Abstract
The presence of a novel form of zebrafish fibronectin (FN2) on the cell surface increased the cell’s susceptibility to infection by infectious hematopoietic necrosis virus (IHNV). Unlike other fibronectins, FN2 possesses a truncated structure and accumulates on the cell surface instead of in the extracellular matrix. Fish embryo cells expressing recombinant FN2 were more susceptible to IHNV infection, with a greater percentage of cells exhibiting cytopathic effect (CPE) compared to nontransfected control cells. Incubation of nontransfected cells with soluble recombinant FN2 increased IHNV infection, as measured by plaque assay. The number of plaques increased in correlation with the amount of protein added and the length of time that cells were incubated with the protein. Incubation of IHNV with soluble FN2 before addition to cells also increased infection. FN2 immobilized on the culture surface inhibited IHNV infection. The results indicate that FN2 present on the cell surface is able to mediate IHNV attachment and cell entry.
Infectious hematopoietic necrosis virus (IHNV) is the cause of a devastating disease of salmon and trout that results in mortality approaching 100% in populations of young fish and approximately 25% in chronically infected larger fish (43). The disease was first reported on the west coast of North America in the 1940s and has since spread to central and eastern United States, Canada, Japan, and southern Europe (27, 35, 44). Most North American salmonid species are susceptible to IHNV infection, although Coho salmon are usually resistant (27, 43, 44).
IHNV is a rhabdovirus consisting of an outer protein coat and a single-stranded RNA (molecular weight, 3.7 × 106) core (22, 26, 31, 34). At least five different isolates of IHNV have been distinguished immunologically and by electrophoretic analysis of virion proteins (14), and the virus has been propagated and isolated from cell lines derived from several different fish species, including Chinook salmon, carp, and fathead minnow (32, 43).
IHNV is shed from infected fish and spread through water-borne contact. The most probable route of transmission is through the gills, but studies have indicated that the esophagus and cardiac stomach may also be ports of entry for the virus (7, 21). The first stage of IHNV replication is cellular attachment through the interaction of the viral glycoprotein G with the appropriate cell surface receptor (9, 25, 33). Although the IHNV cellular receptor is not known, studies conducted with other rhabdoviruses, including fish viral hemorrhagic septicemia virus (VHSV) and mammalian vesicular stomatitis virus (VSV) and rabies virus, have indicated that membrane phospholipids are involved in mediating infection (10, 15–17, 37, 38). The phospholipid-binding region of glycoprotein G has been characterized and shown to consist of hydrophobic amino acid heptad repeats that bind phospholipid in a pH-dependent manner (8, 10).
In addition to membrane phospholipids, several studies have provided evidence for the involvement of cellular proteins in mediating rhabdovirus attachment (3, 19, 29, 30). One of the proteins that has been implicated in virus infection is the extracellular matrix protein fibronectin. Bearzotti et al. (2) demonstrated that antibodies recognizing fibronectin were able to block VHSV infection in fish cell cultures. The virus-blocking activity was species specific, and the authors demonstrated that purified trout fibronectin was able to bind VHSV. Fibronectin has also been implicated in mediating infection by other viruses, including rabies virus (4), human immunodeficiency virus (HIV) (42), hepatitis A (40) and B (5) viruses, cytomegalovirus (1), and Rous sarcoma virus (RSV) (41).
Fibronectin is a high-molecular-weight glycoprotein that is a major constituent of the extracellular matrix and plays an important role in several cellular processes (23). The protein exists as a dimer composed of 250-kDa monomers, each comprised of three repeating units, types I, II, and III. The repeat units are organized into functional domains possessing binding affinities for fibrin, gelatin, heparin, collagen, cells, and other fibronectin molecules (24). In higher vertebrates, fibronectin heterogeneity results from alternative splicing of a common transcript at three exons to generate multiple isoforms of the protein (18).
Using the zebrafish as a model, we have identified and characterized the fibronectins that are expressed during fish embryogenesis as well as in various tissues of the adult fish (45). From this work we have discovered that, in addition to a highly conserved form of fibronectin (FN1), zebrafish express a novel truncated form of the protein (FN2) that has not been identified in other organisms (45). In this study, we demonstrate that FN2 present on the cell surface enhances IHNV infection of fish cells in culture. Cells that express recombinant FN2 or are incubated in the presence of the soluble protein are more susceptible to IHNV infection. Cells expressing zebrafish FN1, which is incorporated into the extracellular matrix, are only slightly more susceptible to IHNV infection than nontransfected control cells.
MATERIALS AND METHODS
Plasmid construction.
FN2 cDNA, previously cloned into the pBluescript-SK phagemid (45), was isolated by digestion with EcoRI and KpnI and ligated into the pBK-RSV plasmid (Stratagene). After ligation, pBK-RSV-FN2 was digested with EcoRI and NheI and filled with Klenow to remove the prokaryote promoter region. The pBK-RSV-FN2 plasmid contained FN2 cDNA under the control of the RSV promoter and simian virus 40 (SV40) polyadenylation signal along with the neo selectable marker gene driven by the SV40 early promoter. The FN1 expression vector was constructed in the same manner using FN1 cDNA isolated from the pBluescript-SK phagemid (45) by digestion with KpnI and SacI and ligated into the pBK-RSV plasmid. After ligation, pBK-RSV-FN1 was digested with NheI and NotI and filled with Klenow to remove the prokaryote promoter region. Recombinant FN1 expression was driven by the RSV promoter.
