Abstract
The effect of the human papillomavirus type 16 (HPV-16) E5 protein on apoptosis was investigated by using the polyclonal HaCaT-cell lines stably transfected either with E5 (HaCaT/E5) or the empty vector (HaCaT/pMSG) as reference. Apoptosis was triggered either by Fas ligand (FasL) or by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and was monitored by detection of cleavage of procaspase-8 and procaspase-3, as well as their substrate poly(ADP-ribose) polymerase (PARP). In contrast to the HaCaT/pMSG control cells we found that apoptosis induced by either of the two ligands is strongly suppressed in the E5-expressing keratinocytes. Fas expression is reduced by about a factor of two in HaCaT/E5 cells, which could be part of the mechanisms that protect the cells from FasL-induced apoptosis. For the TRAIL receptors, no such downregulation was observed. Here, E5 impairs the formation of the death-inducing signaling complex triggered by TRAIL. Apparently, E5 employs different mechanisms to inhibit death receptor signaling. This effect is not restricted to HaCaT/E5 cells since we found that the mouse fibroblast cell line A31-E5 is protected from TRAIL-induced apoptosis, as well but not the E5-lacking control cells A31-Neo. However, no such protection was observed upon FasL-induced apoptosis. Presumably, some of the antiapoptotic mechanisms employed by E5 of the human pathogenic HPV-16 are cell type specific. We propose that inhibition of ligand-mediated apoptosis in human keratinocytes is a primary function of the HPV-16 E5 protein needed to prevent apoptosis at early stages of viral infection.
Human papillomaviruses (HPV) are small double-stranded DNA viruses that infect basal epithelial cells of cutaneous or mucosal tissues through microlesions (28). A copy number of 50 to 100 viral genomes is established in the infected cell and is maintained in the two daughter cells upon cell division. One of the daughter cells remains in the basal layer, whereas the other one migrates up into the suprabasal strata and starts to differentiate. The viral DNA is amplified to high copy numbers when the host cell reaches the granular layer. Particle assembly takes place in the cornified layer, completing the viral life cycle (29, 30).
Substantial research effort has been devoted to the high-risk types HPV-16 and −18, which infect the genital mucosa and are strongly associated with cervix carcinoma (64). In the case of HPV-16, the genome is organized into six early (E1, E2, E4, E5, E6, and E7) and two late (L1 and L2) open reading frames that code for functional and structural proteins, respectively. While E1 and E2 are essential for replication of the viral genome (16), E6 and E7 are responsible for maintaining the correct environment for DNA replication in the host cell by preventing possible cell cycle arrest and intrinsic, p53-dependent apoptosis (28, 41, 59).
Unlike the other viral proteins, E5 is a membrane protein (83 amino acids) that associates with the Golgi apparatus, the endoplasmic reticulum, and the nuclear membrane, as has been shown for an E5-fusion protein (7). E5 rearranges the actin cytoskeleton, inhibits endocytic trafficking (52), and influences signal transduction pathways, leading to a complex pattern of observed effects. Expression of E5 in human keratinocytes increases activation of the signaling cascade originating from the epidermal growth factor receptor (9, 11, 36, 49) and activates c-jun gene expression via the Ras-dependent pathway (3, 5). In mouse fibroblasts E5 is able to modulate membrane signaling phospholipids (10). Upon nonspecific stress induced by hyperosmolar concentrations of sorbitol, E5-expressing human keratinocytes are sensitized to apoptosis (20). When UV radiation is used for stress, E5-expressing human keratinocytes are protected from apoptosis (61).
The exact function of E5 within the HPV-16 life cycle is unknown and many of the effects described above may be due to the localization of this hydrophobic molecule in cellular membranes. In addition to the major oncoproteins E6 and E7, E5 possesses weak oncogenic properties as demonstrated in assays employing rodent cells (14, 25, 27, 36). E5 allows anchorage-independent growth of human keratinocyte colonies in soft agarose (5), enhances the HPV-16-induced immortalization of human keratinocytes (8), and reduces gap-junction-mediated cell communication (32). Large amounts of E5 mRNA have been found in abnormal cervical smears (1) and cervical intraepithelial neoplasia (low-grade CIN 1), which suggests that E5 plays a role in the first steps of cellular transformation (22, 48).
Normal cell and tissue homeostasis reflects a dynamic balance of cell proliferation, differentiation, and apoptosis. Apoptosis is a program for the elimination of cells initiated by specific biological signals. Two main apoptotic routes have been identified (4, 15, 17). In the extrinsic death receptor pathway, receptors are activated specifically by their cognate ligands, e.g., tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) or FasL. FasL is binding to the Fas (CD95) receptor leading to activation and clustering of the death receptors (42, 43, 58). TRAIL interacts with four cellular and one secreted, soluble receptor called osteoprotegerin (13). Among the four cellular receptors only the TRAIL receptors 1 (DR4, TRAIL-R1) and 2 (DR5, TRAIL-R2) contain intracellular death domains (DDs) to transmit the apoptotic signal (23, 33, 34, 43, 45, 47). In contrast, the TRAIL receptors 3 (TRAIL-R3, DcR1) and 4 (TRAIL-R4, DcR2) are not able to mediate apoptosis due to complete or partial absence of cytoplasmic DDs, respectively (34, 35, 45). Upon binding of the ligand to the receptors, homo- and heterotrimeric structures are formed (19, 43). Trimerization of the activated Fas or TRAIL receptors leads to binding of the adaptor protein FADD (Fas-associated DD) and subsequent recruitment of procaspase-8 (58); this protein complex is called death-inducing signaling complex (DISC). DISC formation provides the necessary environment for activation of procaspase-8 by autocatalytic cleavage (24, 40). The intrinsic mitochondrial pathway is used in response to many nonspecific stimuli, e.g., DNA damage, radiation, and osmotic stress (37), resulting in cytochrome c release from the mitochondrial intermembrane space. Cytochrome c associates with Apaf-1 and then with procaspase-9 to form the apoptosome complex, leading to activation of caspase-9. Both pathways converge at the level of caspase-3 activation.
In malignant cells, these physiological apoptotic pathways are often disturbed, which allows an uncontrolled cell proliferation that leads to a variety of diseases, including cancer (50, 53). Fas and TRAIL receptors are expressed by a broad panel of normal epithelial cells (26, 60). Downregulation of Fas expression is a common abnormality in gynecological cancers (12), whereas the expression of TRAIL receptors is not reduced in cervical cancer (38) compared to normal tissue. It is thus plausible that HPV-16, like many other viruses (31, 51), has developed mechanisms to delay apoptosis of the infected cell and one of its early proteins, namely, E6, has been identified to prevent intrinsic, p53-dependent apoptosis (41, 59). We show here that E5 impairs extrinsic apoptosis mediated by TRAIL and FasL in human keratinocytes, and we propose that this is a primary function of the viral protein.
MATERIALS AND METHODS
Cell lines and apoptosis induction.
We used the spontaneously immortalized human keratinocyte cell line HaCaT (2) that had been stably transfected with HPV-16 E5 (HaCaT/E5) or the empty vector pMSG as control (HaCaT/pMSG) (32). In all experiments, including controls, cells were grown in keratinocyte growth medium (BioWhittaker, Heidelberg, Germany) to 70 to 80% confluency and serum-starved for 48 h in Dulbecco modified Eagle medium with penicillin-streptomycin (Gibco, Karlsruhe, Germany). Both cell lines were treated with 1 μM dexamethasone during culture and serum deprivation to induce E5 expression. Under these culture conditions the presence of E5 mRNA in HaCaT/E5 cells was verified routinely by Northern blot or reverse transcription-PCR. For apoptosis induction, cells were treated with 500 ng of TRAIL (KillerTRAIL; Alexis, Grünberg, Germany) or 150 ng of FasL (SuperFas Ligand; Alexis)/ml for 30 min, 1 h, 2 h, 4 h, and 8 h. Ligand-blocking experiments were performed with 3 μg of neutralizing antibodies (2E5; Alexis)/ml directed against TRAIL. Cells were subsequently washed twice with cold phosphate-buffered saline (PBS) and lysed in 1% sodium dodecyl sulfate (SDS) for Western blot analysis. The protein content of all extracts was measured by using the DC protein assay (Bio-Rad, Munich, Germany) according to Lowry. In addition to the keratinocytes, we used the mouse fibroblast cell line A31-E5, which permanently expresses HPV-16 E5 and the A31-Neo cell line as a reference (25). Cells were cultured in Dulbecco modified Eagle medium with 10% fetal calf serum and penicillin-streptomycin (Gibco) and serum starved for 24 h without addition of dexamethasone. Apoptosis was induced with 500 ng of TRAIL or 50 ng of FasL/ml as described above.
