Abstract
Our previous studies provided evidence that E10R, a vaccinia virus protein belonging to the ERV1/ALR family, has a redox function and is required for virion assembly. Repression of E10R prevented the formation of intramolecular disulfide bonds of the G4L glutaredoxin, the L1R membrane protein, and the structurally related F9L protein. Here, we demonstrate an oxidation pathway (E10RSS → G4LSS → L1RSS, F9LSS) in which G4L occupies an intermediate position. By reacting free thiols with 4-acetamido-4′-malemideylstilbene-2,2′-disulfonic acid, alkylated and nonalkylated disulfide-bonded forms of G4L could be resolved from each other by polyacrylamide gel electrophoresis. The cysteines of intracellular G4L were in both disulfide and reduced forms, whereas those of E10R, L1R, and F9L and virion-associated G4L were mostly disulfide bonded. Repression of G4L expression prevented the formation of disulfide bonds in both L1R and F9L but not E10R. Both cysteines of G4L were required for L1R and F9L disulfide bond formation or for trans-complementation of virus infectivity when G4L expression was repressed. No role in the E10R-G4L redox pathway was found for O2L, a nonessential glutaredoxin encoded by vaccinia virus. We suggest that cytoplasmic G4L is a redox shuttle between membrane-associated E10R and L1R or F9L.
For poxviruses to replicate within the cytoplasm of infected cells, they must encode proteins that carry out some processes usually restricted to other cellular compartments. Thus, it has long been known that poxviruses express their own enzymes and factors for genome replication and transcription (14). Virion assembly also occurs in the cytoplasm, raising questions of how certain viral membrane and core proteins acquire stable disulfide bonds (8, 13), which are thought to form exclusively in the relatively oxidizing endoplasmic reticulum of eukaryotes or periplasm of bacteria (18).
Vaccinia virus encodes three proteins, E10R, G4L, and O2L, with CXXC redox motifs. Recently, we reported that E10R and G4L, are required for virion assembly (21, 23). E10R is a member of the ERV1/ALR (essential for respiration and vegetative growth/augmenter of liver regeneration) family common to all sequenced eukaryotes and cytoplasmic DNA viruses (19). Although ERV1/ALR proteins lack the characteristic thioredoxin fold of many other redox proteins, several members of the family have been shown to be flavin adenine dinucleotide-linked sulfhydryl oxidases (6, 11, 12), suggesting a related mechanism of electron transfer for E10R. Vaccinia virus G4L and O2L glutaredoxins, with predicted thioredoxin folds, have both thiol transferase and dehydroascorbate reductase activities in vitro (1, 7). O2L is nonessential for vaccinia virus replication and is thought to serve as a ribonucleotide reductase (15), though it may have additional functions consistent with its late promoter.
Evidence that the E10R protein is a component of a novel cytoplasmic disulfide bond pathway has been obtained (21). When E10R expression was repressed or when the CXXC motif was mutated, the cysteines of three cytoplasmic proteins (G4L, L1R, and F9L) were fully reduced. L1R contains three intramolecular disulfide bonds (21) and is an essential myristoylated membrane component of intracellular mature virions (16, 17, 24). F9L is an uncharacterized protein but is structurally related to L1R and also has intramolecular disulfide bonds (21). Whether E10R directly oxidizes the three viral proteins or acts through intermediates has not yet been determined.
In the present study, we demonstrated that repression of G4L prevented the formation of disulfide bonds in L1R and F9L without affecting oxidation of E10R, leading us to propose a redox pathway (E10RSS → G4LSS → L1RSS, F9LSS) in which G4L is an intermediate.
MATERIALS AND METHODS
Cells and viruses.
Cells and recombinant vaccinia viruses were propagated as previously described (4) using 50 μM isopropyl-β-𝒹-thiogalactopyranoside (IPTG) for inducer-dependent strains. Virus was purified by centrifugation through a 36% (wt/vol) sucrose cushion or by sucrose gradient centrifugation (3).
Antibodies.
Rabbit antiserum was raised to the C-terminal 13 amino acids of the predicted G4L sequence preceded by a cysteine (CEKATYGVWPPVTE). A purified murine monoclonal antibody (MAb) HA.11 to the influenza virus hemagglutinin (HA) epitope tag was obtained from BABco/Covance Research Products Inc, Richmond, Calif.. Rabbit antiserum was raised to purified O2L protein (1).
Plasmids.
The open reading frames (ORFs) of E10R and L1R with C-terminal HA epitope tags were cloned adjacent to the late vaccinia virus P11 promoter for transient-expression experiments (21). The F9L ORF with an HA tag was cloned adjacent to the composite synthetic early and late promoter (21).