To produce FN1 fused to the enhanced green fluorescent protein (EGFP), site-directed mutagenesis was performed (Stratagene QuickChange mutagenesis kit) to create a NotI site between the type III and IV repeats of FN1 cDNA contained in pBK-RSV-FN1 (45). The primers used for site-directed mutagenesis were 5′-GAGCCACTGGCGGGCGGCCGCGAGGAAGTGCCGTC-3′ and 5′-GACGGCACTTCCTCGCGGCCGCCCGCCAGTGGCTC-3′. The PCR program consisted of 95°C for 30 s, followed by 18 cycles of 95°C for 30s, 55°C for 1 min, and 68°C for 26 min. The mutated pBK-RSV-FN1 plasmid was cut with NotI and ligated to EGFP PCR product containing a NotI site at each end and selected for proper orientation.
To construct the FN2-EGFP expression vector, site-directed mutagenesis was performed to create a NotI site following the sequence encoding the 31-amino-acid FN2 signal peptide in pBK-RSV-FN2. The primers used were 5′-GAAACATAAAAGAGGCGGCCGCCAAGCTCAGGAAC-3′ and 5′-GTTCCTGAGCTTGGCGGCCGCCTCTTTTATGTTTC-3′. The PCR program was the same as for FN1-EGFP except that the elongation time was 15 min. The mutated pBK-RSV-FN2 plasmid was cut with NotI and ligated to EGFP PCR product possessing NotI sites on each end.
Cell transfections.
CHSE-214 cells (American Type Culture Collection [ATCC]) grown in LDF medium (20) (20°C) supplemented with 10% fetal bovine serum (FBS) were transfected with pBK-RSV-FN2 or pBK-RSV-FN1 by calcium phosphate-mediated precipitation (11). Plasmid DNA (10 μg) was suspended in 0.5 ml of calcium chloride (0.5 M) and added dropwise with mixing to 0.5 ml of HEPES-buffered saline (50 mM HEPES, 250 mM NaCl, 1.8 mM sodium phosphate). The solution was incubated (30 min at room temperature) and then added to a dish (100 mm) containing a cell monolayer at 90% confluency. After 8 h the medium was changed, and 48 h later G418 (1 mg/ml) was added to the culture. Individual colonies were selected, expanded, and frozen in liquid nitrogen.
CPE and plaque assays.
Cytopathic effect (CPE) assays were conducted according to published methods with slight modifications (13). CHSE cells were seeded into 24- or 12-well plates (Falcon) (0.5 × 106 or 1 × 106 cells/well, respectively) in serum-free LDF medium and grown for 3 days. IHNV (ATCC; 105.75 50% tissue culture infectious doses [TCID50] per 0.2 ml) suspended at the appropriate dilution in LDF was added to each well and incubated for 1 h. The medium was aspirated, and fresh LDF containing 10% FBS was added. The percentage of cells exhibiting CPE was estimated by microscopic examination of the cultures on the days indicated. The initial appearance of CPE in the culture was quantified by counting the number of sites where groups of round translucent detaching cells appeared in the cell monolayer. As CPE spread through the culture and large areas of the monolayer detached from the culture surface, the percentage of cells affected was estimated.
Plaque assays were conducted according to published methods (6). CHSE cells were seeded into 12-well plates (Falcon) (8 × 105 cells/well) in LDF supplemented with 5% fibronectin-depleted FBS. When the cultures reached confluency, the medium was aspirated, and the cultures were rinsed with serum-free LDF. IHNV (100 μl) suspended at the appropriate dilution in LDF was added, and after 30 min (20°C) the medium was aspirated and the well was rinsed three times with fresh medium. Low-melting-point agarose (1 ml, 0.85%) in medium containing 5% fibronectin-depleted FBS was added to each well. After 1 week the cells were fixed and stained (3.7% formaldehyde in phosphate-buffered saline [PBS] containing 0.5% crystal violet) and rinsed, and plaques were counted. Fibronectin-depleted FBS was prepared by adding gelatin-Sepharose A (4 ml) (Pharmacia) to FBS (25 ml) and incubating (4°C) overnight with constant mixing. The gelatin-Sepharose A was removed by centrifugation, and the FBS was filter sterilized.
To examine the effect of immobilized FN2 on IHNV infection, PBS (200 μl) containing the indicated concentration of purified recombinant FN2 was added to each well of a 12-well plate and incubated overnight (4°C). The wells were aspirated, blocked with bovine serum albumin (10 mg/ml in PBS) for 1 h at room temperature and rinsed three times with serum-free LDF. IHNV (100 μl at 5 × 10−4 dilution) was added, and the plate was incubated for 30 min (room temperature). The virus suspension was aspirated, and CHSE cells were added to each well (5 × 105 cells/well) in LDF containing fibronectin-depleted FBS (5%). After the cells attached to the culture surface (approximately 4 h), the medium was aspirated, an agarose overlay was added, and the plaque assay was performed as described above.