Western blot analysis.
For SDS-polyacrylamide gel electrophoresis (PAGE), 50 μg of protein extract from HaCaT/pMSG, and HaCaT/E5 cells (or A31-E5 and A31-Neo, respectively) were separated on the same gel and subsequently transferred onto one nitrocellulose membrane (Schleicher & Schuell, Dabbel, Germany). The membranes were blocked with 10% dry milk in PBS containing 0.1% Tween 20 for several hours at room temperature. The first antibody was incubated at 4°C overnight. Reactions were detected with the ECL system (Amersham-Pharmacia, Freiburg, Germany).
The antibodies used included caspase-3 (BD PharMingen, Heidelberg, Germany), caspase-8 (c15, murine immunoglobulin G2b) (39), and PARP (c-2-10) (21).
DISC analysis by coimmunoprecipitation.
HaCaT/E5 and HaCaT/pMSG cells were cultured and serum starved as described above before treatment with TRAIL at ng/ml for 30 min. Cells were detached from dishes by use of a rubber policeman, washed twice in ice-cold PBS, and lysed in buffer (30 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% NP-40, 10% glycerol) supplemented with protease inhibitors (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. After 15 min of incubation on ice, the lysates were centrifuged at 20,000 × g for 15 min at 4°C to remove nuclei. The protein content of the supernatant was measured as described above, and 700 to 800 μg of cell extract was used for immunoprecipitation with 3 μg of DR4 and DR5 antibody, respectively (HS101 and HS201; Alexis). Precipitation was performed by using magnetic protein G Microbeads and binding to a MACS column in a magnetic field (Miltenyi Biotec, Bergisch Gladbach, Germany). To prevent dissociation of the antibodies into their heavy and light chains, elution buffer without β-mercaptoethanol (58.3 mM Tris-HCl [pH 6.8], 5.73% glycerol, 1.67% SDS, 0.01% bromphenolblue) was used. After separation by SDS-PAGE and subsequent transfer onto nitrocellulose membrane (Schleicher & Schuell, Dabbel, Germany), the blots were cut into an upper and lower part before being probed with DR4 or DR5 antibody and FADD antibody (1F7; Biozol Diagnostica, Eching, Germany) overnight, respectively. The blots were developed by enhanced chemiluminescence as described. For immunoprecipitation of the Fas receptors 655 μg of protein extracts were used, together with 3 μg of Apo-1 antibody (56), and processed for Western blot analysis. For detection of Fas receptor the Fas antibody (C-20; Santa Cruz, Heidelberg, Germany) was used for Western blot. A densitometric quantification of the bands was performed by using ImageQuant software.
FACS analysis.
Surface expression of the death receptors Fas, DR4 (TRAIL-R1), and DR5 (TRAIL-R2) in untreated, serum-starved HaCaT cells was analyzed by fluorescence-activated cell sorting (FACS). Cells were detached from the plate after incubation with PBS containing 0.25% EDTA for 10 min, followed by brief trypsinization. Then, 7.5 × 105 cells were resuspended in 100 μl of PBS and incubated with human CD95 antibody (1 μg/ml; Cymbus Biotechnology) or DR4 and DR5 antibody (each 10 μg/ml; Alexis) for 45 min on ice. After a washing step with PBS, a fluorescein isothiocyanate (FITC)-conjugated secondary antibody was added, and the cells were stained for 45 min on ice. The washed cells were then resuspended in 300 μl of cold PBS and pressed through a syringe with needle. For each sample, 10,000 single events were analyzed with a flow cytometer (Becton Dickinson, Mountain View, Calif.) by using CELLQuest software and the FITC detector filter FL-1.
Indirect immunofluorescence.
Cells were grown on coverslips and cultured and starved as described above before apoptosis was induced by treatment with 500 ng of TRAIL or 150 ng of FasL/ml for 2, 4, and 8 h. The cells were washed in PBS and fixed in methanol and acetone (both −20°C) for 5 min each, before an antibody that specifically detects the cleaved form of caspase-3 (New England Biolabs, Bad-Schwalbach, Germany) was added in a dilution of 1:50 in PBT (PBS containing 0.5% bovine serum albumin, 0.5% Tween 20, and 0.02% NaN3) and incubated overnight at 4°C. After being washed with PBS, an Alexa 488-conjugated secondary antibody was added, together with propidium iodide, as a counterstain and incubated for 1 h. The washed samples were mounted in Fluoprep (bioMerieux, Marcy l'Etoile, France), and the fluorescent cells were visualized for counting with a Leica DMRD microscope (Leica, Bensheim, Germany). Pictures were taken by using the Confocal laser scanning microscope (Leica TCS SP, Bensheim, Germany).
RESULTS
Polyclonal HaCaT/E5 and HaCaT/pMSG cell lines were used for functional studies of the viral E5 protein.
For a first series of experiments, we used the spontaneously immortalized human keratinocyte cell line HaCaT (2) permanently expressing the HPV-16 E5 gene (HaCaT/E5) under control of the dexamethasone-inducible mouse mammary tumor virus (MMTV)-promoter. HaCaT cells transfected with the same recombinant DNA devoid of the E5 reading frame (HaCaT/pMSG) were used as reference.
Activation of caspase-3 upon TRAIL and FasL treatment is strongly reduced in HaCaT/E5 cells.
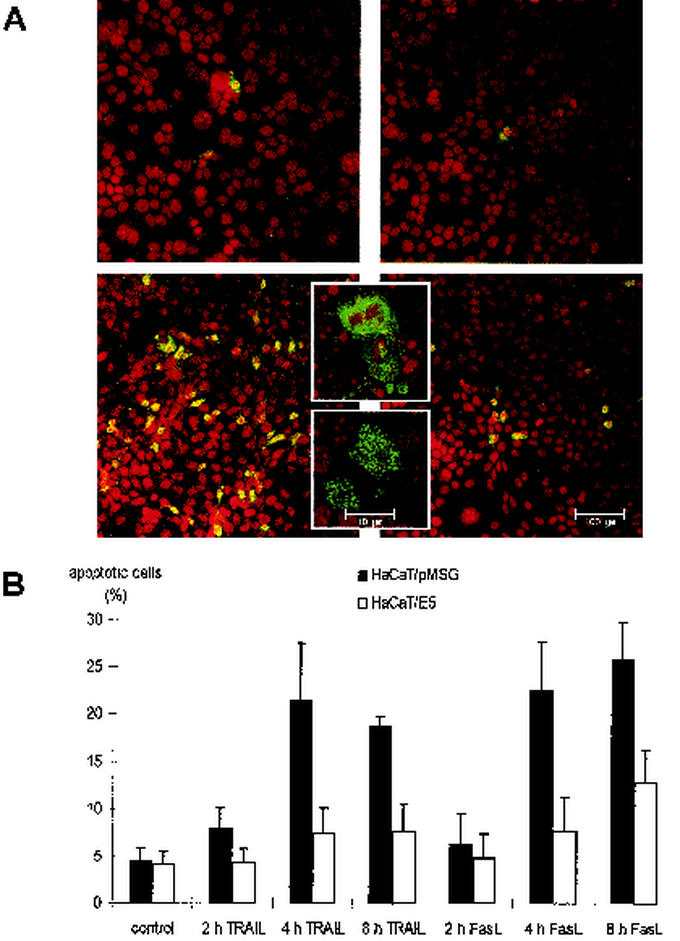
Apoptosis was induced by the addition of either 500 ng of TRAIL or 150 ng of FasL/ml after serum deprivation. Apoptotic cells were visualized by indirect immunofluorescence against activated caspase-3 (Fig. 1A). The upper panel shows untreated HaCaT/pMSG (left) and HaCaT/E5 (right) cells. In the absence of TRAIL very few fluorescent cells were visible, which indicates a low background of apoptotic cells in both cell lines. As expected, a large number of fluorescent cells could be detected in HaCaT/pMSG after addition of TRAIL for 8 h (lower left panel). In contrast, the number of apoptotic cells was only slightly increased in HaCaT/E5 after identical exposure to TRAIL (lower right panel). The fluorescent cells displayed the morphology that is typical of different stages of apoptosis such as condensation of the chromatin and membrane blebbing. For better visualization, two representative cells are shown in higher magnification (inserted boxes in the lower panel). Similar results were obtained after FasL treatment. Figure 1B depicts a histogram of the percentage of apoptotic HaCaT/pMSG and HaCaT/E5 cells, either untreated or treated for 2, 4, and 8 h with TRAIL or FasL. Compared to HaCaT/pMSG, the percentage of apoptotic HaCaT/E5 cells is significantly reduced by more than a factor of 2 after TRAIL or FasL treatment.
