Abstract
1. Seven patients clinically diagnosed as being hypersensitive to carbamazepine and one patient hypersensitive to both carbamazepine and oxcarbazepine have been identified. They have been compared with a control group (hereafter referred to as 'control subjects') comprising five patients on chronic carbamazepine therapy without adverse effects and 12 healthy volunteers who have never been exposed to anticonvulsants. 2. An in vitro cytotoxicity assay employing mononuclear leucocytes as target cells has been used first, to determine the ability of 10 different human livers to bioactivate carbamazepine to a cytotoxic metabolite, and secondly, to compare the cell defences of carbamazepine-hypersensitive patients and control subjects to oxidative drug metabolites generated by a murine microsomal system, using a blinded protocol. 3. With human liver microsomes, the metabolism-dependent cytotoxicity of carbamazepine increased with increasing microsomal protein concentration. At a protein concentration of 2 mg per incubation, the cytotoxicity of carbamazepine with human liver microsomes (n = 10 livers) increased from 7.2 +/- 0.8% (baseline) to 16.4 +/- 2.1% (with NADPH; P = 0.002). 4. In the presence of phenobarbitone-induced mouse microsomes and NADPH, the mean increase in cytotoxicity above the baseline with carbamazepine was significantly greater (P less than 0.001) for the cells from the carbamazepine-hypersensitive patients (7.9 +/- 0.8%) than from control subjects (2.6 +/- 0.3%). 5. In the presence of phenobarbitone-induced mouse microsomes and NADPH, there was no significant difference in cytotoxicity between the cells from carbamazepine hypersensitive patients and from control subjects in the presence of either phenytoin or oxcarbazepine.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

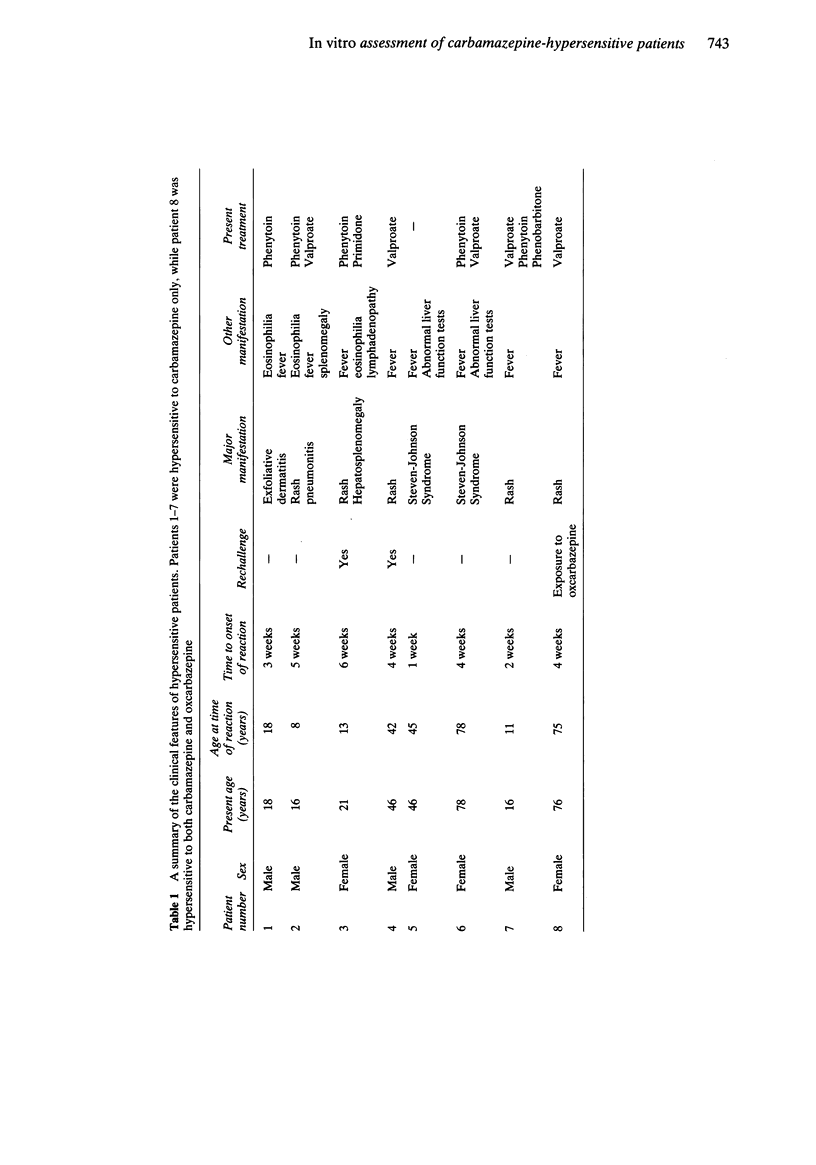


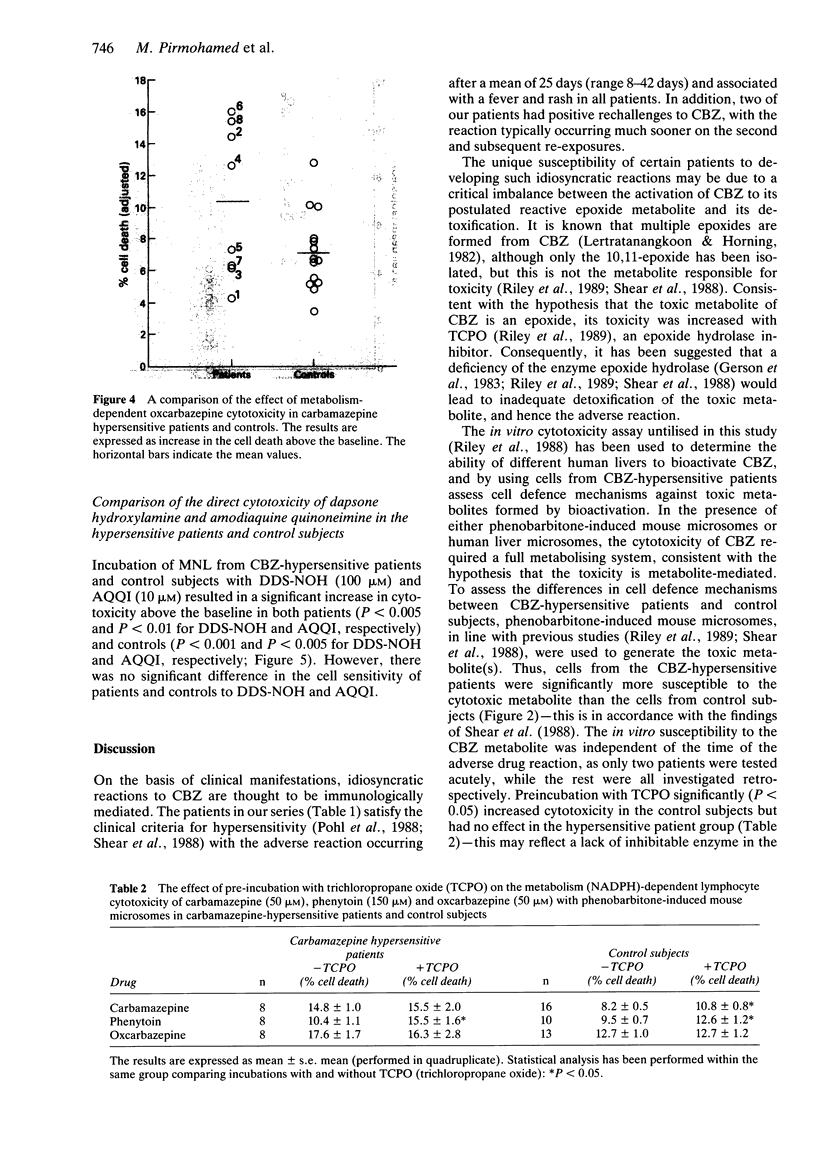
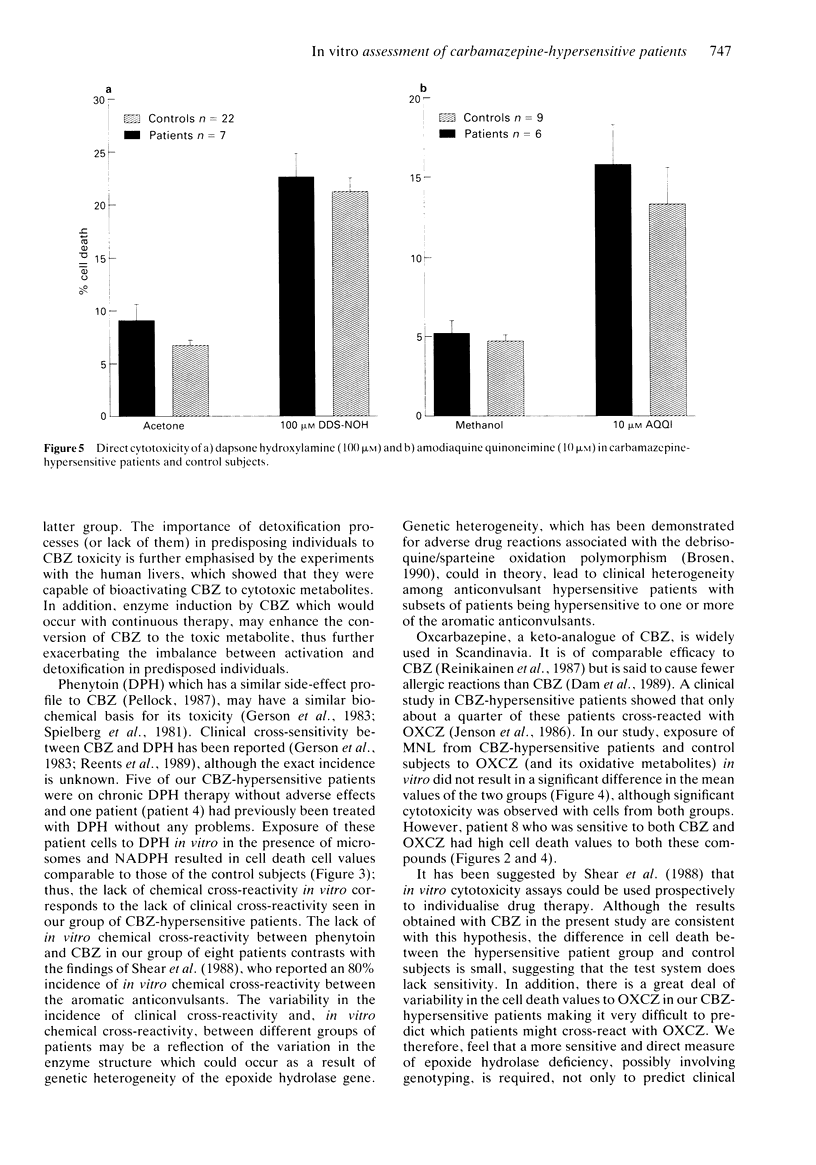