For the experiments involving the addition of soluble protein, purified recombinant FN2, suspended at the appropriate concentration in LDF (200 μl), was added to a confluent culture of CHSE contained in a single well of a 12-well plate. Cells were incubated (20°C) for 15, 30, 60, or 120 min in the presence of the protein, then aspirated, and rinsed with LDF. IHNV (100 μl at 10−5 dilution) was added to each well and incubated for 30 min (20°C), and a plaque assay was performed. To incubate the virus with soluble protein, IHNV (10−5) was suspended in LDF (100 μl) containing 0, 0.1, 1.0, 10, or 20 μg of FN2 per ml and incubated for 1 h (room temperature). The suspension was added to a confluent monolayer of CHSE in a single well of a 12-well plate and incubated for 30 min (20°C) before a plaque assay was performed.
Purification of soluble FN2.
Recombinant FN2 protein was synthesized using a commercial baculovirus expression system (Bac-to-Bac; Life Technologies) using sf9 cells as previously described (45). The recombinant protein was purified by affinity chromatography on a fish gelatin-Sepharose 4B column. Medium containing the protein was added to the column, and after washing, the FN2 was eluted (3 M guanidine-HCl in Tris-buffered saline), dialyzed into PBS, and concentrated (Centricon YM-30; Millipore). Purification of FN2 was monitored by silver-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis using anti-rat fibronectin antiserum (Life Technologies).
RESULTS
Characteristics of FN2.
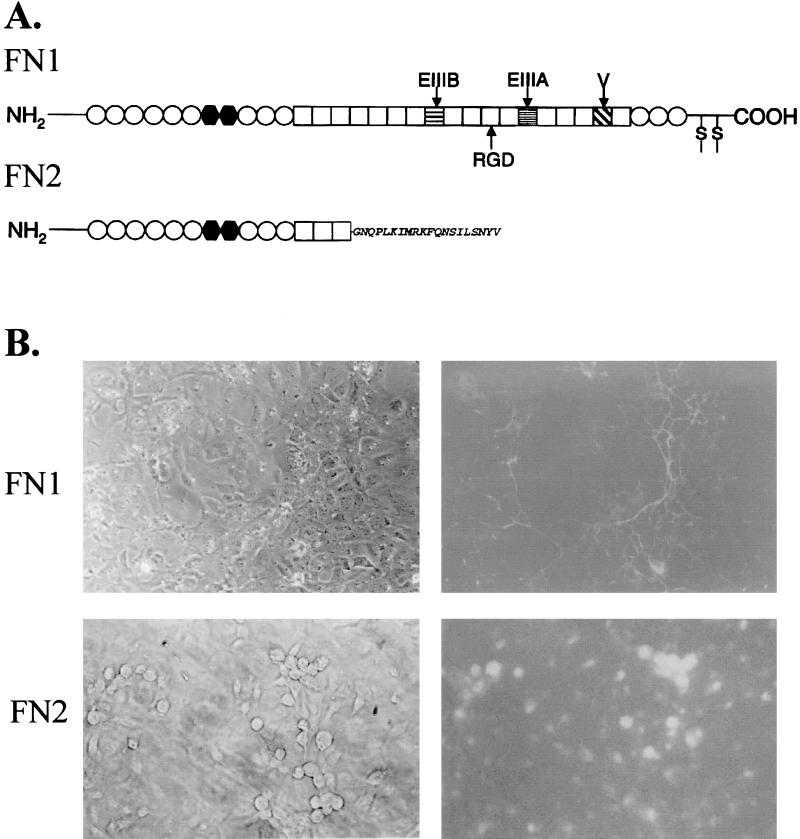
In addition to a highly conserved fibronectin (FN1), zebrafish express a novel form of the protein (FN2) that possesses a truncated structure (45). While FN1 contains the typical number of type I, II, and III repeats that are present in other fibronectins, FN2 is comprised of only the N-terminal 9 type I, 2 type II, and the first 3 type III units (Fig. 1A). The truncated FN2 protein lacks the additional 12 to 14 type III repeats that are found in other fibronectins and terminates in a unique 20 amino acid C-terminal tail that does not contain the cysteine residues usually involved in fibronectin dimer formation (Fig. 1A).
FIG. 1.
(A) Schematic diagram showing the organization of zebrafish FN1 and FN2 (45). The type I (○), II (•), and III (□) repeats along with EIIIA, EIIIB, and the variable region (V) are shown. The amino acid sequence of the FN2 C-terminal tail is indicated. (B) Cellular distribution of recombinant FN1 and FN2, each synthesized as a fusion protein with GFP in CHSE-214 cells. Photomicrographs of the same field examined by light (left) and UV (right) phase-contrast microscopy showing the distribution of the GFP-labeled proteins. Magnification, ×200.
In addition to its truncated structure, FN2 differs from FN1 in its cellular distribution. Recombinant FN2 synthesized in Chinook salmon embryo cells (CHSE-214) accumulates on the cell surface and, unlike other fibronectins, including zebrafish FN1, is not incorporated into the extracellular matrix (Fig. 1B). The same cellular distribution of FN2 was obtained when the protein was labeled with EGFP (Fig. 1B) or with a 6-residue histidine tag (data not shown) fused to the N-terminal region of the protein.