FIG. 1.
Activation of caspase-3 after TRAIL and FasL treatment. (A) Activation of caspase-3 in HaCaT/pMSG (left) and E5 cells (right) is shown by indirect immunofluorescence with a confocal laser scanning microscope. (Upper panel) Fluorescent cells in the absence of TRAIL; (lower panel) fluorescent cells after the addition of TRAIL for 8 h. The inset boxes in the lower panel show two representative cells at different stages of apoptosis. (B) Quantitative analysis of apoptotic HaCaT/pMSG (▪) and E5 (□) cells, either untreated (control) or treated for 2, 4, and 8 h with TRAIL or FasL. Each data point is the mean value of four measurements of randomly chosen fields in one experiment. Exact sampling theory (Student t distribution with 3 degrees of freedom) is used for error estimation. The error bars represent the interval in which the true (population) mean is found with a probability of 95%. The experiment was repeated three times with similar results.
Cleavage of procaspase-8 and -3 and PARP after TRAIL treatment is undetectable in HaCaT/E5 cells.
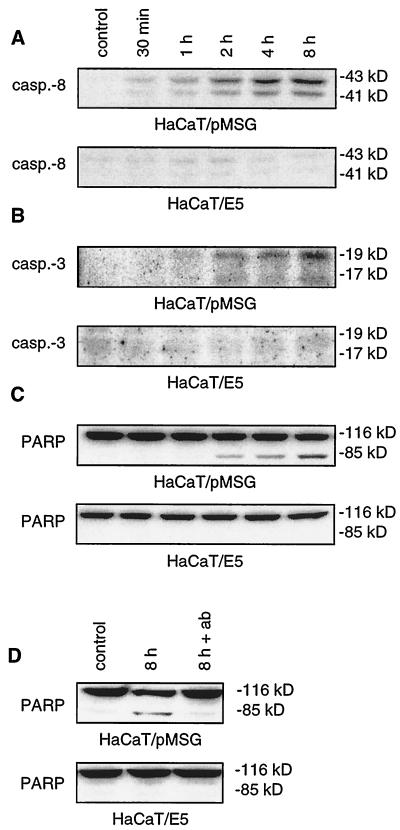
Evidence for apoptotic signaling along the death receptor pathway after addition of TRAIL is provided in Fig. 2. A total of 500 ng of TRAIL/ml was applied for 30 min, 1 h, 2 h, 4 h, and 8 h after serum deprivation. The effect of TRAIL on our cells was monitored by the 43- and 41-kDa fragments of procaspase-8 (Fig. 2A). In HaCaT/pMSG cells cleavage started 1 h after the addition of TRAIL and steadily increased over the course of the experiment. For the E5-expressing cells, no such cleavage could be observed. Activation of the initiator caspase-8 was followed by cleavage of the downstream procaspase-3, as shown in Fig. 2B. In HaCaT/pMSG, but not in HaCaT/E5 cells, activation of procaspase-3 was detected after 2 h and increased until the end of the experiment. Evidence for the catalytic competence of activated caspase-3, namely, the proteolytic cleavage of its substrate poly(ADP-ribose) polymerase (PARP), is given in Fig. 2C. Identically stimulated HaCaT/E5 cells show neither caspase-3 activation nor PARP cleavage. To prove that the observed effects were due to TRAIL-mediated apoptosis, neutralizing antibody against TRAIL was added at 3 μg/ml together with the ligand. Consistent with the above findings, PARP cleavage occurred after 8 h of TRAIL treatment in HaCaT/pMSG cells but was undetectable in the presence of neutralizing antibody (Fig. 2D). For HaCaT/E5 cells, no PARP cleavage was observed in either case.
FIG. 2.
Cleavage of procaspase-8 and -3 and PARP after TRAIL treatment. HaCaT/pMSG and E5 were serum starved, and apoptosis was induced by 500 ng of TRAIL/ml as described in Materials and Methods. Then, 50 μg of each protein extract were separated by SDS-PAGE and immunoblotted with antibodies recognizing caspase-8 and -3 and PARP. Panels A and C derive from the same blot with uncleaved PARP also serving as an internal loading control. (A) In HaCaT/pMSG cells cleavage of the initiator procaspase-8 starts 1 h after the addition of TRAIL and increases over the course of the experiment. No such cleavage can be observed for HaCaT/E5 cells. (B) Cleavage of the downstream procaspase-3 occurs after 2 h and increases until the end of the experiment in HaCaT/pMSG cells, whereas no significant activation can be detected in HaCaT/E5. (C) In HaCaT/pMSG cells, but not in HaCaT/E5 cells, cleavage of PARP starts after 2 h and strongly increases over the course of the experiment. (D) The apoptotic effect of TRAIL on PARP cleavage in HaCaT/pMSG cells can be blocked by neutralizing antibody (ab) directed against the ligand (lane 3).
Cleavage of procaspase-8 and PARP after FasL treatment is strongly reduced in HaCaT/E5 cells.
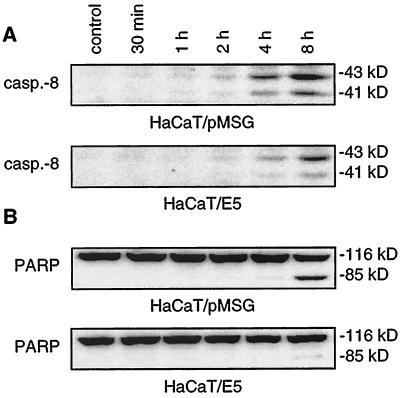
Involvement of the death receptor pathway was also shown for FasL-mediated apoptosis (Fig. 3). A total of 150 ng of FasL/ml was added for 30 min, 1 h, 2 h, 4 h, and 8 h after serum deprivation. In HaCaT/pMSG cells cleavage of procaspase-8 started 4 h after the addition of FasL and increased strongly after 8 h (Fig. 3A). For the E5-expressing cells no significant cleavage could be observed after 4 h, and only a faint cleavage of procaspase-8 occurred after 8 h. Similar to the TRAIL experiments, we found a strong reduction of PARP cleavage for the E5-expressing cells (Fig. 3B).
FIG. 3.
Cleavage of procaspase-8 and PARP after FasL treatment. HaCaT/pMSG and E5 were serum starved, and apoptosis was induced by 150 ng of FasL/ml as described in Materials and Methods. Then, 50 μg of each protein extract were separated by SDS-PAGE and immunoblotted with antibodies recognizing caspase-8 and PARP. Both panels derive from the same blot with the uncleaved PARP serving as an additional loading control. (A) In HaCaT/pMSG cells cleavage of the procaspase-8 starts after 4 h and increases strongly after 8 h. Only a faint cleavage is seen after 4 h in HaCaT/E5 cells that slightly increases after 8 h. (B) PARP cleavage occurs after 4 h and increases strongly after 8 h in HaCaT/pMSG cells, whereas only faint cleavage is detected after 8 h in HaCaT/E5.
Surface expression of Fas, but not of DR4 and DR5, is reduced in HaCaT/E5 cells.