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brosen K. Recent developments in hepatic drug oxidation. Implications for clinical pharmacokinetics. Clin Pharmacokinet. 1990 Mar;18(3):220–239. doi: 10.2165/00003088-199018030-00004. [DOI] [PubMed] [Google Scholar]
- Chadwick D., Shaw M. D., Foy P., Rawlins M. D., Turnbull D. M. Serum anticonvulsant concentrations and the risk of drug induced skin eruptions. J Neurol Neurosurg Psychiatry. 1984 Jun;47(6):642–644. doi: 10.1136/jnnp.47.6.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. D., Breckenridge A. M., Park B. K. Bioactivation of dapsone to a cytotoxic metabolite by human hepatic microsomal enzymes. Br J Clin Pharmacol. 1989 Oct;28(4):389–395. doi: 10.1111/j.1365-2125.1989.tb03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill W. E. Drugs 5 years later: carbamazepine. Ann Intern Med. 1973 Dec;79(6):844–847. doi: 10.7326/0003-4819-79-6-844. [DOI] [PubMed] [Google Scholar]
- Dam M., Ekberg R., Løyning Y., Waltimo O., Jakobsen K. A double-blind study comparing oxcarbazepine and carbamazepine in patients with newly diagnosed, previously untreated epilepsy. Epilepsy Res. 1989 Jan-Feb;3(1):70–76. doi: 10.1016/0920-1211(89)90070-3. [DOI] [PubMed] [Google Scholar]
- Farrell G., Prendergast D., Murray M. Halothane hepatitis. Detection of a constitutional susceptibility factor. N Engl J Med. 1985 Nov 21;313(21):1310–1314. doi: 10.1056/NEJM198511213132102. [DOI] [PubMed] [Google Scholar]
- Gerson W. T., Fine D. G., Spielberg S. P., Sensenbrenner L. L. Anticonvulsant-induced aplastic anemia: increased susceptibility to toxic drug metabolites in vitro. Blood. 1983 May;61(5):889–893. [PubMed] [Google Scholar]
- Horowitz S., Patwardhan R., Marcus E. Hepatotoxic reactions associated with carbamazepine therapy. Epilepsia. 1988 Mar-Apr;29(2):149–154. doi: 10.1111/j.1528-1157.1988.tb04411.x. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W. Arene oxides: a new aspect of drug metabolism. Science. 1974 Aug 16;185(4151):573–582. doi: 10.1126/science.185.4151.573. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larrey D., Berson A., Habersetzer F., Tinel M., Castot A., Babany G., Lettéron P., Freneaux E., Loeper J., Dansette P. Genetic predisposition to drug hepatotoxicity: role in hepatitis caused by amineptine, a tricyclic antidepressant. Hepatology. 1989 Aug;10(2):168–173. doi: 10.1002/hep.1840100208. [DOI] [PubMed] [Google Scholar]
- Lertratanangkoon K., Horning M. G. Metabolism of carbamazepine. Drug Metab Dispos. 1982 Jan-Feb;10(1):1–10. [PubMed] [Google Scholar]
- Maggs J. L., Tingle M. D., Kitteringham N. R., Park B. K. Drug-protein conjugates--XIV. Mechanisms of formation of protein-arylating intermediates from amodiaquine, a myelotoxin and hepatotoxin in man. Biochem Pharmacol. 1988 Jan 15;37(2):303–311. doi: 10.1016/0006-2952(88)90733-2. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Park B. K. Metabolic basis of adverse drug reactions. J R Coll Physicians Lond. 1986 Jul;20(3):195–200. [PMC free article] [PubMed] [Google Scholar]
- Pellock J. M. Carbamazepine side effects in children and adults. Epilepsia. 1987;28 (Suppl 3):S64–S70. doi: 10.1111/j.1528-1157.1987.tb05780.x. [DOI] [PubMed] [Google Scholar]
- Pohl L. R., Satoh H., Christ D. D., Kenna J. G. The immunologic and metabolic basis of drug hypersensitivities. Annu Rev Pharmacol Toxicol. 1988;28:367–387. doi: 10.1146/annurev.pa.28.040188.002055. [DOI] [PubMed] [Google Scholar]
- Purba H. S., Maggs J. L., Orme M. L., Back D. J., Park B. K. The metabolism of 17 alpha-ethinyloestradiol by human liver microsomes: formation of catechol and chemically reactive metabolites. Br J Clin Pharmacol. 1987 Apr;23(4):447–453. doi: 10.1111/j.1365-2125.1987.tb03074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reents S. B., Luginbuhl W. E., Davis S. M. Phenytoin-carbamazepine cross-sensitivity. DICP. 1989 Mar;23(3):235–236. doi: 10.1177/106002808902300308. [DOI] [PubMed] [Google Scholar]