Effect of FN2 expression on IHNV infection.
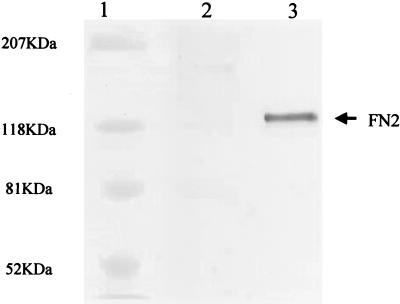
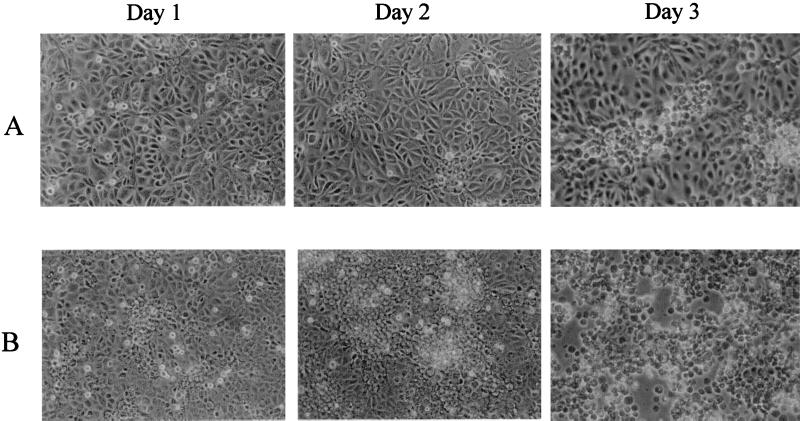
To investigate the effect that FN2 expression has on rhabdovirus infection, CHSE cells transfected with FN2 cDNA under the control of a viral promoter (Fig. 2) were infected with different doses of IHNV. The results demonstrate that cells expressing recombinant FN2 were more susceptible to IHNV infection (Table 1). Six days following exposure to a low dose of IHNV (10−7), approximately 50% of the transfected cells exhibited CPE, while CPE was not detected in the nontransfected control cultures (Table 1). At higher doses (10−2 and 10−3), a greater percentage of cells exhibiting CPE was detected in the transfected cultures compared to the controls as early as 24 h following infection (Table 1, Fig. 3). CPE was characterized by the presence of translucent floating cells and rounded loose cell aggregates that had detached from the culture surface (Fig. 3).
FIG. 2.
Expression of recombinant FN2 by CHSE-214 cells. FN2 was detected by Western blot analysis of proteins present in medium taken from a 7-day-old culture of CHSE-214 cells expressing the pBK-RSV-FN2 plasmid (lane 3). Although recombinant FN2 initially localizes on the cell surface, it accumulates in the medium after several days of constitutive expression. Nontransfected CHSE did not produce detectable amounts of FN2 (lane 2). Molecular size markers are shown in lane 1.
TABLE 1.
Percentage of cells exhibiting CPE following IHNV exposure
| Virus dilution | Cells | % of cells with CPE on day post-IHNV exposure:
|
|||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 6 | ||
| 10−2 | CHSE | 0 | 5–10 | 30–40 | |
| CHSE-FN2 | 40–50 | 70–80 | 100 | ||
| CHSE-FN1 | 5 | 10–20 | 50–60 | ||
| 10−3 | CHSE | 0 | <5 | 5 | |
| CHSE-FN2 | 5 | 30–40 | 70–80 | ||
| CHSE-FN1 | 0 | 10 | 10 | ||
| 10−7 | CHSE | 0 | |||
| CHSE-FN2 | 50–60 | ||||
FIG. 3.
Phase-contrast photomicrograph showing nontransfected (A) and FN2-transfected (B) CHSE-214 cells 1, 2, and 3 days following IHNV infection (10−2). Individual cells exhibiting CPE initially appear round and translucent. By day 3, 100% of the FN2-transfected cells exhibit CPE, indicated by aggregates of rounded cells detaching from the culture surface, while a small percentage of cells in the control culture are just beginning to exhibit CPE. Magnification, ×144.