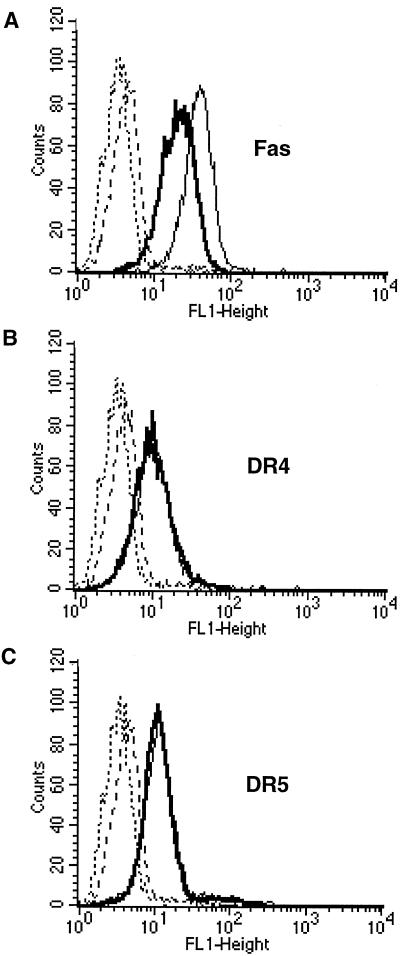
Since the antiapoptotic effect of E5 has been observed at the level of caspase-8 activation (Fig. 2A and 3A), we expected that E5 would interfere with the beginning of the death-signaling pathway. Therefore, we analyzed surface expression of the death receptors Fas, DR4 (TRAIL-R1) and DR5 (TRAIL-R2) by using the FACS method. We found that Fas surface expression is reduced by a factor of 1.8 in untreated HaCaT/E5 compared to HaCaT/pMSG cells (Fig. 4A). Presumably, the reduced amount of Fas receptors is expected to render the HaCaT/E5 cells less susceptible to FasL-induced apoptosis. In contrast, no differences in the expression of DR4 and DR5 were detectable (Fig. 4B and C), indicating that a different mechanism must be responsible for the inhibition of TRAIL-mediated apoptosis in HaCaT/E5.
FIG. 4.
Surface expression of the death receptors Fas, DR4, and DR5. HaCaT/pMSG and E5 were serum starved, and surface expression of the death receptors was analyzed by FACS as described in Materials and Methods. The death receptor fluorescence of HaCaT/pMSG (solid light line) and HaCaT/E5 (solid bold line) is compared to the fluorescence of the secondary FITC-conjugated antibody alone (dashed and dotted lines, respectively). Ordinate, number of cells; abscissa, fluorescence intensity. (A) Fas surface expression is reduced by a factor of 1.8 in HaCaT/E5 compared to HaCaT/pMSG cells. (B and C) No differences in the expression of DR4 and DR5 are detectable between the two cell lines.
TRAIL-induced DISC formation is impaired in HaCaT/E5 cells.
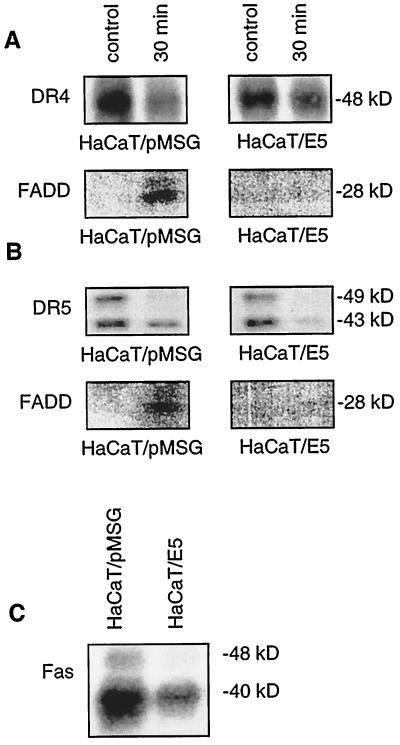
We studied DISC assembly by coimmunoprecipitation of the death receptors DR4 and DR5 with their adaptor protein FADD. A total of 500 ng of TRAIL/ml was added for 30 min after serum deprivation to HaCaT/pMSG and HaCaT/E5 cells. Then, 700 and 800 μg of cell extract were used for immunoprecipitation with 3 μg of DR4 and DR5 antibody, respectively. The amounts of DR4 and DR5 receptors in untreated HaCaT/pMSG and HaCaT/E5 cells were similar, as expected from the FACS analysis (Fig. 4B and C) and declined after the TRAIL treatment (upper panels of Fig. 5A and B), possibly due to receptor degradation. For each receptor a very faint, but nonetheless identifiable FADD-band appeared only in the HaCaT/pMSG cells after induction of DISC formation by TRAIL (lower panels of Fig. 5A and B). This means that the resistance of HaCaT/E5 toward TRAIL could be attributed to the inability of the cells to form a functional DISC, which is necessary for transmission of the apoptotic signal.
FIG. 5.
TRAIL-induced DISC formation is impaired in HaCaT/E5 cells. HaCaT/pMSG and E5 were serum starved and treated with 500 ng of TRAIL/ml for 30 min upon immunoprecipitation of DR4 and DR5 prior to separation by SDS-PAGE and subsequent Western blot with antibodies recognizing DR4, DR5, and FADD. (A) Despite similar amounts of DR4 in both cell lines (upper panels), binding of FADD to the receptor is only observed in HaCaT/pMSG cells after TRAIL treatment (lower, left panel). (B) Although expression of DR5 is similar in both cell lines (upper panels), TRAIL-induced functional DISC assembly is only detected in HaCaT/pMSG (lower, left panel). (C) In untreated HaCaT/E5 cells, Fas expression is reduced by more than a factor of 2 compared to HaCaT/pMSG, as monitored by immunoprecipitation.
Fas expression is strongly reduced in HaCaT/E5 cells.
In contrast to the death receptors DR4 and DR5, we found a pronounced reduction in the expression of Fas receptors by more than a factor of 2 in untreated HaCaT/E5 cells compared to untreated HaCaT/pMSG cells (Fig. 5C). This indicates that E5 is able to downregulate the total amount of Fas receptors and not only their surface expression (Fig. 4A). Although it is clear that DISC formation must have occurred after addition of FasL, we were unable to detect coimmunoprecipitated FADD in either HaCaT/E5 or HaCaT/pMSG cells despite several attempts with different amounts of cell extracts (up to 1 mg) and different incubation times (5 min, 30 min, and 8 h).
The antiapoptotic effects in HaCaT/E5 cells depend on E5 expression.
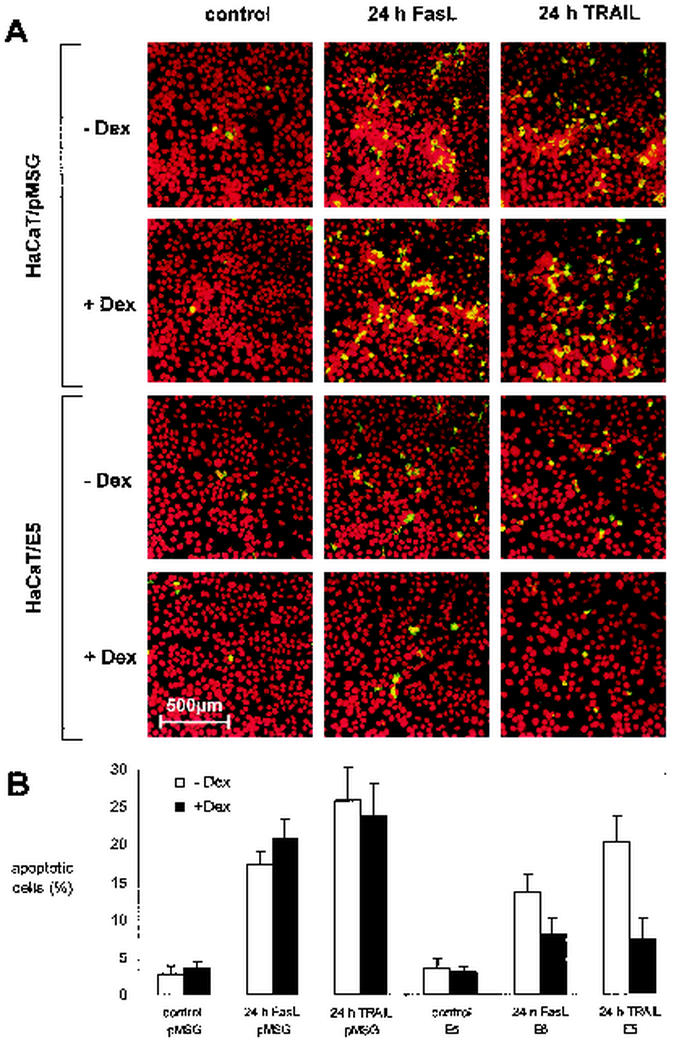
Since E5 expression is controlled by the dexamethasone-inducible MMTV-promoter, the antiapoptotic effects observed in HaCaT/E5 cells should disappear if the promoter is not activated. To verify this expectation we compared HaCaT/E5 and HaCaT/pMSG cells with or without dexamethasone (+Dex, −Dex) induction. Apoptosis was induced by the addition of either 150 ng of FasL or 500 ng of TRAIL/ml for 24 h after serum deprivation in the presence or absence of 1 μM dexamethasone. Apoptotic cells were visualized by indirect immunofluorescence against activated caspase-3 (Fig. 6A). In the absence of FasL or TRAIL very few fluorescent cells were visible, which indicates a low background of apoptotic cells (left column; control). After the addition of FasL or TRAIL, a large number of fluorescent cells could be detected in the E5-lacking HaCaT/pMSG cells with insignificant differences between the cells with or without dexamethasone treatment (upper part of the middle and right columns). A different picture emerges for the HaCaT/E5 cells after identical exposure to FasL or TRAIL (lower part of the middle and right columns). In HaCaT/E5+Dex the amount of apoptotic cells was reduced by about a factor of two compared to HaCaT/E5-Dex. A quantification of the results is shown in Fig. 6B. Upon comparing HaCaT/E5-Dex with HaCaT/pMSG-Dex, we observed a small reduction in the number of apoptotic cells after FasL or TRAIL treatment. This could be due to a residual amount of E5 in the HaCaT/E5-Dex cells, perhaps because of the low degradation rate of the membrane protein or the leakiness of the MMTV-promoter (32).