Plasmids containing the wild-type G4L ORF controlled by the P11 (pUC19 G4L P11) promoter or the native promoter (pUC19 G4L native) were constructed using standard techniques. Cysteine-to-serine point mutations in either or both of the conserved cysteines were introduced into the G4L sequence by a series of PCRs using the pUC-G4L native plasmid as the template. All constructs were sequenced to confirm that the point mutations were present and that no changes had been introduced during the cloning procedures.
Transient expression of vaccinia virus ORFs in vaccinia virus-infected cells.
BS-C-1 cells were infected with vG4Li, unless otherwise specified at a multiplicity of 5 in the presence or absence of 50 μM IPTG for 1 h. vG4Li is a conditional lethal recombinant virus that is dependent on IPTG for the expression of G4L (23). This virus expresses the Escherichia coli lac repressor and the bacteriophage T7 RNA polymerase and has the endogenous copy of G4L removed and replaced with a copy of the G4L ORF with an N-terminal HA epitope tag under the control of a T7 promoter. E. coli lac operator sequences precede both the T7 RNA polymerase and the G4L ORFs. The cells were washed with OptiMEM (Life Technologies/Invitrogen) and transfected with 1 μg of plasmid in the presence or absence of 50 μM IPTG. After 5 h, the medium was removed and replaced with complete Eagle’s minimal essential medium (EMEM) containing 2.5% fetal calf serum with or without IPTG.
Alkylation of free thiols with AMS or NEM.
At 24 h after infection or mock infection, cell monolayers were treated with 10% trichloroacetic acid (TCA) in phosphate-buffered saline using a modification of previously described procedures to prevent disulfide interchange (9, 10). TCA precipitates were washed three times with ice-cold acetone, and the pellets were dissolved in 50 mM Tris-HCl (pH 8.0)-0.1% sodium dodecyl sulfate (SDS) containing either 40 mM N-ethylmalemide (NEM) (Sigma) or 40 mM 4-acetamido-4′-malemideylstilbene-2,2′-disulfonic acid (AMS) (Molecular Probes). The soluble proteins were mixed with an equal volume of nonreducing loading buffer (4% SDS, 0.125 M Tris-HCl [pH 6.8], 20% glycerol, 0.004% bromophenol blue) unless otherwise specified.
Western blotting.
Samples alkylated with NEM or AMS were analyzed by nonreducing SDS-polyacrylamide gel electrophoresis (PAGE) using 10 to 20% polyacrylamide gradient Tris-glycine gels and Tris-glycine-SDS running buffer; 16% or 10 to 20% polyacrylamide gradient Tricine gels and Tricine-SDS running buffer; or Novex NuPAGE (bis[2-hydroxyethyl]imino-tris[hydroxymethyl]methane-HCl) gels buffered with 3-(N-morpholino)propanesulfonic acid (MOPS)-SDS running buffer (Invitrogen). Proteins were electrophoretically transferred to a polyvinylidene difluoride (Millipore 0.45 μm) or nitrocelluose (Osmonics 0.45 μm) membrane, which was then blocked overnight in 2.5% nonfat milk in TBS-T (100 mM Tris-HCl [pH 7.5], 150 mM NaCl, and 0.1% [vol/vol] Tween 20). The membranes were incubated with either the G4L peptide antiserum or MAb HA.11 at a 1:1,000 dilution for 1 h. The blots were washed in TBS-T and incubated with the secondary antibody, either an anti-rabbit or an anti-mouse immunoglobulin (Ig) conjugated to horseradish peroxidase (HRPO; Amersham). Proteins were detected by chemiluminescence (West-Pico; Pierce), and the figures were produced using Adobe Photoshop version 6.
Complementation assays.
BS-C-1 cells were infected with vG4Li at a multiplicity of 5 in the presence or absence of 50 μM IPTG for 1 h. The cells were washed with OptiMEM (Life Technologies/Invitrogen) and transfected with 1 μg of pUC-G4L native plasmids containing the G4L ORF in which one or both of the conserved cysteines was mutated to serine, or pUC19 using Lipofectamine 2000 (Life Technologies/Invitrogen) in the presence or absence of 50 μM IPTG. After 5 h, the medium was removed and replaced with complete EMEM containing 2.5% fetal calf serum with or without IPTG. At 24 h after infection, the cells were harvested, frozen and thawed three times, and sonicated for 30 s, and the virus titers were determined by plaque assay.
Plaque assay and one-step virus growth.
BS-C-1 cell monolayers in six-well tissue culture plates were infected with 10-fold serial dilutions of virus. After 1 h, the inocula were removed, and the monolayers were washed twice with medium and then incubated for 2 days at 37°C with complete EMEM containing 2.5% fetal calf serum and 50 μM IPTG. The monolayers were stained with crystal violet, and the plaques were counted.