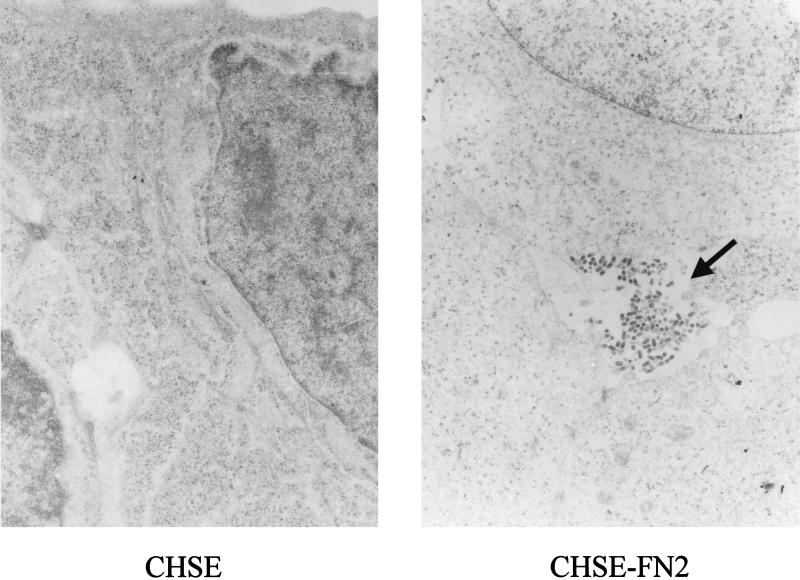
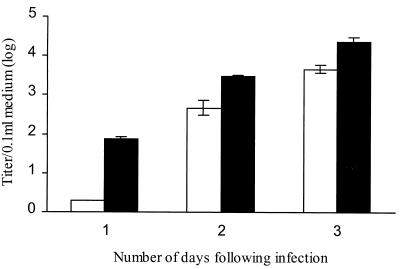
Cells expressing recombinant FN1 were only slightly more susceptible to IHNV infection than the control (Table 1). Increased IHNV susceptibility of the transfected cells was also indicated by the ability of the virus to infect and replicate more rapidly in the FN2-expressing cells. Virus particles were detected by electron microscopy in the FN2-transfected cultures 24 h after infection, compared to 48 h in the controls (Fig. 4). Also, the virus titer in medium obtained from transfected cultures 24 h after virus exposure was more than 30 times higher than the titer of control medium (Fig. 5). The virus titers of the media were approximately the same by 3 days after infection.
FIG. 4.
Electron photomicrograph showing the presence of IHNV particles (arrow) in a CHSE-FN2 culture 1 day following exposure to the virus. Virus particles were not detected in a nontransfected CHSE-214 culture (left) exposed to IHNV on the same day. Magnification, ×7,800.
FIG. 5.
IHNV titer in medium obtained from FN2-transfected (solid bars) and nontransfected (open bars) CHSE cells. Medium was collected from each culture 1, 2, or 3 days following IHNV infection, and virus titer was measured by plaque assay as described in Materials and Methods. The mean titer of triplicate samples is shown with the standard error.
Effect of exogenous recombinant FN2 on the level of IHNV infection.
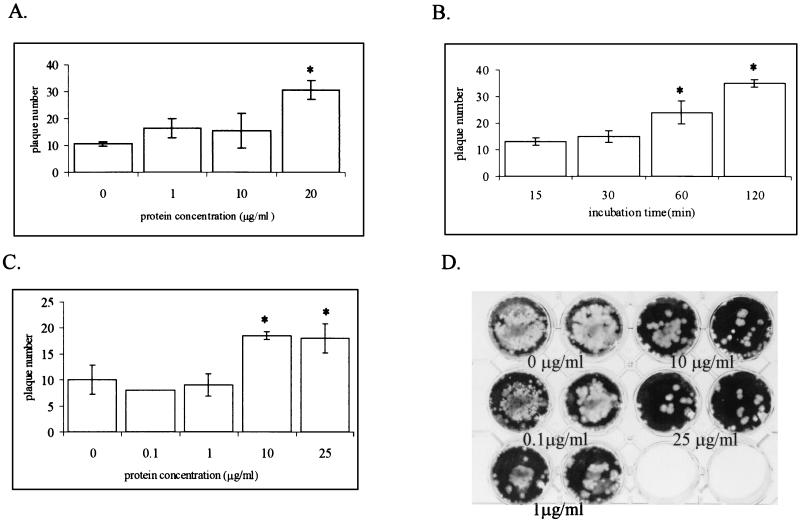
Recombinant FN2 protein added to CHSE cultures affected the cells’ susceptibility to IHNV infection. Incubation of CHSE cells with soluble recombinant FN2 before IHNV exposure increased the frequency of infection measured by plaque assay (Fig. 6). The number of plaques observed was positively correlated to the amount of FN2 added to the culture medium (Fig. 6A) and to the length of time that the cells were incubated in the presence of the protein before virus exposure (Fig. 6B). Incubation of the virus with soluble FN2 before its addition to the cells also resulted in an increase in the number of plaques (Fig. 6C). In contrast, the presence of FN2 immobilized on the culture surface inhibited IHNV infection (Fig. 6D). The number of plaques decreased with increasing amounts of protein immobilized on the culture surface.
FIG. 6.
Effect of exogenously added recombinant FN2 on IHNV infection. (A) CHSE-214 cells were incubated (30 min, room temperature) with soluble recombinant FN2 at the indicated concentration before being exposed to IHNV (10−5). The number of plaques produced in the culture was determined as described in Materials and Methods. (B) CHSE-214 cells were incubated with soluble recombinant FN2 (10 μg/ml) for the length of time indicated before being exposed to IHNV (10−5), and the number of plaques produced was determined. (C) IHNV (10−5) was incubated with the indicated concentration of soluble recombinant FN2 before being added to CHSE-214 cells, and the number of plaques produced in the culture was determined. For each experiment, the mean number of plaques produced in two cultures along with the standard error is shown. Plaque numbers that are significantly different from the control, determined by the Duncan’s multiple-range test (α = 0.05), are indicated (*). (D) Photograph showing plaque assays that were performed on CHSE-214 cultures grown on plates containing immobilized FN2 at the indicated concentration.