FIG. 6.
Activation of caspase-3 in HaCaT cells after FasL and TRAIL treatment with or without dexamethasone (+Dex, −Dex) induction. (A) Activation of caspase-3 in HaCaT/pMSG (upper part) and E5 cells (lower part) in the presence (+Dex) or absence (−Dex) of 1 μM dexamethasone is shown by indirect immunofluorescence with a confocal laser scanning microscope. Apoptosis was induced by the addition of either 150 ng of FasL or 500 ng of TRAIL/ml for 24 h after serum deprivation. (B) Quantitative analysis of apoptotic, uninduced (−Dex; white) or induced (+Dex; black) HaCaT/pMSG (left) and E5 (right) cells, either untreated (control) or treated for 24 h with FasL or TRAIL. Each data point is the mean value of five to six measurements of randomly chosen fields in one experiment. Exact sampling theory (Student t distribution with 4 to 5 degrees of freedom) is used for error estimation. The error bars represent the interval in which the true (population) mean is found with a probability of 95%.
The reduced surface expression of Fas in HaCaT/E5 cells is not the sole reason for protection from FasL-mediated apoptosis.
From Fig. 4A and also from Fig. 6, we expected to observe a strong E5-concentration-dependent effect on Fas surface expression in the HaCaT/E5 cells. To this end, we compared HaCaT/E5 and HaCaT/pMSG cells with or without induction of the MMTV-promoter by 1 μM dexamethasone. Contrary to our expectation, we found that the reduced surface expression of Fas remained unaltered in HaCaT/E5 cells even when the MMTV-promoter was not induced (Table 1). We have repeated the FACS analysis twice, obtaining essentially the same results as already shown in Fig. 4A. However, we found that the total amount of Fas protein changed upon treatment with dexamethasone in both cell lines (Table 1). In HaCaT/E5+Dex cells the total amount of Fas protein was lower in the presence of dexamethasone than in the absence of the inducer, which indicates that the total Fas content is E5 concentration dependent. In contrast, we observed an increase of the total amount of Fas protein in the HaCaT/pMSG+Dex cells. This indicates that the synthetic glucocorticoid causes additional effects besides induction of the MMTV-promoter that ultimately lead to an increase of Fas protein. In both HaCaT/pMSG-Dex and HaCaT/E5-Dex we found approximately the same total amount of Fas protein, whereas a pronounced reduction of Fas levels was observed in HaCaT/E5+Dex but not in HaCaT/pMSG+Dex. This reduction is consistent with the results shown in Fig. 5C. Finally, Northern blot analysis showed that induction of the MMTV-promoter by dexamethasone hardly altered Fas mRNA expression. Between the two cell lines no significant differences in Fas mRNA expression were detectable (Table 1).
TABLE 1.
Effect of dexamethasone on Fas expression in HaCaT/pMSG and HaCaT/E5 cells as determined by using HaCaT/pMSG-Dex as a reference
| Dexa combination | Mean value ± SEM
|
||
|---|---|---|---|
| Fas mRNAb | Total Fas proteinb | Fas surface expressionc | |
| pMSG − Dex | 1.00 | 1.00 | 1.00 |
| pMSG + Dex | 0.73 ± 0.04 | 1.41 ± 0.16 | 0.93 ± 0.13 |
| E5 − Dex | 0.69 ± 0.14 | 1.15 ± 0.16 | 0.44 ± 0.06 |
| E5 + Dex | 0.68 ± 0.14 | 0.80 ± 0.10 | 0.51 ± 0.10 |
Dex, dexamethasone.
Mean values derived from three independent measurements.
Mean values derived from two independent measurements.
E5-expressing mouse fibroblasts are protected from TRAIL- but not from FasL-mediated apoptosis.
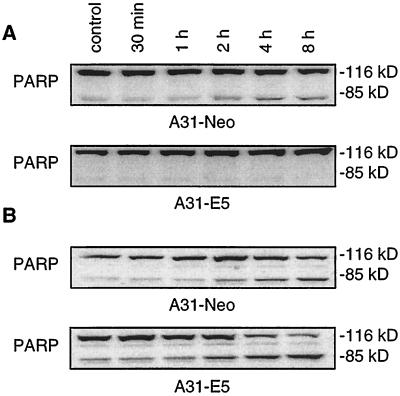
To address the question whether the antiapoptotic effect of E5 is restricted to HaCaT cells, we compared the mouse fibroblast cell line A31-E5 with the E5-lacking control cells A31-Neo. A total of 500 ng of TRAIL/ml was added for 30 min, 1 h, 2 h, 4 h, and 8 h after serum deprivation. The apoptotic effect of TRAIL was monitored by PARP cleavage (Fig. 7A). In A31-Neo cells cleavage started 2 h after the addition of TRAIL and increased to a constant level at 4 and 8 h. For the E5-expressing cells no such cleavage could be observed.
FIG. 7.
PARP cleavage of mouse fibroblasts A31 after TRAIL and FasL treatment. A31-Neo and A31-E5 were serum starved and apoptosis was induced by either 500 ng of TRAIL or 50 ng of FasL/ml as described in Materials and Methods. Then, 50 μg of each protein extract was separated by SDS-PAGE and immunoblotted with antibodies detecting PARP. (A) In A31-Neo cells, cleavage starts 2 h after the addition of TRAIL and increases to a constant level at 4 and 8 h, whereas no significant cleavage can be detected in the A31-E5 cells. (B) Upon addition of FasL both cell lines show PARP cleavage that starts after 2 h and steadily increases until the end of the experiment.
A different picture emerges when apoptosis is induced by FasL (Fig. 7B). Upon the addition of 50 ng of FasL/ml, both A31-E5 and A31-Neo cells showed PARP cleavage that started after 2 h and steadily increased over the course of the experiment. Apparently, E5 of the human papillomavirus is unable to protect mouse fibroblasts from FasL-mediated apoptosis. This observation is consistent with the hypothesis that human pathogenic E5 employs different mechanisms to protect from TRAIL- and FasL-induced apoptosis in human target cells.
DISCUSSION
We have shown here that the viral protein HPV-16 E5 protects the human keratinocyte cell line HaCaT from TRAIL- and FasL-mediated apoptosis and that this effect correlates with the level of E5 expression. E5 is a difficult protein to work with since there are no antibodies available that allow a direct detection of this strongly hydrophobic protein. For this reason, our studies refer to only three states of E5 expression: high levels (upon dexamethasone induction; HaCaT/E5+Dex), residual amounts (no dexamethasone induction; HaCaT/E5-Dex), or absolutely no E5 (HaCaT/pMSG). Evidence for some residual E5 in HaCaT/E5-Dex cells comes from faint E5 mRNA bands in Northern blots after long exposure times due to the leakiness of the MMTV-promoter (32). By comparative analysis of cells in these three states of E5 expression we were able to assign the viral protein a new, inhibitory role in TRAIL- and FasL-mediated apoptosis.
Many viruses have developed antiapoptotic mechanisms (31, 51) that allow the host cell to stay alive long enough for the virus to replicate. We propose that the antiapoptotic effect of E5, namely, to impair TRAIL- and FasL-mediated apoptosis, is a primary function of the viral protein in human keratinocytes. In fact, at early stages of HPV-16 infection, when the viral genome is episomal, E5 is one of the most abundant viral mRNA transcripts (22, 48).
