fig. 5A) shows that our pΔXcm plasmid expresses a truncated stable intron as well as an exon 1-exon 2 mRNA. This plasmid is somewhat similar to the pLAT3.3 plasmid described previously (19), except that pLAT3.3 does not have the ability to produce a stable intron (lacks splice acceptor site or branch point region). Together these data indicate that the 5′ sequences of the 2.0-kb LAT, either within or outside the context of the excised stable intron, would appear to be important for the antiapoptotic effect in vitro.
Several open reading frames are found in the region of the LAT that shows protection from apoptosis. However, although several translation products have been described in tissue culture experiments (10, 24, 47), as yet no protein products have been detected from any of the LAT transcripts in vivo during latency. Formally, it is possible that the protective effect from this region is due to LAT RNA sequences and not a result of protein products. It is interesting that recently Lock et al. (28) showed that expression of a reading frame in the exon 1 region of the LAT conferred nuclear localization on chimeric GFP sequences. Exon 1 may function to localize a protein involved in protection from apoptosis to the nucleus. The role of the intron is not clear, though it was recently shown that it is associated with ribosomal proteins during lytic infection (1).
Our pΔBstE plasmid, which does not have the splice donor junction sequence that is required to produce a 2.0-kb LAT stable intron, was unable to protect cells from apoptosis. Therefore, it is possible that the ability to splice out the intron is important for maximum antiapoptotic effect. Inman et al. (19) used truncations of their Apa-LAT construct (pLAT3.3) that removed the splice acceptor and branch point sequences. Nevertheless, their pLAT3.3 plasmid was as effective as their full-length Apa-LAT plasmid at preventing apoptosis. It is possible that pLAT3.3 is able to undergo splicing from downstream splice acceptor and branch point elements. In addition, their pLAT2.6 construct containing LAT nucleotides 1 to 811 shows intermediate levels of protection in their system. However, when the 2.0-kb intron region was deleted from their constructs, the protective activity was eliminated. Therefore, splicing of the intron region may be necessary for the prevention of apoptosis in vitro. Our pΔSty/pCons cotransfection results indicate that although the exon 1 region of the LAT is essential, the spliced 2.0-kb intron contributes to the effect, thus supporting the hypothesis that splicing may be an important factor in inhibiting apoptosis.
The N/H region of LAT inhibits apoptosis in productively infected tissue culture cells and in trigeminal ganglia of acutely infected mice in vivo.
HSV-1 contains factors that induce as well as inhibit apoptosis in a cell type-dependent manner (4, 5, 13, 14, 54). Viral gene products that have been implicated in protecting cells from apoptosis include the US3, US5, ICP4, and ICP27 proteins and others (3, 20, 26, 27, 55). In the context of virus infection in HeLa cells, the ΔSty virus, containing the same deletion as the pΔSty plasmid, was as protective as the wild-type virus against apoptosis induced by anti-Fas (Fig. 6). However, the LAT deletion virus 17N/H was slightly more apoptotic than the wild-type and ΔSty viruses. These data indicate that in productively infected tissue culture cells, the region of LAT deleted in the N/H virus exerts an antiapoptotic effect. However, since the cell death exhibited by this LAT deletion virus was not as great as that seen with anti-Fas alone, it is possible that it is the US3, US5, ICP4, and ICP27 proteins that afford protection from apoptosis during tissue culture infection and that LAT contributes to this effect. Furthermore, consistent with our results in tissue culture cells, we showed that the 17ΔSty virus was as effective as strain 17 at protecting the trigeminal ganglia from virus-induced apoptosis (Fig. 7Aiii and Biii). In contrast, trigeminal ganglia from 17N/H-infected mice showed dual staining for apoptosis and viral antigens, but the amount of staining was decreased. Therefore, these data demonstrate that although the exon region of LAT does not protect cells from apoptosis in the context of the virus infection, both in vitro and in vivo, the region of LAT spanning the 2.0-kb intron appears to have an antiapoptotic function during the acute infection.
Previous studies by Perng et al. measured apoptosis in the trigeminal ganglia of rabbits during acute infection with the LAT deletion virus in HSV-1 strain McKrae. They found that the LAT deletion virus dLAT2903, a McKrae virus deleted in the LAT promoter, exon 1, and the 5′ half of the LAT intron, showed a greater percentage of apoptotic cells than its wild-type counterpart (36). Our data from mice lytically infected with HSV-1 strain 17 and mutant viruses show similar results. Unfortunately, due to the low percentage of positively stained cells in 17N/H sections, quantitation of our results is somewhat difficult. However, dual staining in the sections (Fig. 7) clearly shows that the cells infected with 17N/H are undergoing apoptosis, while tissue sections from animals infected with the 17 and ΔSty viruses do not exhibit this dual pattern of staining.