DISCUSSION
As a component of plasma and a major constituent of the extracellular matrix, fibronectin is widely distributed in most tissues. Although different isoforms of fibronectin exist, the protein possesses the same basic repeat structure in fish (45), amphibians (12), birds (36), and mammals (24). In higher vertebrates, alternative splicing of a common fibronectin transcript results in the complete inclusion or exclusion of the EIIIA and EIIIB repeats, and splicing within the V region generates multiple isoforms of the mature protein. While EIIIA−, EIIIB−, and V region splice variants have not been detected in zebrafish, a novel form of fibronectin, FN2, has been identified that is structurally unique and is generated by a new alternative splicing pattern not previously described for other fibronectins (45).
In addition to possessing a truncated structure, FN2 exhibits several unique characteristics. The lack of cysteines in the C-terminal tail suggests that FN2 exists as a monomer, and reverse transcription-PCR and RNase protection analyses have demonstrated that, in vivo, FN2 mRNA is consistently present at a lower abundance than the highly conserved FN1 mRNA (45). The results of this study reveal that, unlike other fibronectins, FN2 accumulates on the cell surface and is not incorporated into the extracellular matrix. The absence of matrix-associated FN2 is not surprising, since the protein lacks the RGD cell adhesion site that is normally present in III10 and shown to be required for matrix assembly (39).
Though the function of FN2 is not known, the results of this work demonstrate that its presence on the cell surface makes the cell more susceptible to IHNV infection, indicating that FN2 may mediate virus attachment. The involvement of zebrafish fibronectin in IHNV infection is consistent with recent work demonstrating that the virus is able to infect and replicate in zebrafish (28). Localization of FN2 on the cell surface is important for IHNV infection, since recombinant zebrafish FN1 that was incorporated into the extracellular matrix had only a slight effect on virus infection. These results are consistent with those of Bearzotti et al. (2), who have shown that monoclonal antibodies recognizing cell surface fibronectin were able to block fish rhabdovirus infection in cell culture.
The authors selected three different monoclonal antibodies based on their ability to block VHSV infection and demonstrated by Western blot analysis and immunoprecipitation that all three recognized trout fibronectin. The antibodies were raised against rainbow trout gonad cells and cell membranes, and immunofluorescent staining revealed that the recognized antigens were restricted to the cell surface and not observed in the extracellular region (2). This pattern of fibronectin staining would be expected if the antibodies recognized FN2 on the surface of the trout cells. We have also found that incubating CHSE-214 cells with anti-rat fibronectin antiserum before adding IHNV inhibited infection (data not shown). Bearzotti et al. (2) demonstrated that fibronectin purified from muscle by gelatin chromatography possessed VHVS binding activity. FN2 possesses the gelatin-binding domain located in the N-terminal type I repeats, and the protein is purified by gelatin chromatography.
Fibronectin is able to bind to several viruses (1, 4, 5, 40–42), and a recent study has shown that a virus-binding domain is located in the type III1 repeat (42). A peptide containing the III1 region efficiently bound to HIV in vitro, and when the virus was incubated with the III1 fragment before being added to lymphocytes, HIV attachment and internalization were enhanced (42). HIV infection was also enhanced by incubation of the virus with multimeric but not dimeric fibronectin, indicating that the III1 binding site may be exposed in fibronectin fibrils to increase virus attachment to the extracellular matrix (42). Incubation of lymphocytes with III1 before exposure to HIV did not make the cells more susceptible to infection (42). We also observed an increase in infection when IHNV was incubated with FN2 before being added to the cells. However, unlike the results with HIV, we detected an increase in IHNV susceptibility when the CHSE cells were incubated with soluble FN2 before virus exposure.
Although FN2 possesses the III1 repeat, the protein lacks the RGD cell binding site and the C-terminal cysteines that are required for multimeric fibril formation. Consistent with these structural characteristics, recombinant FN2 localizes on the cell surface and is not incorporated into the matrix. The results demonstrating that cells expressing recombinant FN2 along with nonexpressing cells that are incubated with soluble FN2 both exhibit increased susceptibility to IHNV infection indicate that the protein is able to associate with the cell surface and in the process expose the virus-binding site.
Surprisingly, immobilized FN2 inhibited IHNV infection of the CHSE cells. The inhibition may be due to a change in cell shape, resulting in reduced accessibility of the viral receptor in the presence of immobilized FN2. Previously, we demonstrated that immobilized FN2 is able to enhance CHSE attachment and spreading through the N-terminal type I and II repeats (45). It is also possible that the immobilized FN2 is able to form a complex with other cell surface components involved in IHNV attachment and make them inaccessible to the virus.
The results of this study demonstrate that a unique form of fibronectin is involved in mediating IHNV infection. The relatively low abundance and cell surface distribution of zebrafish FN2 may allow the protein to modulate IHNV infection in vivo and play a role in determining the host range of the virus.