RESULTS
Redox states of G4L and O2L glutaredoxins.
The G4L and O2L glutaredoxins contain two and three cysteines, respectively. To determine whether the cysteines were reduced or disulfide bonded (oxidized), intact vaccinia virus-infected cells were treated with TCA, which prevents disulfide interchange (9). The pellets were then dissolved in SDS containing NEM (with a mass of 125 kDa) or AMS (with a mass of 536 kDa), which react with free thiols. Because of its low molecular weight, NEM causes only a small retardation in protein mobility. However, AMS causes a readily detectable >0.5-kDa decrease in mobility for each free cysteine alkylated. Specific antibodies were used to detect G4L and O2L by Western blotting.
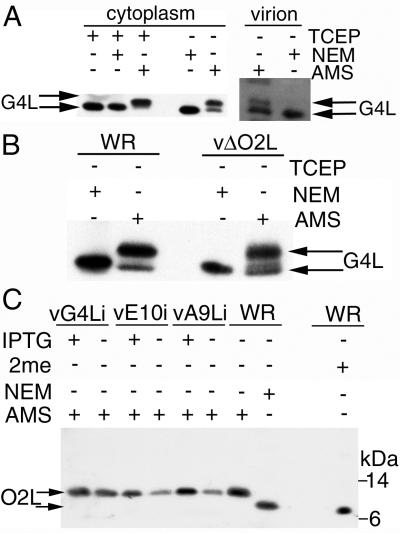
First, we determined the relative mobilities of G4L following complete alkylation with NEM or AMS. Infected cell lysates were either (i) treated with tris-(2-carboxyethyl)phosphine (TCEP) to reduce the disulfide bonds, (ii) reduced with TCEP and then alkylated with NEM, or (iii) reduced with TCEP and alkylated with AMS. G4L that had been reduced or reduced and treated with NEM migrated with almost the same mobility (Fig. 1A). Alkylation of reduced G4L protein with AMS, however, caused a significant retardation in migration (Fig. 1A). When the cell lysates were directly treated with AMS, omitting the reduction step, two bands were detected: the faster one represented G4L which was disulfide bonded and hence not alkylated, whereas the slower one represented G4L with free thiols that were alkylated (Fig. 1A). Accordingly, cytoplasmic G4L existed in reduced and oxidized forms. The predominance of the alkylated form indicated that the majority of cytoplasmic G4L was reduced under these conditions. Only a single G4L band was detected when the cell lysate was treated with NEM because of the inability to resolve the nonalkylated and alkylated species (Fig. 1A). A similar analysis of G4L from sucrose gradient-purified virions indicated that the cysteines were mostly nonreactive with AMS, indicating that they were disulfide bonded (Fig 1A). However, disulfide bonding could have occurred after cell lysis during virion purification.
FIG. 1.
Redox status of G4L and O2L. (A) BS-C-1 cells were infected at a multiplicity of 5 with vaccinia virus WR. After 24 h, the cells were TCA precipitated, and the pelleted proteins were solubilized with SDS, treated or not with TCEP, and alkylated with NEM or AMS. Proteins were resolved by electrophoresis in a 10 to 20% polyacrylamide gradient Tricine gel and detected by Western blotting with G4L-specific rabbit antiserum, followed by an anti-rabbit MAb conjugated to peroxidase. Sucrose gradient-purified virions (approximately 108 PFU) were solubilized in 50 mM Tris-HCl (pH 8.0)-0.1% SDS containing NEM or AMS and analyzed as above. The upper arrow points to G4L alkylated with AMS; the lower arrow points to reduced G4L or G4L alkylated with NEM. (B) BS-C-1 cells were infected and the proteins were alkylated as in panel A. Proteins were resolved on a 12% polyacrylamide NuPAGE gel with MOPS buffer and detected as in panel A. The upper arrow points to G4L alkylated with AMS; the lower arrow points to G4L alkylated with NEM. (C) BS-C-1 cells were infected with standard vaccinia virus (WR), vA9Li, vE10Ri, or vG4Li in the presence (+) or absence (−) of IPTG. After 24 h, the cells were TCA precipitated and the solubilized proteins were alkylated with NEM or AMS or reduced with β-mercaptoethanol (2me). Proteins were resolved as in panel B, and O2L was detected with a rabbit antiserum and an anti-rabbit HRPO-conjugated secondary antibody. The upper arrow points to the O2L protein alkylated with AMS; the lower arrow points to reduced O2L or O2L alkylated with NEM. The positions of 14- and 6-kDa protein markers are indicated on the right.