Function of LAT and reactivation from latency.
Although several independent groups (19, 36), including ours, have demonstrated that LAT functions as an antiapoptotic factor, this work has raised some speculation. A recent study suggested that although LAT is able to protect neurons from death following virus infection, this death is due to mechanisms other than apoptosis (44). Nevertheless, it is evident from these studies that LAT plays a role in preventing the host immune response from responding to the virus infection.
It has been suggested that LAT promotes survival of neurons to maintain the host for latent virus and facilitate efficient reactivation (19, 36). Because stress, the trigger for HSV-1 reactivation, also triggers increased levels of corticosteroids, which promote apoptosis, it is thought that LAT functions to reduce the programmed cell death triggered during reactivation. The results in Fig. 7 indicate that LAT contributes to the protection of cells against apoptosis during acute infection of the peripheral nervous system of mice. However, it is possible that during reactivation, LAT may play a more significant role.
During acute infection the virus enters the cell with gD and gJ and produces ICP27 at the immediate-early and US3 at the early stage of the lytic cycle gene expression cascade, However, during latency and reactivation, none of these gene products are present. Thus, there may be a greater need for a LAT antiapoptotic gene product at this time. While we have made several attempts to look at the apoptotic phenotype of the mutant viruses ΔSty and 17N/H in latent and reactivating trigeminal ganglia from mice, technical difficulties with regard to colocalization of latent infectivity and apoptosis assays have so far precluded our efforts. Still, our data are consistent with the ability of these viruses to reactivate from latency, as determined previously (30). The wild-type and ΔSty viruses protect cells from apoptosis in the trigeminal ganglia of mice, and both reactivate efficiently from latency. On the other hand, the N/H virus, which is deficient at protecting cells from apoptosis, reactivates poorly.
Since the LATs are abundantly transcribed during latent infections, their role in the establishment and maintenance of latency and reactivation from latency has been examined in detail. Early studies proposed that the 2.0-kb LAT is involved in an antisense suppression mechanism because it overlaps the 3′ end of ICP0 mRNA (50). Other studies with several LAT deletion viruses suggest that LATs play a role in efficient reactivation from latency (6, 18, 25, 48, 52). Further work suggested that LATs play a role in promoting efficient establishment of latency in trigeminal ganglia (15, 29, 37, 43). However, for most of these roles, the mechanisms for the potential functions for LATs remain elusive.
In terms of its antiapoptotic roles, it is possible that the LATs protect cells from apoptosis by interacting directly with members of the apoptotic pathway. Previous results and data in this paper show that certain LAT sequences can protect cells from a host of apoptotic inducers. Therefore, it is thought that LAT may affect a downstream regulator of apoptosis (36). Alternatively, perhaps LAT affects apoptosis indirectly by interacting with certain ribosomal proteins or members of the translational machinery to inhibit apoptosis. We have recently found that the association of the 2-kb LAT intron is similar to that of rRNAs for the translation apparatus and not like that of cellular or viral mRNAs (1). Therefore, it is possible that the function of LAT is similar to that of the rRNAs, which play both structural and functional roles in the translation complex (35).
The association of LAT with ribosomal proteins may serve to stabilize the translational machinery and aid in the production of specific antiapoptotic factors, or it may inhibit the production of apoptotic proteins. Reports have suggested a correlation between levels of ribosomal proteins and activation of apoptosis. For example, inhibiting the expression of enhanced levels of certain ribosomal proteins has been correlated to apoptotic induction in certain cell lines (32–34). In these cases, it is possible that disrupting the balance of factors involved in the translation complex leads to a reduction in the expression of certain cellular antiapoptotic factors. Therefore, LAT may serve to stabilize the translational complex by interacting with certain ribosomal proteins, thereby leading to preferential expression of specific cellular proteins to aid in cell survival. However, further work must be done to determine at what step during the viral life cycle LAT inhibits apoptosis and then to dissect the exact mechanism by which LAT promotes cell survival.
Acknowledgments
We acknowledge the help of Wen Kang with the figures and critical discussions on the manuscript and the excellent technical help of Vikram Suri. We thank Jeffrey Cohen for his generous gift of the pC8 and pCIp35 plasmids.
This work was supported by Public Health Service Program Project grant NS33768 from the National Institutes of Health.