REFERENCES
- 1.Agbanyo, F., and S. Wasi. 1994. Human cytomegalovirus interaction with platelets and adhesive glycoproteins: significance in viral pathogenesis. J. Infect. Dis. 170:1120–1127. [DOI] [PubMed] [Google Scholar]
- 2.Bearzotti, M., B. Delmas, A. Lamoureux, A.-M. Loustau, S. Chilmonczyk, and M. Bremont. 1999. Fish rhabdovirus cell entry is mediated by fibronectin. J. Virol. 73:7703–7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bracci, L., G. Antoni, M. Cusi, L. Lozzi, N. Niccolai, S. Petreni, M. Rustici, A. Santucci, P. Soldani, and P. Valensin. 1988. Antipeptide monoclonal antibodies inhibit the binding of rabies virus glycoprotein and alpha-bungarotoxin to the nicotinic acetylcholine receptor. Mol. Immunol. 25:881–888. [DOI] [PubMed] [Google Scholar]
- 4.Broughan, J., and W. Wunner. 1995. Characterization of protein involvement in rabies virus binding to BHK-21 cells. Arch. Virol. 140:75–93. [DOI] [PubMed] [Google Scholar]
- 5.Budkowska, A., P. Bedossa, F. Groh, A. Louise, and J. Pillot. 1995. Fibronectin of human liver sinusoids binds hepatitis B virus: identification by an anti-idiotypic antibody bearing the internal image of the pre-S2 domain. J. Virol. 69:840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burke, J., and D. Mulcahy. 1980. Plaquing procedure for infectious hematopoietic necrosis virus. Appl. Environ. Microbiol. 39:872–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chilmonczyk, S., and D. Monge. 1980. Rainbow trout gill pillar cells: demonstration of inert particle phagocytosis and involvement in viral infection. J. Reticuloendothel. Soc. 28:327–332. [PubMed] [Google Scholar]
- 8.Coll, J. 1995. Low-pH increases the binding of haemorrhagic septicemia rhabdovirus to membrane phospholipids. J. Fish Dis. 18:519–527. [Google Scholar]
- 9.Coll, J. 1995. The glycoprotein G of rhabdoviruses. Arch. Virol. 140:827–851. [DOI] [PubMed] [Google Scholar]
- 10.Coll, J. 1997. Synthetic peptides from the heptad repeats of the glycoproteins of rabies, vesicular stomatitis and fish rhabdoviruses bind phosphatidylserine. Arch. Virol. 142:2089–2097. [DOI] [PubMed] [Google Scholar]
- 11.Collodi, P. 1998. DNA transfer to blastula-derived cultures from zebrafish, p.69–92. In K. Ravid and I. Freshney (ed.), DNA transfer to cultured cells. Wiley-Liss, New York, N.Y.
- 12.DeSimone, D., P. Norton, and R. O. Hynes. 1992. Identification and characterization of alternatively spliced fibronectin mRNAs expressed in early Xenopus embryos. Dev. Biol. 149:357–369. [DOI] [PubMed] [Google Scholar]
- 13.Engelking, H., and J. Leong. 1981. IHNV persistently infects Chinook salmon embryo cells. Virology 109:47–58. [DOI] [PubMed] [Google Scholar]
- 14.Engelking, H., J. Harry, and J. Leong. 1991. Comparison of representative strains of infectious hematopoietic necrosis virus by serological neutralization and cross-protection assays. Appl. Environ. Microbiol. 57:1372–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estepa, A., and J. Coll. 1996. Pepscan mapping and fusion-related properties of the major phosphatidylserine-binding domain of the glycoprotein of viral hemorrhagic septicemia virus, a salmonid rhabdovirus. Virology 216:60–70. [DOI] [PubMed] [Google Scholar]
- 16.Estepa, A., and J. Coll. 1996. Phosphatidylserine binding to solid-phase rhabdoviral peptides: a new method to study phospholipid/viral protein interactions. J. Virol. Methods 61:37–45. [DOI] [PubMed] [Google Scholar]
- 17.Estepa, A., M. Fernandez-Alonso, and J. Coll. 1999. Structure, binding and neutralization of VHSV with synthetic peptides. Virus Res. 63:27–34. [DOI] [PubMed] [Google Scholar]
- 18.ffrench-Constant, C. 1995. Alternative splicing of fibronectin- Many different proteins but few different functions. Exp. Cell Res. 221:261–271. [DOI] [PubMed] [Google Scholar]
- 19.Gastka, M., J. Horvath, and T. L. Lentz. 1996. Rabies virus binding to the nicotinic acetylcholine receptor alpha subunit demonstrated by virus overlay protein-binding assay. J. Gen. Virol. 77:2437–2440. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh, C., Y. Liu, C. Ma, and P. Collodi. 1997. Cell cultures derived from early zebrafish embryos differentiate in vitro into neurons and astrocytes. Cytotechnology 23:221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helmick, C., J. Bailey, S. LaPatra, and S. Ristow. 1995. The esophagus/cardiac stomach region: site of attachment and internalization of infectious hematopoietic necrosis virus in challenged juvenile rainbow trout Oncorhynchus mykiss and coho salmon O. kisutch. Dis. Aquat. Org. 23:189–199. [Google Scholar]
- 22.Hsu, Y., H. Engelking, and J. Leong. 1985. Analysis of quantity and synthesis of the virion proteins of infectious hematopoietic necrosis virus. Fish Pathol. 20:331–338. [Google Scholar]
- 23.Hynes, R. O. 1990. Fibronectins. Springer Verlag, New York, N.Y.
