Abstract
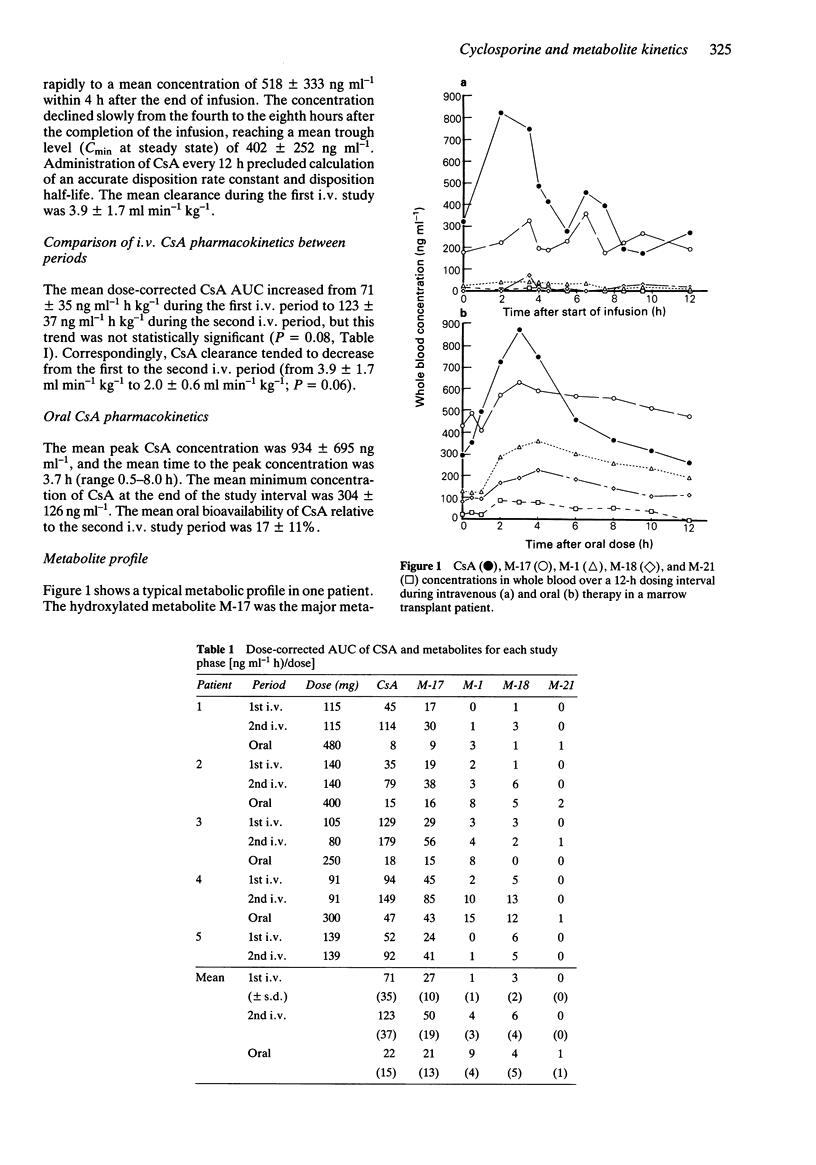
1. The pharmacokinetics of cyclosporine (CsA) and the time course of CsA metabolites were studied in five bone marrow transplant patients after intravenous (i.v.) administration on two separate occasions and once after oral CsA administration. 2. Cyclosporine and cyclosporine metabolites were measured in whole blood by h.p.l.c. 3. Cyclosporine clearance after i.v. administration decreased from 3.9 +/- 1.7 ml min-1 kg-1 to 2.0 +/- 0.6 ml min-1 kg-1 after 14 days of treatment. The mean +/- s.d. absolute oral bioavailability of cyclosporine was 17 +/- 11%. 4. Hydroxylated CsA (M-17) was the major metabolite in blood. There were no significant differences in the mean metabolite/CsA AUC ratios between the first and second i.v. studies. 5. After oral administration, the metabolite to CsA AUC ratios were higher for most metabolites compared to those observed in the second i.v. study, suggesting a contribution of intestinal metabolism to the clearance of CsA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson K., Biggs J. C., Britton K., Short R., Mrongovius R., Concannon A., Dodds A. Oral administration of cyclosporin A for recipients of allogeneic marrow transplants: implications of clinical gut dysfunction. Br J Haematol. 1984 Feb;56(2):223–231. doi: 10.1111/j.1365-2141.1984.tb03950.x. [DOI] [PubMed] [Google Scholar]
- Augustine J. A., Zemaitis M. A. The effects of cyclosporin A (CsA) on hepatic microsomal drug metabolism in the rat. Drug Metab Dispos. 1986 Jan-Feb;14(1):73–78. [PubMed] [Google Scholar]
- Awni W. M., Kasiske B. L., Heim-Duthoy K., Rao K. V. Long-term cyclosporine pharmacokinetic changes in renal transplant recipients: effects of binding and metabolism. Clin Pharmacol Ther. 1989 Jan;45(1):41–48. doi: 10.1038/clpt.1989.7. [DOI] [PubMed] [Google Scholar]
- Burckart G. J., Starzl T. E., Venkataramanan R., Hashim H., Wong L., Wang P., Makowka L., Zeevi A., Ptachcinski R. J., Knapp J. E. Excretion of cyclosporine and its metabolites in human bile. Transplant Proc. 1986 Dec;18(6 Suppl 5):46–49. [PMC free article] [PubMed] [Google Scholar]
- Cole E., Cheung F., Wong P. Y., Fung L. S., Skorecki K., Levy G. A. Toxic effects on renal cells in culture--a comparison of cyclosporin A and its metabolites. Transplant Proc. 1989 Feb;21(1 Pt 1):943–945. [PubMed] [Google Scholar]
- Freed B. M., Rosano T. G., Lempert N. In vitro immunosuppressive properties of cyclosporine metabolites. Transplantation. 1987 Jan;43(1):123–127. doi: 10.1097/00007890-198701000-00027. [DOI] [PubMed] [Google Scholar]
- Keown P. A., Laupacis A., Carruthers G., Stawecki M., Koegler J., McKenzie F. N., Wall W., Stiller C. R. Interaction between phenytoin and cyclosporine following organ transplantation. Transplantation. 1984 Sep;38(3):304–306. [PubMed] [Google Scholar]
- Maurer G., Lemaire M. Biotransformation and distribution in blood of cyclosporine and its metabolites. Transplant Proc. 1986 Dec;18(6 Suppl 5):25–34. [PubMed] [Google Scholar]
- Maurer G. Metabolism of cyclosporine. Transplant Proc. 1985 Aug;17(4 Suppl 1):19–26. [PubMed] [Google Scholar]