REFERENCES
- 1.Ahmed, M., and N. W. Fraser. 2001. The herpes simplex virus type 1 2-kilobase latency-associated transcript associates with ribosomal proteins and splicing factors. J. Virol. 75:12070–12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfonso, C. L., J. G. Neilan, G. F. Kutish, and D. L. Rock. 1996. An African swine fever virus Bcl-2 homolog, 5-HL, suppresses apoptotic cell death. J. Virol. 70:4858–4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubert, M., and J. Blaho. 1999. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J. Virol. 73:2803–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubert, M., J. O’Toole, and J. A. Blaho. 1999. Induction and prevention of apoptosis in human HEp-2 cells by herpes simplex virus type 1. J. Virol. 73:10359–10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubert, M., S. A. Rice, and J. A. Blaho. 2001. Accumulation of herpes simplex virus type 1 early and leaky-late proteins correlates with apoptosis prevention in infected human HEp-2 cells. J. Virol. 75:1013–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Block, T. M., S. L. Deshmane, J. Masonis, J. Maggioncalda, T. Valyi-Nagy, and N. W. Fraser. 1992. An HSV LAT null mutant reactivates slowly from latent infection and makes small plaques on CV-1 monolayers. Virology 192:618–630. [DOI] [PubMed] [Google Scholar]
- 7.Crook, N. E., R. J. Clem, and R. A. Miller. 1993. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J. Virol. 67:2168–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derfuss, T., H. Fickenscher, M. S. Kraft, G. Henning, D. Lengenfelder, B. Fleckenstein, and E. Meinl. 1998. Antiapoptotic activity of the herpesvirus saimiri-encoded Bcl-2 homolog: stabilization of mitochondria and inhibition of caspase 3-like activity. J. Virol. 72:5897–5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobson, A. T., F. Sederati, G. Devi-Rao, J. Flanagan, M. J. Farrell, J. G. Stevens, E. K. Wagner, and L. T. Feldman. 1989. Identification of the latency-associated transcript promoter by expression of rabbit beta-globin mRNA in mouse sensory nerve ganglia latently infected with a recombinant herpes simplex virus. J. Virol. 63:3844–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doerig, C., L. I. Pizer, and C. L. Wilcox. 1991. An antigen encoded by the latency-associated transcript in neuronal cell cultures latently infected with herpes simplex virus type 1. J. Virol. 65:2724–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrell, M. J., A. T. Dobson, and L. T. Feldman. 1991. Herpes simplex virus latency-associated transcript is a stable intron. Proc. Natl. Acad. Sci. USA 88:790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser, N. W., T. M. Block, and J. G. Spivack. 1992. The latency-associated transcripts of herpes simplex virus: RNA in search of function. Virology 191:1–8. [DOI] [PubMed] [Google Scholar]
- 13.Galvan, V., R. Brandimarti, and B. Roizman. 1999. Herpes simplex virus type 1 blocks caspase-3-independent and caspase-dependent pathways to cell death. J. Virol. 73:3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galvan, V., and B. Roizman. 1998. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type-dependent manner. Proc. Natl. Acad. Sci. USA 95:3931–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garber, D., P. Schaffer, and D. Knipe. 1997. A LAT-associated function reduces productive-cycle gene expression during acute infection of murine sensory neurons with herpes simplex virus type 1. J. Virol. 71:5885–5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goins, W. F., L. R. Sternberg, K. D. Croen, P. R. Krause, R. L. Hendricks, D. J. Fink, S. E. Straus, M. Levine, and J. C. Glorioso. 1994. A novel latency-active promoter is contained within the herpes simplex virus type 1 UL flanking repeats. J. Virol. 68:2239–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson, S., D. Huen, M. Rowe, C. Dawson, G. Johnson, and A. Rickinson. 1993. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B-cells from programmed cell death. Proc. Natl. Acad. Sci. USA 90:8479–8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill, J. M., F. Sederati, R. T. Javier, E. K. Wagner, and J. G. Stevens. 1990. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology 174:117–125. [DOI] [PubMed] [Google Scholar]
- 19.Inman, M., G.-C. Perng, G. Herderson, H. Ghiasi, A. B. Nesburn, S. L. Wechsler, and C. Jones. 2001. Region of herpes simplex virus type 1 latency-associated transcript sufficient for wild-type spontaneous reactivation promotes cell survival in tissue culture. J. Virol. 75:3636–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jerome, K. R., R. Fox, Z. Chen, A. E. Sears, H.-Y. Lee, and L. Corey. 1999. Herpes simplex virus inhibits apoptosis through the action of two genes, Us5 and Us5. J. Virol. 73:8950–8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koyama, A. H., and A. Adachi. 1997. Induction of apoptosis by herpes simplex virus type 1. J. Gen. Virol. 78:2909–2912. [DOI] [PubMed] [Google Scholar]