- Reinikainen K. J., Keränen T., Halonen T., Komulainen H., Riekkinen P. J. Comparison of oxcarbazepine and carbamazepine: a double-blind study. Epilepsy Res. 1987 Sep;1(5):284–289. doi: 10.1016/0920-1211(87)90003-9. [DOI] [PubMed] [Google Scholar]
- Riley R. J., Kitteringham N. R., Park B. K. Structural requirements for bioactivation of anticonvulsants to cytotoxic metabolites in vitro. Br J Clin Pharmacol. 1989 Oct;28(4):482–487. doi: 10.1111/j.1365-2125.1989.tb03530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R. J., Maggs J. L., Lambert C., Kitteringham N. R., Park B. K. An in vitro study of the microsomal metabolism and cellular toxicity of phenytoin, sorbinil and mianserin. Br J Clin Pharmacol. 1988 Nov;26(5):577–588. doi: 10.1111/j.1365-2125.1988.tb05298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear N. H., Spielberg S. P. Anticonvulsant hypersensitivity syndrome. In vitro assessment of risk. J Clin Invest. 1988 Dec;82(6):1826–1832. doi: 10.1172/JCI113798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg S. P. Acetaminophen toxicity in human lymphocytes in vitro. J Pharmacol Exp Ther. 1980 May;213(2):395–398. [PubMed] [Google Scholar]
- Spielberg S. P., Gordon G. B., Blake D. A., Goldstein D. A., Herlong H. F. Predisposition to phenytoin hepatotoxicity assessed in vitro. N Engl J Med. 1981 Sep 24;305(13):722–727. doi: 10.1056/NEJM198109243051302. [DOI] [PubMed] [Google Scholar]
- Spielberg S. P. In vitro assessment of pharmacogenetic susceptibility to toxic drug metabolites in humans. Fed Proc. 1984 May 15;43(8):2308–2313. [PubMed] [Google Scholar]
- Stephan W. C., Parks R. D., Tempest B. Acute hypersensitivity pneumonitis associated with carbamazepine therapy. Chest. 1978 Oct;74(4):463–464. doi: 10.1378/chest.74.4.463. [DOI] [PubMed] [Google Scholar]
- Uetrecht J., Zahid N., Shear N. H., Biggar W. D. Metabolism of dapsone to a hydroxylamine by human neutrophils and mononuclear cells. J Pharmacol Exp Ther. 1988 Apr;245(1):274–279. [PubMed] [Google Scholar]