AMS could also be used to retard the mobility of reduced O2L protein (Fig. 1C). The cellular form of the O2L protein, in contrast to G4L, was fully reduced, since only a slow-migrating alkylated form was detected after AMS treatment (Fig. 1C). Because O2L was fully reduced, we considered that it might regulate the redox state of G4L. However, G4L was still alkylated with AMS and hence predominantly reduced in cells infected with the O2L deletion mutant vΔO2L (Fig. 1B). These data demonstrated that both reduced and disulfide-bonded forms of G4L exist in the cytoplasm and that O2L was not required to maintain G4L in a reduced state.
Using the inducible conditional lethal mutant vG4Li (23) described in Materials and Methods, we found that repression of G4L expression did not influence the oxidation state of O2L (Fig. 1C). Repression of A9L, a protein that is necessary for virion assembly (25), also did not affect O2L (Fig. 1C) or G4L (not shown). Furthermore, repression of the E10R redox protein, which was previously shown to prevent oxidation of G4L (21), had no effect on O2L. In addition, the absence of O2L did not influence the redox state of E10R protein (data not shown). Thus, the O2L glutaredoxin is not a component of a redox pathway involving E10R and G4L.
G4L is required to form disulfide bonds in the L1R and F9L proteins.
We previously reported that expression of the E10R protein was required for disulfide bonds in the L1R membrane protein and the structurally related F9L protein, as well as the G4L glutaredoxin (21). It remained to be determined, however, whether G4L expression was needed for oxidation of L1R or F9L.
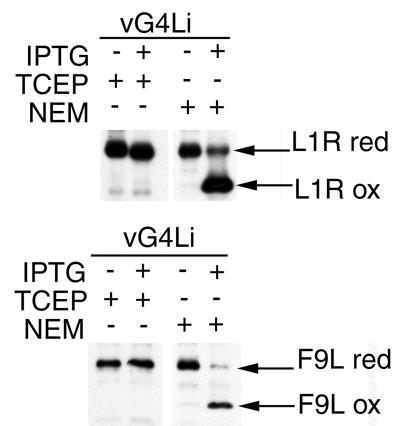
The L1R protein contains six cysteines forming three intramolecular disulfide bonds, which evidently give it a compact structure, as this form has a markedly faster electrophoretic mobility than the reduced form (21, 24). A similar explanation may account for the faster electrophoretic mobility of the unreduced form of F9L compared to the reduced form (21). To determine if G4L was required for the formation of L1R or F9L disulfide bonds, cells were infected with vG4Li in the presence or absence of inducer and transfected with plasmids containing influenza virus HA epitope-tagged L1R or F9L ORFs regulated by vaccinia virus late promoters. HA-tagged proteins were expressed because of the unavailability of antibodies that reacted with reduced and disulfide-bonded forms of the L1R or F9L proteins. Although AMS treatment was not needed to resolve reduced and disulfide-bonded forms of the L1R and F9L proteins, TCA treatment and alkylation with NEM were still used to prevent disulfide interchange.
When G4L expression was induced, both the L1R-HA and F9L-HA proteins migrated rapidly as disulfide-bonded forms, with only faint slow bands representing reduced proteins (Fig. 2). When expression of G4L was repressed by omission of IPTG or when proteins were treated with the reducing agent TCEP, the L1R and F9L proteins migrated entirely in the reduced forms (Fig. 2). Thus, G4L was required to form the intramolecular disulfide bonds of both L1R and F9L proteins. In the absence of the O2L glutaredoxin, however, L1R protein was disulfide bonded (data not shown) confirming that O2L has no role in the redox cascade.
FIG. 2.
Effect of G4L repression on formation of disulfide bonds in L1R and F9L proteins. BS-C-1 cells were infected with vG4Li in the presence (+) or absence (−) of inducer. Cells were transfected with plasmids containing C-terminal HA epitope-tagged copies of the L1R or F9L ORF regulated by late vaccinia virus promoters. After 24 h, the cells were TCA precipitated and the proteins were solubilized in SDS and alkylated with NEM or reduced with TCEP. The proteins were resolved on 10 to 20% polyacrylamide gradient Tris-glycine gels. L1R and F9L were detected by Western blotting using MAb HA.11 and an anti-mouse Ig HRPO-conjugated secondary antibody. The reduced (red) and oxidized (ox) forms of L1R and F9L are indicated.
G4L expression was not required for E10R disulfide bond formation.
The E10R protein contains two cysteines that can form an intramolecular disulfide bond and one that remains as a free thiol (21). Under conditions of natural infection, the E10R protein is mostly disulfide bonded, but significant amounts of the reduced form are detected when the E10R protein is overexpressed (21). To assess if G4L influenced the redox state of E10R, cells were infected with vG4Li in the absence of inducer or with wild-type vaccinia virus (strain WR) and transfected with a plasmid containing the E10R ORF with a C-terminal HA epitope tag. Proteins were modified with NEM or AMS, resolved by SDS-PAGE, and detected by Western blotting.