- 22.Koyama, A. H., and Y. Miwa. 1997. Suppression of apoptotic DNA fragmentation in herpes simplex virus type 1-infected cells. J. Virol. 71:2567–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krummenacher, C., J. M. Zabolotny, and N. W. Fraser. 1997. Selection of a nonconsensus branch point is influenced by an RNA stem-loop structure and is important to confer stability to the herpes simplex virus 2-kilobase latency-associated transcript. J. Virol. 71:5849–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagunoff, M., and B. Roizman. 1994. Expression of a herpes simplex virus 1 open reading frame antisense to the g134.5 gene and transcribed by an RNA 3′ coterminal with the unspliced latency-associated transcript. J. Virol. 68:6021–6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leib, D. A., C. L. Bogard, M. Kosz-Vnenchak, K. A. Hicks, D. M. Coen, D. M. Knipe, and P. A. Schaffer. 1989. A deletion mutant of the latency associated transcript of herpes simplex virus type 1 reactivates from the latent infection. J. Virol. 63:2893–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leopardi, R., and B. Roizman. 1996. The herpes simplex virus major regulatory protein ICP4 blocks apoptosis induced by the virus or by hyperthermia. Proc. Natl. Acad. Sci. USA 93:9583–9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leopardi, R., C. Van Sant, and B. Roizman. 1997. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci.USA 94:7891–7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lock, M., C. Miller, and N. W. Fraser. 2001. Analysis of protein expression from within the region encoding the 2.0-kb latency-associated transcript of herpes simplex virus type 1. J. Virol. 75:3413–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mador, N., D. Goldenberg, O. Cohen, A. Panet, and I. Steiner. 1998. Herpes simplex virus type 1 latency-associated transcripts suppress viral replication and reduce immediate-early gene mRNA levels in a neuronal cell line. J. Virol. 72:5067–5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maggioncalda, J., A. Mehta, Y. H. Su, N. W. Fraser, and T. M. Block. 1996. Correlation between herpes simplex virus type 1 rate of reactivation from latent infection and the number of infected neurons in trigeminal ganglia. Virology 225:72–81. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell, W. J., I. Steiner, S. M. Brown, A. R. MacLean, J. H. Subak-Sharpe, and N. W. Fraser. 1990. A herpes simplex virus type 1 variant, deleted in the promoter region of the latency-associated transcripts, does not produce any detectable minor RNA species during latency in the mouse trigeminal ganglion. J. Gen. Virol. 71:953–957. [DOI] [PubMed] [Google Scholar]
- 32.Naora, H., and H. Naora. 1999. Involvement of ribosomal proteins in regulating cell growth and apoptosis: translational modulation or recruitment for extraribosomal activity? Immunol. Cell Biol. 77:197–205. [DOI] [PubMed] [Google Scholar]
- 33.Naora, H., T. Nishida, Y. Shindo, M. Adachi, and H. Naora. 1998. Antisense sequences of the nbl gene induce apoptosis in the human promyelocytic leukemia cell line HL-60. Leukemia. 12:532–541. [DOI] [PubMed] [Google Scholar]
- 34.Naora, H., I. Takai, M. Adachi, and H. Naora. 1998. Altered cellular responses by varying expression of a ribosomal protein gene: sequential coordination of enhancement and suppression of ribosomal protein S3a gene expression induces apoptosis. J. Cell Biol. 141:741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neumann, F., P. HEmmerich, A. von Mikecz, H. H. Peter, and U. Krawinkel. 1995. Human ribosomal protein L7 inhibits cell-free translation in reticulocyte lysates and affects the expression of nuclear proteins upon stable transfection in Jurkat T-lymphoma cells. Nucleic Acids Res. 23:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perng, G.-C., C. Jones, J. Ciacci-Zanella, M. Stone, G. Herderson, A. Yukht, S. M. Slanina, F. M. Hofman, H. Ghiasi, A. B. Nesburn, and S. L. Wechsler. 2000. Virus-induced neuronal apoptosis blocked by herpes simplex virus latency-associated transcript. Science 287:1500–1503. [DOI] [PubMed] [Google Scholar]
- 37.Perng, G.-C., S. M. Slanina, A. Yukht, H Ghiasi, A. B. Nesburn, and S. L. Wechsler. 2000. The latency-associated transcript gene enhances establishment of herpes simplex virus type 1 latency in rabbits. J. Virol. 74:1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Randazzo, B. P., S. Kesari, R. M. Gesser, D. Alsop, J. C. Ford, S. M. Brown, A. R. MacLean, and N. W. Fraser. 1995. Treatment of experimental intracranial murine melanoma with a neuro-attenuated herpes simplex virus-1 mutant. Virology 211:94–101. [DOI] [PubMed] [Google Scholar]
- 39.Rock, D. L., A. B. Nesburn, H. Ghiasi, J. Ong, T. L. Lewis, J. R. Lokensgard, and S. M. Wechsler. 1987. Detection of latency related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J. Virol. 61:3820–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodier, F., R. Bertrand, M. Bossolasco, and A. M. Mes-Masson. 2000. Polyomavirus large T-antigen protects mouse cells from Fas-, TNF-alpha- and taxol-induced apoptosis. Oncogene 19:6261–6270. [DOI] [PubMed] [Google Scholar]
- 41.Roizman, B., and A. E. Sears. 1996. Herpes simplex viruses and their replication, p.2231–2295. In B. Fields, D. Knipe, and P. Howley (ed.), Fundamental virology 3rd ed. Lippincott-Raven. Phildelphia, Pa..