As already mentioned HPV-16 belongs to the high-risk types which infect the genital mucosa. Proliferation and regression of gynecological tissues such as ovary, endometrium and cervix is hormone dependent, and Fas is constitutively expressed in these cells (26), which are the natural target of the virus. Analysis of Fas expression in gynecological cancers revealed that downregulation of this death receptor is a common abnormality in these tissues (12). The decreased levels of Fas receptor and the resultant resistance to FasL-mediated apoptosis could enable tumor cells to escape immunosurveillance (63). It is known that virus infection can lead to a loss of cell surface Fas mediated by viral proteins, as has been described for adenovirus (46, 54). Generally, a reduction in the number of death receptors lowers the probability that the ligand molecules reach their target, rendering these cells less susceptible to apoptosis. The observation of a reduced Fas surface expression in HaCaT/E5 cells (Fig. 4A) suggests that HPV-16 E5 exploits this principle to protect infected human keratinocytes from FasL-mediated apoptosis. However, to demonstrate the capability of E5 to protect HaCaT cells, we used a large amount of Fas ligand that presumably exceeds the physiological concentration by high margin. (According to the manufacturer [Alexis], 30 to 150 times lower concentrations than used in our experiments are sufficient to induce apoptosis in A20 B lymphoma cells.) This leads to saturation of the receptors with ligand molecules, which could explain why apoptosis occurs in HaCaT/E5-Dex (Fig. 6), despite a reduced Fas surface expression. Apparently, additional mechanisms must exist that prevent transmission of the apoptotic signals in a E5-concentration-dependent manner (Fig. 6). HaCaT/E5+Dex cells contain less total Fas than HaCaT/E5-Dex or HaCaT/pMSG cells (Table 1), which could be due to a forced degradation of Fas protein since the amount of Fas mRNA remains essentially unaffected by E5 expression. Such a forced degradation of Fas that inhibits apoptosis has been previously reported for adenovirus-infected cells (54). It remains unclear, however, by what mechanism a small amount of E5 in HaCaT/E5-Dex is able to maintain the same low surface expression of Fas as was observed in HaCaT/E5+Dex cells. It is conceivable that the reduction in surface expression and total amount of Fas leads in combination to efficient protection from FasL-mediated apoptosis under physiological conditions.
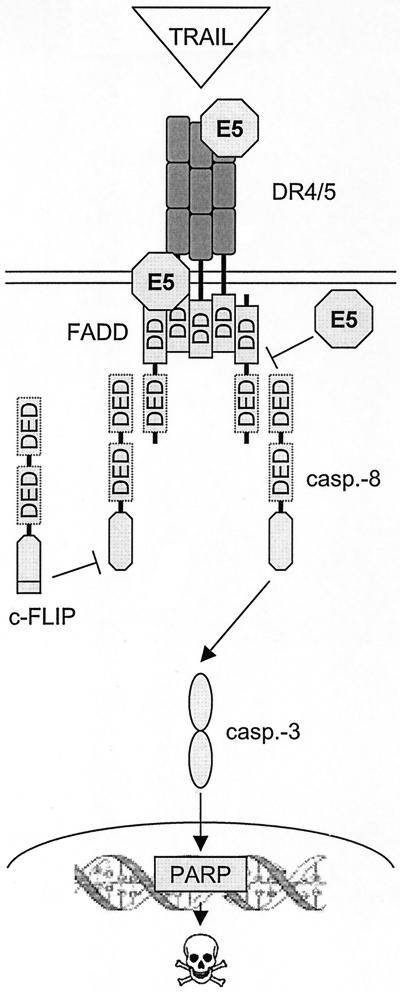
Besides Fas, TRAIL, and the TRAIL receptors have been shown to play a role in a number of viral infections (6, 44, 55, 57). For example, in adenovirus infected cells, TRAIL-induced apoptosis is inhibited by an enhanced internalization of DR4 (55). The presence of TRAIL receptors 1, 2, and 3 has also been reported for normal cervical epithelium as well as for tumor cells. Downregulation of the TRAIL receptors is not observed in the tumor cells (38), which is in contrast to Fas (12). We have shown that HPV-16 E5 inhibits TRAIL-mediated apoptosis by a mechanism different from Fas since the amount of surface and intracellular DR4 and DR5 is similar in HaCaT/E5 and HaCaT/pMSG cells (Fig. 4 and 5). The differences between the two cell lines with respect to TRAIL-mediated apoptosis were observed at the level of caspase-8 activation (Fig. 2A and 3A), which leads us to expect that E5 acts on DISC formation that is upstream in the death-signaling pathway (Fig. 8).
FIG. 8.
Model for the mechanism by which HPV-16 E5 impairs DISC formation. Three possible sites of E5 interactions with DISC are indicated that could intercept apoptosis signaling upstream of caspase-8 activation. The model is described in the text.
The binding of TRAIL to DR4 and DR5 induces receptor trimerization, generating interaction surfaces for DD-containing adaptor proteins such as FADD. In a homotypic interaction, the DD of FADD binds to the DD of the receptor. FADD recruits procaspase-8 through interactions of the death effector domain (DED). The induced proximity of caspase-8 zymogen in the DISC facilitates autocatalysis, which in turn leads to activation of downstream effector caspases such as caspase-3 that execute the apoptotic death program. One of the substrates of caspase-3 is the DNA repair enzyme poly(ADP-ribose) polymerase (PARP). Apoptotic signaling can be intercepted by c-FLIP, which is structurally similar to caspase-8 and binds to DED of FADD with a higher affinity than caspase-8 (24, 40). The binding of c-FLIP prevents further recruitment of caspase-8 into the complex. c-FLIP is subsequently cleaved, resulting in the release of a caspase-like, but proteolytically inactive, subunit.
Although E5 does not downregulate DR4 and DR5 expression, it is conceivable that E5 reduces the functionality of these receptors. DR4 and DR5 have been found to primarily associate with the Golgi network (62). The hydrophobic E5 molecule, which is located in the membranes of endoplasmic reticulum and Golgi (7), may be able to bind to the receptors during their posttranslational processing and transport to the cell surface. This could result in the receptor's inability to bind TRAIL or to transmit the apoptotic signal via the FADD protein. Alternatively, E5 could be responsible for an overexpression of apoptosis inhibitors. We regard this scenario as less likely because we have shown that c-FLIP expression remains unaltered in both HaCaT/E5 and HaCaT/pMSG cells (data not shown).
Consistent with the high degree of homology between human and murine proteins, including the death receptors, HaCaT/pMSG and A31-Neo cells undergo apoptosis upon induction by the human TRAIL and Fas ligands. Apparently, the murine receptors bind the human homologues of their cognate ligands equally well. Moreover we found that HPV-16 E5 protects A31 cells from TRAIL-mediated apoptosis (Fig. 7A), a finding which demonstrates that this effect of the viral protein is not restricted to HaCaT cells. In contrast, no protection of the A31-E5 cells was observed upon addition of FasL (Fig. 7B). It is conceivable that the intricate mechanisms employed by HPV-16 E5 for protection from FasL-mediated apoptosis are optimized for human keratinocytes and do not apply to the murine A31 cells. This would be consistent with the finding that E5 impairs TRAIL- and FasL-mediated apoptosis by different mechanisms.
The presence of Fas and TRAIL receptors in the target tissue makes it plausible that HPV-16 has developed mechanisms to escape immunosurveillance that otherwise would be likely to detect infected cells at an early stage in the viral life cycle. In addition to the known function of E6 in preventing intrinsic, p53-dependent apoptosis, we have shown here for the first time that E5 protects from extrinsic ligand-mediated apoptosis, as demonstrated for FasL and TRAIL. On the other hand, suppression of apoptosis can lead to transformed cells that are no longer capable to support viral reproduction. However, it is known that only a small fraction of HPV-16-infected cells become malignant (1, 18). Taken together, it appears to be a selective advantage for this small virus to include in its genome the code for two proteins, namely, E5 and E6, that ensure that the correct environment for viral replication is maintained.
Acknowledgments
We thank Stefanie Rösch and Jürgen Fischer for excellent technical assistance, John Wray for critical reading of the manuscript, and Cornelia Oetke for help with the FACS. We are grateful to Pascal Tomakidi and Wolfgang Kabsch for valuable discussions.