Because alkylation with NEM does not significantly alter the size of the E10R protein, only a single band was resolved from cells infected with WR (Fig. 3A) or vG4Li (not shown). After AMS treatment, however, two more slowly migrating bands were detected (Fig. 3A). The lower band, containing E10R with only one AMS residue, was derived from disulfide-bonded E10R; the upper band, containing E10R with three AMS residues, was derived from reduced E10R. Importantly, both bands were detected regardless of whether G4L was repressed or not (Fig. 3B). Thus, E10R was required for oxidation of G4L, but G4L was not required for the oxidation of E10R, suggesting a directed pathway of disulfide bond formation.
FIG. 3.
Effect of G4L repression on the formation of disulfide bonds in E10R. (A) BS-C-1 cells were infected with standard vaccinia virus (WR) and transfected with a plasmid containing the E10R gene with a C-terminal HA epitope tag under the control of the vaccinia virus P11 promoter. At 24 h after infection, cells were lysed directly in 10 mM Tris-HCl (pH 7.5)-10 mM KCl-0.5 mM EDTA-1 mM CaCl2-0.2% (vol/vol) NP-40-40 mM NEM or AMS-1 μg of micrococcal nuclease per μl. Cells were allowed to lyse on ice for 15 min, and an equal volume of nonreducing loading buffer was added. Proteins were resolved by electrophoresis on a 16% polyacrylamide-Tricine gel and detected by Western blotting with an anti-HA MAb and anti-mouse Ig HRPO-conjugated secondary antibody. Arrows point to E10R proteins alkylated with three residues of AMS (upper), one residue of AMS (middle), or NEM (lower). (B) BS-C-1 cells were infected with standard vaccinia virus (WR) or vG4Li in the absence of IPTG and transfected with a plasmid containing the E10R gene as described for panel A. Proteins were alkylated and detected as described for panel A. The bands corresponding to modification with three AMS residues (upper) and one AMS residue (lower) are indicated.
Effect of G4L cysteine-to-serine mutations on formation of L1R and F9L disulfide bonds.
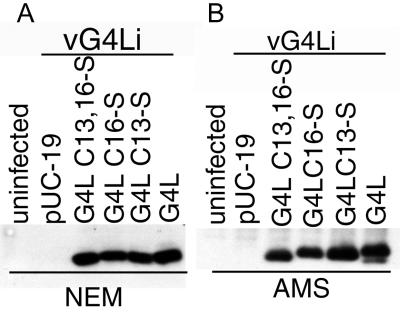
Plasmids were constructed containing the G4L ORF, regulated by its natural promoter, with one or both cysteines of the conserved CXXC motif mutated to serine. Cells were infected with vG4Li in the absence of inducer, which prevented transcription of the endogenous G4L ORF, and transfected with the plasmids. After 24 h, the cells were treated with TCA, and the precipitated proteins were solubilized with SDS in the presence of NEM or AMS. Wild-type and mutated forms of the G4L protein were expressed at similar levels. After treatment with NEM, the two species with one cysteine migrated at similar positions; the wild-type protein migrated slightly slower, and the one with no cysteines slightly faster (Fig. 4A). These differences were more apparent when AMS was used for alkylation (Fig. 4B). Moreover, with AMS it was possible to detect alkylated and nonalkylated species of wild-type G4L protein. In cells transfected with the Cys16Ser form, a band of approximately 28 kDa that may represent a G4L protein dimer or a “trapped” mixed disulfide of G4L with an unidentified protein was also seen (not shown).
FIG. 4.
Expression of G4L with cysteine-to-serine mutations. BS-C-1 cells were infected with vG4Li in the absence of inducer and transfected with the pUC19 vector plasmid or plasmids containing an unmutated or mutated G4L ORF under its natural promoter. The mutations consisted of Cys13Ser (C13-S), Cys16Ser (C16-S), or Cys13,16Ser (C13,16-S). At 24 h after infection, the cells were treated with TCA and the proteins were solubilized with SDS and NEM (A) or AMS (B), resolved on a 10 to 20% polyacrylamide gradient Tricine gel, and detected by Western blotting with G4L rabbit antiserum and an anti-rabbit Ig HRPO-conjugated secondary antibody.