- 42.Roy, D. J., B. C. Ebrahimi, B. M. Dutia, A. A. Nash, and J. P. Stewart. 2000. Murine gammaherpesvirus M11 gene product inhibits apoptosis and is expressed during persistence. Arch. Virol. 145:2411–2420. [DOI] [PubMed] [Google Scholar]
- 43.Sawtell, N. M., and R. L. Thompson. 1992. Herpes simplex virus type 1 latency-associated transcription unit promotes anatomical site dependent establishment and reactivation from latency. J. Virol. 66:2157–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawtell, N. M., and R. L. Thompson. 2001. Herpes simplex virus type 1 latency-associated transcript gene promotes neuronal survival. J. Virol. 75:6660–6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skowronski, J., D. Parks, and R. Mariani. 1993. Altered T cell activation and development in transgenic mice expressing the HIV-1 nef gene. EMBO J. 12:703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spivack, J. G., and N. W. Fraser. 1987. Detection of herpes simplex type 1 transcripts during latent infection in mice. J. Virol. 61:3841–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spivack, J. G., G. M. Woods, and N. W. Fraser. 1991. Identification of a novel latency-specific splice donor signal within the herpes simplex virus type 1 2.0 kb latency-associated transcript (LAT): translation inhibition of LAT open reading frames by the intron within the 2.0 kb LAT. J. Virol. 65:6800–6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steiner, I., J. G. Spivack, R. P. Lirette., S. M. Brown, A. R. MacLean, J. Subak-Sharpe, and N. W. Fraser. 1989. Herpes simplex virus type 1 latency-associated transcripts are evidently not essential for latent infection. EMBO J. 8:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevens, J. G. 1989. Human herpesviruses: a consideration of the latent state. Microbial Rev. 53:318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevens, J. G., E. K. Wagner, G. B. Devi-Rao, M. L. Cook, and L. T. Feldman. 1987. RNA complementary to a herpes virus gene mRNA is prominent in latently infected neurons. Science 235:1056–1059. [DOI] [PubMed] [Google Scholar]
- 51.Tewari, M., W. G. Telford, R. A. Miller, and V. M. Dixit. 1995. CrmA, a poxvirus-encoded serpin, inhibits cytotoxic T-lymphocyte-mediated apoptosis. J. Biol. Chem. 270:22705–22708. [DOI] [PubMed] [Google Scholar]
- 52.Trousdale, M., I. Steiner, J. G. Spivack, S. L. Deshmane, S. M. Brown, A. S. MacLean, J. H. Subak-Sharpe, and N. W. Fraser. 1991. Evidence that the herpes simplex virus type 1 latency-associated transcripts play a role in reactivation of latent infection in vivo. J. Virol. 65:6989–6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zabolotny, J. M., C. Krummenacher, and N. W. Fraser. 1997. The herpes simplex virus type 1 2.0-kilobase latency-associated transcript is a stable intron which branches at a guanosine. J. Virol. 71:4199–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zachos, G., M. Koffa, C. M. Preston, J. B. Clements, and J. Conner. 2001. Herpes simplex virus type 1 blocks the apoptotic host cell defense mechanisms that target Bcl-2 and manipulates activation of p38 mitogen-activated protein kinase to improve viral replication. J. Virol. 72:2710–2728. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Zhou, G., V. Galvan, G. Campadelli-Fuime, and B. Roizman. 2000. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J. Virol. 74:11782–11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu, J., W. Kang, M. E. Marquart, J. Hill, X. D. Zheng, T. Block, and N. W. Fraser. 1999. Identification of a novel 0.7-kb polyadenylated transcript in the LAT promoter region of HSV-1 that is strain specific and may contribute to virulence. Virology 265:296–307. [DOI] [PubMed] [Google Scholar]