REFERENCES
- 1.Biswas, C., B. Kell, C Mant, R. J. Jewers, J. Cason, P. Muir, K. S. Raju, and J. M. Best. 1997. Detection of human papillomavirus type 16 early-gene transcription by reverse transcription-PCR is associated with abnormal cervical cytology. J. Clin. Microbiol. 35:1560-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boukamp, P., R. T. Petrussevska, D. Breitkreutz, J. Hornung, A. Markham, and N. E. Fusenig. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106:761-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouvard, V., G. Matlashewski, Z. M. Gu, A. Storey, and L. Banks. 1994. The human papillomavirus type 16 E5 gene cooperates with the E7 gene to stimulate proliferation of primary cells and increases viral gene expression. Virology 203:73-80. [DOI] [PubMed] [Google Scholar]
- 4.Budihardjo, I., H. Oliver, M. Lutter, X. Luo, and X. Wang. 1999. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 15:269-290. [DOI] [PubMed] [Google Scholar]
- 5.Chen, S.-L., C.-H. Huang, T.-C. Tsai, K.-Y. Lu, and Y.-P. Tsao. 1996. The regulation mechanism of c-jun and junB by human papillomavirus type 16 E5 oncoprotein. Arch. Virol. 141:791-800. [DOI] [PubMed] [Google Scholar]
- 6.Clarke, P., S. M. Meintzer, S. Gibson, C. Widmann, T. P. Garrington, G. L. Johnson, and K. L. Tyler. 2000. Reovirus-induced apoptosis is mediated by TRAIL. J. Virol. 74:8135-8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conrad, M., V. J. Bubb, and R. Schlegel. 1993. The human papillomavirus type 6 and 16 E5 proteins are membrane-associated proteins which associate with the 16-kilodalton pore-forming protein. J. Virol. 67:6170-6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conrad-Stöppler, M., S. W. Straight, G. Tsao, R. Schlegel, and D. J. McCance. 1996. The E5 gene of HPV-16 enhances keratinocyte immortalization by full-length DNA. Virology 223:251-254. [DOI] [PubMed] [Google Scholar]
- 9.Crusius, K., E. Auvinen, B. Steuer, H. Gaissert, and A. Alonso. 1998. The human papillomavirus type 16 E5-protein modulates ligand-dependent activation of the EGF receptor family in the human epithelial cell line HaCaT. Exp. Cell Res. 241:76-83. [DOI] [PubMed] [Google Scholar]
- 10.Crusius, K., M. Kaszkin, V. Kinzel, and A. Alonso. 1999. The human papillomavirus type 16 E5 protein modulates phospholipase C-γ-1 activity and phosphatidyl inositol turnover in mouse fibroblasts. Oncogene 18:6714-6718. [DOI] [PubMed] [Google Scholar]
- 11.Crusius, K., I. Rodriguez, and A. Alonso. 2000. The human papillomavirus type 16 E5 protein modulates ERK1/2 and p38 MAP kinase activation by an EGFR-independent process in stressed human keratinocytes. Virus Genes 20:65-69. [DOI] [PubMed] [Google Scholar]
- 12.Das, H., T. Koizumi, T. Sugimoto, S. Chakraborty, T. Ichimura, K. Hasegawa, and R. Nishimura. 2000. Quantitation of Fas and Fas ligand gene expression in human ovarian, cervical and endometrial carcinomas using real-time quantitative RT-PCR. Br. J. Cancer 82:1682-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emery, J. G., P. McDonnell, M. B. Burke, K. C. Deen, S. Lyn, C. Silverman, E. Dul, E. R. Appelbaum, C. Eichman, R. DiPrinzio, R. A. Dodds, I. E. James, M. Rosenberg, J. C. Lee, and P. R. Young. 1998. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J. Biol. Chem. 273:14363-14367. [DOI] [PubMed] [Google Scholar]
- 14.Faulkner-Valle, G., and L. Banks. 1995. The human papillomavirus (HPV)-6 and HPV-16 E5 proteins co-operate with HPV-16 E7 in the transformation of primary rodent cells. J. Gen. Virol. 76:1239-1245. [DOI] [PubMed] [Google Scholar]
- 15.Fesik, S. W. 2000. Insights into programmed cell death through structural biology. Cell 103:273-282. [DOI] [PubMed] [Google Scholar]
- 16.Frattini, M. G., and L. A. Laimins. 1994. The role of the E1 and E2 proteins in the replication of human papillomavirus type 31b. Virology 204:799-804. [DOI] [PubMed] [Google Scholar]
- 17.Hengartner, M. O. 2000. The biochemistry of apoptosis. Nature 407:770-776. [DOI] [PubMed] [Google Scholar]
- 18.Hildesheim, A., M. H. Schiffman, P. E. Gravitt, A. G. Glass, C. E. Greer, T. Zhang, D. R. Scott, B. B. Rush, P. Lawler, M. E. Sherman, R. J. Kurman, and M. M. Manos. 1994. Persistence of type-specific human papillomavirus infection among cytologically normal women. J. Infect. Dis. 169:235-240. [DOI] [PubMed] [Google Scholar]
- 19.Hymowitz, S. G., H. W. Christinger, G. Fuh, M. Ultsch, M. O'Connell, R. F. Kelley, A. Ashkenazi, and A. M. de Vos. 1999. Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol. Cell 4:563-571. [DOI] [PubMed] [Google Scholar]
- 20.Kabsch, K., and A. Alonso. 2002. The human papillomavirus type 16 (HPV-16) E5 protein sensitizes human keratinocytes to apoptosis induced by osmotic stress. Oncogene 21:947-953. [DOI] [PubMed] [Google Scholar]
- 21.Kaufmann, S. H., S. Desnoyers, Y. Ottaviano, N. E. Davidson, and G. G. Poirier. 1993. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: An early marker of chemotherapy-induced apoptosis. Cancer Res. 53:3976-3985. [PubMed] [Google Scholar]
- 22.Kell, B., R. J. Jewers, J. Cason, F. Pakarian, J. N. Kaye, and J. M. Best. 1994. Detection of E5 oncoprotein in human papillomavirus type 16-positive cervical scrapes using antibodies raised to synthetic peptides. J. Gen. Virol. 75:2451-2456. [DOI] [PubMed] [Google Scholar]
- 23.Kischkel, F. C., D. A. Lawrence, A. Chuntharapai, P. Schow, K. J. Kim, and A. Ashkenazi. 2000. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity 12:611-620. [DOI] [PubMed] [Google Scholar]
- 24.Krueger, A., S. Baumann, P. H. Krammer, and S. Kirchhoff. 2001. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol. Cell. Biol. 21:8247-8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leechanachai, P., L. Banks, F. Moreau, and G. Matlashewski. 1992. The E5 gene from human papillomavirus type 16 is an oncogene which enhances growth factor-mediated signal transduction to the nucleus. Oncogene 7:19-25. [PubMed] [Google Scholar]
- 26.Leithäuser, F., J. Dhein, G. Mechtersheimer, K. Koretz, S. Brüderlein, C. Henne, A. Schmidt, K.-M. Debatin, P. H. Krammer, and P. Möller. 1993. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab. Investig. 69:415-429. [PubMed] [Google Scholar]
- 27.Leptak, C., S. Ramon y Cajal, R. Kulke, B. H. Horwitz, D. J. Riese, I. I., G. P. Dotto, and D. DiMaio. 1991. Tumorigenic transformation of murine keratinocytes by the E5 genes of bovine papillomavirus type 1 and human papillomavirus type 16. J. Virol. 65:7078-7083. (Erratum, J. Virol. 66: 1833, 1992.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMurray, H. R., D. Nguyen, T. F. Westbrook, and D. J. McAnce. 2001. Biology of human papillomaviruses. Int. J. Exp. Pathol. 82:15-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayer, T. J., and C. Meyers. 1998. Temporal and spatial expression of the E5a protein during the differentiation-dependent life cycle of human papillomavirus type 31b. Virology 248:208-217. [DOI] [PubMed] [Google Scholar]
- 30.Meyers, C., T. J. Mayer, and M. A. Ozbun. 1997. Synthesis of infectious human papillomavirus type 18 in differentiating epithelium transfected with viral DNA. J. Virol. 71:7381-7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Brien, V. 1998. Viruses and apoptosis. J. Gen. Virol. 79:1833-1845. [DOI] [PubMed] [Google Scholar]