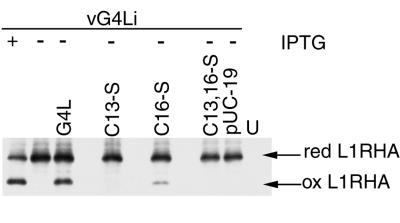
Having demonstrated similar expression of wild-type and mutated forms of G4L by transfection, further experiments were carried out to determine whether G4L proteins lacking one or both cysteines retained function. As a control, cells were infected with vG4Li in the presence or absence of IPTG and transfected with a plasmid containing an HA epitope-tagged L1R ORF. The cells were TCA precipitated, treated with NEM, and analyzed by SDS-PAGE and Western blotting. In the presence of IPTG, both the reduced and disulfide-bonded forms of L1R were detected (Fig. 5). In the absence of IPTG, only the reduced form of the L1R protein was seen.
FIG. 5.
Effect of G4L cysteine-to-serine mutations on the formation of disulfide bonds in the L1R protein. BS-C-1 cells were uninfected (U) or infected with vG4Li in the presence (+) or absence (−) of IPTG and cotransfected with plasmids containing HA-tagged L1R and either pUC19, wild-type G4L, or mutated G4L as described in the legend to Fig. 4. At 24 h after infection, proteins were TCA precipitated, solubilized with SDS, alkylated with NEM, and resolved by electrophoresis on a Tris-glycine-10 to 20% polyacrylamide gradient gel. Proteins were detected by Western blotting with MAb HA.11. The reduced (red) and oxidized (ox) forms of L1R are indicated.
To ascertain the importance of the cysteines, additional cells were infected with vG4Li in the absence of IPTG and transfected with a plasmid containing the epitope-tagged L1R ORF and either the pUC19 vector plasmid or a plasmid with the unmutated or cysteine-to-serine-mutated G4L ORF. Only the reduced form of L1R was detected when pUC19 or the G4L plasmid with the mutations Cys13Ser or Cys13,16Ser was transfected (Fig. 5). Oxidized L1R was detected when the plasmid containing the Cys16Ser mutation was transfected, but this amount was much less than occurred after transfection of the unmutated ORF.
The ability of the mutated G4L proteins to induce F9L HA disulfide bonds was also tested. The oxidized form of F9L HA was detected in the permissive infection or when the unmutated G4L ORF was transfected into a nonpermissive infection, but only the reduced form of F9L was found when either the Cys13Ser, Cys16Ser, or the Cys13,16Ser forms of G4L were expressed (data not shown). Similar results for L1R and F9L disulfide bonds were obtained when G4L expression was controlled by the strong P11 promoter (data not shown). Together these data indicate that both cysteines are required for efficient disulfide bond formation in F9L and L1R.
Effect of G4L cysteine-to-serine mutations on complementation of virus infectivity.
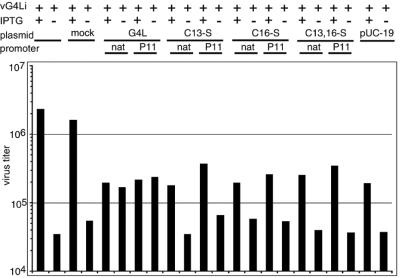
Previously, we showed that a nonpermissive infection of vG4Li in the absence of IPTG could be complemented by transfection of an expression plasmid containing an intact G4L ORF (23). We now wanted to determine whether the two cysteine residues of G4L were necessary for this function. To test this, cells were infected with vG4Li in the presence or absence of IPTG and transfected with the empty plasmid vector (pUC19) or a plasmid containing the wild-type or mutated G4L ORF under the native or strong P11 viral promoter. We found that Lipofectamine 2000 alone had no effect on virus titers, but addition of either plasmid DNA (pUC19) or plasmids containing the G4L ORF considerably reduced the virus yield in the presence of IPTG. Nevertheless, transfection of the intact G4L ORF in the absence of IPTG increased the virus titer to nearly the value obtained with IPTG (Fig. 6). However, this was not the case upon transfection of plasmids containing the G4L ORF with any of the cysteine-to-serine mutations or the empty vector alone (Fig. 6). The inability of G4L with cysteine-to-serine mutations to rescue the nonpermissive infection correlated with the inability of the mutated protein to induce disulfide bonds in L1R or F9L.
FIG. 6.
trans-Complementation of virus infectivity. BS-C-1 cells were infected with vG4Li in the presence (+) or absence (−) of 50 μM IPTG and mock transfected with Lipofectamine 2000 alone (mock) or with pUC19 vector plasmid or plasmids containing the wild-type or mutated G4L ORF under its natural (nat) or the P11 promoter as described in the legend to Fig. 4. At 24 h after infection, the cells were harvested and the virus titers were determined by plaque assay in the presence of 50 μM IPTG.