- 24.Hynes, R. O., and K. Yamada. 1982. Fibronectins: multifunctional modular glycoproteins. J. Cell Biol. 95:369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koener, J., C. Passavant, G., Kurath, and J. Leong. 1987. Nucleotide sequence of a cDNA clone carrying the glycoprotein G gene of infectious hematopoietic necrosis virus, a fish rhabdovirus. J. Virol. 61:1342–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurath, G., and J. Leong. 1985. Characterization of infectious hematopoietic necrosis virus mRNA species reveals a nonvirion rhabdovirus protein. J. Virol. 53:462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaPatra, S. 1998. Factors affecting pathogenicity of infectious hematopoietic necrosis virus (IHNV) for salmonid fish. J. Aquat. Anim. Health 10:121–131. [Google Scholar]
- 28.LaPatra, S. E., L. Barone, G. Jones, and L. Zon. 2000. Effects of infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus infection on hematopoietic precursors of the zebrafish. Blood Cells Mol. Dis. 26:445–452. [DOI] [PubMed] [Google Scholar]
- 29.Lentz, T., T. Burrage, A., Smith, J. Crick, and G. Tignor. 1982. Is the acetycholine receptor a rabies receptor? Science 215:182–184. [DOI] [PubMed] [Google Scholar]
- 30.Lentz, T., J. J. Benson, D. Klimowicz, P. Wilson, and E. Hawrot. 1986. Binding of rabies virus to purified Torpedo acetycholine receptor. Mol. Brain Res. 1:211–219. [DOI] [PubMed] [Google Scholar]
- 31.Leong, J., Y. Hsu, and H. Engelking. 1983. Synthesis of the structural proteins of infectious hematopoietic necrosis virus, p.66–71. In J. Crosa (ed.), Bacterial and viral diseases of fish: molecular studies. University of Washington Press, Seattle, Wash.
- 32.Lorenzen, E., B. Carstensen, and N. Olesen. 1999. Inter-laboratory comparison of cell lines for susceptibility to three viruses: VHSV, IHNV and IPNV. Dis. Aquat. Org. 37:81–88. [DOI] [PubMed] [Google Scholar]
- 33.Lorenzen, N., N. Olesen, and C. Koch. 1999. Immunity to VHS virus in rainbow trout. Aquaculture 172:41–61. [Google Scholar]
- 34.Meyers, T., and J. Winton. 1996. Viral hemorrhagic septicemia virus in North America. Annu. Rev. Fish Dis. 5:3–24. [Google Scholar]
- 35.Noble, A. C., and S. T. Summerfelt. 1996. Diseases encountered in rainbow trout cultured in recirculating systems. Annu. Rev. Fish Dis. 6:65–92. [Google Scholar]
- 36.Norton, P. A., and R. O. Hynes. 1987. Alternative splicing of chicken fibronectin in embryos and in normal and transformed cells. Mol. Cell. Biol. 7:4297–4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nunez, E., A. Fernandez, A. Estepa, J. Gonzalez-Ros, F. Gavilanes, and J. Coll. 1998. Phospholipid interactions of a peptide from the fusion-related domain of the glycoprotein of VHSV, a fish rhabdovirus. Virology 243:322–330. [DOI] [PubMed] [Google Scholar]
- 38.Schlegal, R., T. Tralka, M., Willingham, and I. Pastan. 1983. Inhibition of VSV binding and infectivity by phosphatidylserine: is phosphatidylserine a VSV-binding site? Cell 32:639–646. [DOI] [PubMed] [Google Scholar]
- 39.Schwarzbauer, J. E., and J. L. Sechler. 1999. Fibronectin fibrillogenesis: a paradigm for extracellular matrix assembly. Curr. Opin. Cell Biol. 11:622–627. [DOI] [PubMed] [Google Scholar]
- 40.Seelig, R., G. Pott, H. Seelig, H. Liehr, P. Metzger, and R. Waldherr. 1984. Virus-binding activity of fibronectin: masking of hepatitis A virus. J. Virol. Methods 8:335–347. [DOI] [PubMed] [Google Scholar]
- 41.Stanislawski, L. 1983. Attachment of rous sarcoma virus to the fibronectin matrix of infected chick embryo fibroblasts. J. Ultrastruct. Res. 82:134–142. [DOI] [PubMed] [Google Scholar]
- 42.Tellier, M., G. Greco, M. Klotman, A. Mosoian, A. Cara, W. Arap, E. Ruoslahti, R. Pasqualini, and L. Schnapp. 2000. Superfibronectin, a multimeric form of fibronectin, increases HIV infection of primary CD4+ lymphocytes. J. Immunol. 164:3236–3245. [DOI] [PubMed] [Google Scholar]
- 43.Winton, J. 1991. Recent advances in detection and control of infectious hematopoietic necrosis virus in aquaculture. Annu. Rev. Fish Dis. 1:83–93. [Google Scholar]
- 44.Wolf, K. 1988. Fish viruses and fish viral diseases, p.83–114. Comstock Publishing Associates, Cornell University Press, Ithaca, N.Y.
- 45.Zhao, Q., X. Liu, and P. Collodi. 2001. Identification and characterization of a novel fibronectin in zebrafish. Exp. Cell Res. 268:211–219. [DOI] [PubMed] [Google Scholar]