- Pang K. S. Metabolite pharmacokinetics: the area under the curve of metabolite and the fractional rate of metabolism of a drug after different routes of administration for renally and hepatically cleared drugs and metabolites. J Pharmacokinet Biopharm. 1981 Aug;9(4):477–487. doi: 10.1007/BF01060890. [DOI] [PubMed] [Google Scholar]
- Ptachcinski R. J., Venkataramanan R., Burckart G. J. Clinical pharmacokinetics of cyclosporin. Clin Pharmacokinet. 1986 Mar-Apr;11(2):107–132. doi: 10.2165/00003088-198611020-00002. [DOI] [PubMed] [Google Scholar]
- Rosano T. G., Freed B. M., Cerilli J., Lempert N. Immunosuppressive metabolites of cyclosporine in the blood of renal allograft recipients. Transplantation. 1986 Sep;42(3):262–267. doi: 10.1097/00007890-198609000-00007. [DOI] [PubMed] [Google Scholar]
- Rosano T. G., Freed B. M., Pell M. A., Lempert N. Cyclosporine metabolites in human blood and renal tissue. Transplant Proc. 1986 Dec;18(6 Suppl 5):35–40. [PubMed] [Google Scholar]
- Rosenfeld C., Shadduck R. K., Przepiorka D., Mangan K. F., Colvin M. Autologous bone marrow transplantation with 4-hydroperoxycyclophosphamide purged marrows for acute nonlymphocytic leukemia in late remission or early relapse. Blood. 1989 Aug 15;74(3):1159–1164. [PubMed] [Google Scholar]
- Venkataramanan R., Habucky K., Burckart G. J., Ptachcinski R. J. Clinical pharmacokinetics in organ transplant patients. Clin Pharmacokinet. 1989 Mar;16(3):134–161. doi: 10.2165/00003088-198916030-00002. [DOI] [PubMed] [Google Scholar]
- Wallemacq P. E., Lhoëst G., Latinne D., De Bruyère M. Isolation, characterization and in vitro activity of human cyclosporin A metabolites. Transplant Proc. 1989 Feb;21(1 Pt 1):906–910. [PubMed] [Google Scholar]
- Wang C. P., Burckart G. J., Ptachcinski R. J., Venkataramanan R., Schwinghammer T., Hakala T., Griffith B., Hardesty R., Shadduck R., Knapp J. Cyclosporine metabolite concentrations in the blood of liver, heart, kidney, and bone marrow transplant patients. Transplant Proc. 1988 Apr;20(2 Suppl 2):591–596. [PMC free article] [PubMed] [Google Scholar]
- Zeevi A., Eiras G., Burckart G., Makowka L., Venkataramanan R., Wang C. P., Van Thiel D. H., Murase N., Starzl T. E., Duquesnoy R. Immunosuppressive effect of cyclosporine metabolites from human bile on alloreactive T cells. Transplant Proc. 1988 Apr;20(2 Suppl 2):115–121. [PMC free article] [PubMed] [Google Scholar]
- Zeevi A., Venkataramanan R., Burckart G., Wang C. P., Murase N., Van Thiel D. H., Starzl T. E., Makowka L., Duquesnoy R. J. Sensitivity of activated human lymphocytes to cyclosporine and its metabolites. Hum Immunol. 1988 Feb;21(2):143–153. doi: 10.1016/0198-8859(88)90089-4. [DOI] [PubMed] [Google Scholar]
- van der Heyden A. A., van Oers M. H., Cornelissen P., Yong S. L., Wilmink J. M., Schellekens P. T. The influence of cyclosporine A treatment on immune responsiveness in vitro and in vivo in kidney transplant recipients. Transplant Proc. 1988 Apr;20(2 Suppl 2):190–195. [PMC free article] [PubMed] [Google Scholar]