DISCUSSION
Further evidence that vaccinia virus encodes proteins that comprise a novel redox pathway was obtained in this study. Previously, we reported that E10R, a member of the ERV1/ALR family, was required for the formation of intramolecular disulfide bonds in the G4L glutaredoxin, the L1R viral membrane protein, and F9L, a protein structurally related to L1R (21). Our present focus was on the role of the G4L glutaredoxin. G4L is one of two functional glutaredoxins encoded by vaccinia virus. G4L has orthologs in all sequenced poxviruses and is essential for vaccinia virus replication; the other glutaredoxin, O2L, is present only in orthopoxviruses and is nonessential for vaccinia virus (15, 23). It has been suggested that O2L has a role in DNA replication by serving as a cofactor for ribonucleotide reductase (15), though it has a late promoter (1). In the current study, we recovered both reduced and oxidized forms of G4L from vaccinia virus-infected cells, whereas only the reduced form of O2L was detected. In addition, the redox states of G4L and O2L were unaffected by the absence of the other.
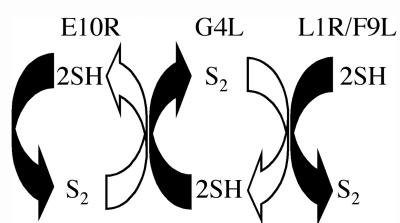
Two findings suggested that G4L might function in cooperation with E10R. First, when expression of either protein was repressed, virus assembly was interrupted at a similar immature stage (20, 23). Second, G4L was not disulfide bonded when E10R was repressed (21). Our current findings that repression of G4L prevented the formation of disulfide bonds in L1R and F9L but not E10R are compatible with a redox pathway in which the glutaredoxin occupies an intermediate position (Fig. 7). Whether one or more additional intermediate redox proteins function between E10R and G4L or between G4L and L1R or F9L remains to be determined. However, vaccinia virus does not encode detectable homologs of other thiol oxidoreductases. We suspect that E10R is linked to flavin adenine dinucleotide, which provides electron transfer, as this cofactor was recently found associated with some other members of the ERV1/ALR family (6, 11, 12). Disulfide bond formation in the endoplasmic reticulum of Saccharomyces cerevisiae, which is catalyzed by the Ero1p and protein disulfide isomerase, also depends on flavin adenine dinucleotide (5, 22). The DsbA-DsbB de novo disulfide bond pathway in the E. coli periplasm, however, depends on ubiquinone and cytochromes (2).
FIG. 7.
Relationship of E10R, G4L, L1R, and F9L proteins in a redox pathway. E10RSS reacts directly or indirectly with G4L2SH, resulting in the transfer of a disulfide bond. Three molecules of G4LSS then interact directly or indirectly through another redox protein with 1 molecule of L1R6SH or F9L6SH to form L1R3S-S or F9L3S-S. Arrows indicating oxidation are solid, and arrows indicating reduction are open.
Previously, we found that both cysteine residues of the E10R protein were required for redox function (21), and the present mutagenesis studies of G4L also showed that both cysteine residues were required for disulfide bond formation in L1R and F9L and for rescue of infectivity when endogenous G4L expression was repressed. We are trying to determine whether G4L with one of the two cysteines mutated will form stable covalent intermediate with other redox proteins, allowing us to confirm and extend the redox pathway depicted in Fig. 7.
REFERENCES
- 1.Ahn, B. Y., and B. Moss. 1992. Glutaredoxin homolog encoded by vaccinia virus is a virion-associated enzyme with thioltransferase and dehydroascorbate reductase activities. Proc. Natl. Acad. Sci. USA 89:7060–7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bader, M., W. Muse, D. P. Ballou, C. Gassner, and J. C. Bardwell. 1999. Oxidative protein folding is driven by the electron transport system. Cell 98:217–227. [DOI] [PubMed] [Google Scholar]
- 3.Earl, P. L., Cooper, N., and B. Moss. 1991. Preparation of cell cultures and vaccinia virus stocks, p.16.16.1–16.16.7. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. Greene Publishing Associates and Wiley International Science, New York, N.Y.
- 4.Earl, P. L., N. Cooper, and B. Moss. 1991. Generation of recombinant vaccinia viruses, p.16.17.1–16.17.16. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. Greene Publishing Associates and Wiley International Science, New York, N.Y.
- 5.Frand, A. R., J. W. Cuozzo, and C. A. Kaiser. 2000. Pathways for protein disulphide bond formation. Trends Cell Biol. 10:203–210. [DOI] [PubMed] [Google Scholar]
- 6.Gerber, J., U. Muhlenhoff, G. Hofhaus, R. Lill, and T. Lisowsky. 2001. Yeast ERV2p is the first microsomal FAD-linked sulfhydryl oxidase of the Erv1p/Alrp protein family. J. Biol. Chem. 276:23486–23491. [DOI] [PubMed] [Google Scholar]
- 7.Gvakharia, B. O., E. K. Koonin, and C. K. Mathews. 1996. Vaccinia virus G4L gene encodes a second glutaredoxin. Virology 226:408–411. [DOI] [PubMed] [Google Scholar]
- 8.Ichihashi, Y. 1981. Unit complex of vaccinia polypeptides linked by disulfide bridges. Virology 113:277–284. [DOI] [PubMed] [Google Scholar]
- 9.Kishigami, S., Y. Akiyama, and K. Ito. 1995. Redox states of DsbA in the periplasm of Escherichia coli. FEBS Lett. 364:55–58. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi, T., S. Kishigami, M. Sone, H. Inokuchi, T. Mogi, and K. Ito. 1997. Respiratory chain is required to maintain oxidized states of the DsbA-DsbB disulfide bond formation system in aerobically growing Escherichia coli cells. Proc. Natl. Acad. Sci. USA 94:11857–11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, J., G. Hofhaus, and T. Lisowsky. 2000. Erv1p from Saccharomyces cerevisiae is a FAD-linked sulfhydryl oxidase. FEBS Lett. 477:62–66. [DOI] [PubMed] [Google Scholar]
- 12.Lisowsky, T., J. E. Lee, L. Polimeno, A. Francavilla, and G. Hofhaus. 2001. Mammalian augmenter of liver regeneration protein is a sulfhydryl oxidase. Dig. Liver Dis. 33:173–180. [DOI] [PubMed] [Google Scholar]
- 13.Locker, J. K., and G. Griffiths. 1999. An unconventional role for cytoplasmic disulfide bonds in vaccinia virus proteins. J. Cell Biol. 144:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moss, B. 2001. Poxviridae: the viruses and their replication, p.2849–2883. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 15.Rajagopal, I., B. Y. Ahn, B. Moss, and C. K. Mathews. 1995. Roles of vaccinia virus ribonucleotide reductase and glutaredoxin in DNA precursor biosynthesis. J. Biol. Chem. 270:27415–27418. [DOI] [PubMed] [Google Scholar]
- 16.Ravanello, M. P., and D. E. Hruby. 1994. Characterization of the vaccinia virus L1R myristylprotein as a component of the intracellular virion envelope. J. Gen. Virol. 75:1479–1483. [DOI] [PubMed] [Google Scholar]
- 17.Ravanello, M. P., and D. E. Hruby. 1994. Conditional lethal expression of the vaccinia virus L1R myristylated protein reveals a role in virion assembly. J. Virol. 68:6401–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rietsch, A., and J. Beckwith. 1998. The genetics of disulfide bond metabolism. Annu. Rev. Genet. 32:163–184. [DOI] [PubMed] [Google Scholar]
- 19.Senkevich, T. G., E. V. Koonin, J. J. Bugert, G. Darai, and B. Moss. 1997. The genome of molluscum contagiosum virus: analysis and comparison with other poxviruses. Virology 233:19–42. [DOI] [PubMed] [Google Scholar]
- 20.Senkevich, T. G., A. S. Weisberg, and B. Moss. 2000. Vaccinia virus E10R protein is associated with the membranes of intracellular mature virions and has a role in morphogenesis. Virology 278:244–252. [DOI] [PubMed] [Google Scholar]
- 21.Senkevich, T. G., C. L. White, E. V. Koonin, and B. Moss. 2000. A viral member of the ERV1/ALR protein family participates in a cytoplasmic pathway of disulfide bond formation. Proc. Natl. Acad. Sci. USA 97:12068–12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tu, B. P., S. C. Ho-Schleyer, K. J. Travers, and J. S. Weissman. 2000. Biochemical basis of oxidative protein folding in the endoplasmic reticulum. Science 290:1571–1574. [DOI] [PubMed] [Google Scholar]
- 23.White, C. L., A. S. Weisberg, and B. Moss. 2000. A glutaredoxin, encoded by the G4L gene of vaccinia virus, is essential for virion morphogenesis. J. Virol. 74:9175–9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolffe, E. J., S. Vijaya, and B. Moss. 1995. A myristylated membrane protein encoded by the vaccinia virus L1R open reading frame is the target of potent neutralizing monoclonal antibodies. Virology 211:53–63. [DOI] [PubMed] [Google Scholar]
- 25.Yeh, W. W., B. Moss, and E. J. Wolffe. 2000. The vaccinia virus A9L gene encodes a membrane protein required for an early step in virion morphogenesis. J. Virol. 74:9701–9711. [DOI] [PMC free article] [PubMed] [Google Scholar]