- 32.Oelze, I., J. Kartenbeck, K. Crusius, and A. Alonso. 1995. Human papillomavirus type 16 E5 protein affects cell-cell communication in an epithelial cell line. J. Virol. 69:4489-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan, G., K. O'Rourke, A. M. Chinnaiyan, R. Gentz, R. Ebner, J. Ni, and V. M. Dixit. 1997. The receptor for the cytotoxic ligand TRAIL. Science 276:111-113. [DOI] [PubMed] [Google Scholar]
- 34.Pan, G., J. Ni, Y. F. Wei, G.-L. Yu, R. Gentz, and V. M. Dixit. 1997. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277:815-818. [DOI] [PubMed] [Google Scholar]
- 35.Pan, G., J. Ni, G.-L. Yu, Y. F. Wei, and V. M. Dixit. 1998. TRUNDD, a new member of the TRAIL receptor family that antagonizes TRAIL signaling. FEBS Lett. 424:41-45. [DOI] [PubMed] [Google Scholar]
- 36.Pim, D., M. Collins, and L. Banks. 1992. Human papillomavirus type 16 E5 gene stimulates the transforming activity of the epidermal growth factor receptor. Oncogene 7:27-32. [PubMed] [Google Scholar]
- 37.Rich, T., R. L. Allen, and A. H. Wyllie. 2000. Defying death after DNA damage. Nature 407:777-783. [DOI] [PubMed] [Google Scholar]
- 38.Ryu, H. S., K. H. Chang, S. J. Chang, M. S. Kim, H. J. Joo, and K. S. Oh. 2000. Expression of TRAIL (TNF-related apoptosis-inducing ligand) receptors in cervical cancer. Int. J. Gynecol. Cancer 10:417-424. [DOI] [PubMed] [Google Scholar]
- 39.Scaffidi, C., J. P. Medema, P. H. Krammer, and M. E. Peter. 1997. FLICE is predominantly expressed as two functionally active isoforms, caspase-8/a and caspase-8/b. J. Biol. Chem. 272:26953-26958. [DOI] [PubMed] [Google Scholar]
- 40.Scaffidi, C., I. Schmitz, P. H. Krammer, and M. E. Peter. 1999. The role of c-FLIP in modulation of CD95-induced apoptosis. J. Biol. Chem. 274:1541-1548. [DOI] [PubMed] [Google Scholar]
- 41.Scheffner, M., B. A. Werness, J. M. Huibregtse, A. J. Levine, and P. M. Howley. 1990. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63:1129-1136. [DOI] [PubMed] [Google Scholar]
- 42.Schneider, P., J.-L. Bodmer, N. Holler, C. Mattmann, P. Scuderi, A. Terskikh, M. C. Peitsch, and J. Tschopp. 1997. Characterization of Fas (Apo-1, CD95)-Fas Ligand interaction. J. Biol. Chem. 272:18827-18833. [DOI] [PubMed] [Google Scholar]
- 43.Schneider, P., M. Thome, K. Burns, J.-L. Bodmer, K. Hofmann, T. Kataoka, N. Holler, and J. Tschopp. 1997. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-κB. Immunity 7:831-836. [DOI] [PubMed] [Google Scholar]
- 44.Sedger, L. M., D. M. Shows, R. A. Blanton, J. J. Peschon, R. G. Goodwin, D. Cosman, and S. R. Wiley. 1999. IFN-γ mediates a novel antiviral activity through dynamic modulation of TRAIL and TRAIL receptor expression. J. Immunol. 163:920-926. [PubMed] [Google Scholar]
- 45.Sheridan, J. P., S. A. Marsters, R. M. Pitti, A. Gurney, M. Skubatch, D. Baldwin, L. Ramakrishnan, C. L. Gray, K. Baker, W. I. Wood, A. D. Goddard, P. Godowski, and A. Ashkenazi. 1997. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277:818-821. [DOI] [PubMed] [Google Scholar]
- 46.Shisler, J., C. Yang, B. Walter, C. F. Ware, and L. R. Gooding. 1997. The adenovirus E3-10.4K/14.5K complex mediates loss of cell surface Fas (CD95) and resistance to Fas-induced apoptosis. J. Virol. 71:8299-8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sprick, M. R., M. A. Weigand, E. Rieser, C. T. Rauch, P. Juo, J. Blenis, P. H. Krammer, and H. Walczak. 2000. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 12:599-609. [DOI] [PubMed] [Google Scholar]
- 48.Stoler, M. H., C. R. Rhodes, A. Whitbeck, S. M. Wolinsky, L. T. Chow, and T. R. Broker. 1992. Human papillomavirus type 16 and 18 gene expression in cervical neoplasias. Hum. Pathol. 23:117-128. [DOI] [PubMed] [Google Scholar]
- 49.Straight, S. W., P. M. Hinkle, R. J. Jewers, and D. J. McCance. 1993. The E5 oncoprotein of human papillomavirus type 16 transforms fibroblasts and effects the downregulation of the epidermal growth factor receptor in keratinocytes. J. Virol. 67:4521-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strasser, A., L. O'Connor, and V. M. Dixit. 2000. Apoptosis signaling. Annu. Rev. Biochem. 69:217-245. [DOI] [PubMed] [Google Scholar]
- 51.Teodoro, J. G., and P. E. Branton. 1997. Regulation of apoptosis by viral gene products. J. Virol. 71:1739-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomsen, P., B. van Deurs, B. Norrild, and L. Kayser. 2000. The HPV16 E5 oncogene inhibits endocytic trafficking. Oncogene 19:6023-6032. [DOI] [PubMed] [Google Scholar]
- 53.Thompson, C. B. 1995. Apoptosis in the pathogenesis and treatment of disease. Science 267:1456-1462. [DOI] [PubMed] [Google Scholar]
- 54.Tollefson, A. E., T. W. Hermiston, D. L. Lichtenstein, C. F. Colle, R. A. Tripp, T. Dimitrov, K. Toth, C. E. Wells, P. C. Doherty, and W. S. M. Wold. 1998. Forced degradation of Fas inhibits apoptosis in adenovirus-infected cells. Nature 392:726-730. [DOI] [PubMed] [Google Scholar]
- 55.Tollefson, A. E., K. Toth, K. Doronin, M. Kuppuswamy, O. A. Doronina, T. W. Hermiston, C. A. Smith, and W. S. M. Wold. 2001. Inhibition of TRAIL-induced apoptosis and forced internalization of TRAIL receptor 1 by adenovirus proteins. J. Virol. 75:8875-8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trauth, B. C., C. Klas, A. M. J. Peters, S. Matzku, P. Möller, W. Falk, K.-M. Debatin, and P. H. Krammer. 1989. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science 245:301-305. [DOI] [PubMed] [Google Scholar]
- 57.Vidalain, P.-O., O. Azocar, B. Lamouille, A. Astier, C. Rabourdin-Combe, and C. Servet-Delprat. 2000. Measles virus induces functional TRAIL production by human dendritic cells. J. Virol. 74:556-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walczak, H., and P. H. Krammer. 2000. The CD95(APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp. Cell Res. 256:58-66. [DOI] [PubMed] [Google Scholar]
- 59.Werness, B. A., A. J. Levine, and P. M. Howley. 1990. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248:76-79. [DOI] [PubMed] [Google Scholar]
- 60.Wiley, S. R., K. Schooley, P. J. Smolak, W. S. Din, C.-P. Huang, J. K. Nicholl, G. R. Sutherland, T. D. Smith, C. Rauch, C. A. Smith, and R. G. Goodwin. 1995. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3:673-682. [DOI] [PubMed] [Google Scholar]
- 61.Zhang, B., D. F. Spandau, and A. Roman. 2002. E5 protein of human papillomavirus type 16 protects human foreskin keratinocytes from UV B-irradiation-induced apoptosis. J. Virol. 76:220-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, X. D., T. Nguyen, W. D. Thomas, J. E. Sanders, and P. Hersey. 2000. Mechanisms of resistance of normal cells to TRAIL induced apoptosis vary between different cell types. FEBS Lett. 482:193-199. [DOI] [PubMed] [Google Scholar]
- 63.Zörnig, M., A. Grzeschiczek, M.-B. Kowalski, K.-U. Hartmann, and T. Möröy. 1995. Loss of Fas/Apo-1 receptor accelerates lymphomagenesis in Eμ L-myc transgenic mice but not in animals infected with MoMuLV. Oncogene 10:2397-2401. [PubMed] [Google Scholar]
- 64.Zur Hausen, H. 1991. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology 184:9-13. [DOI] [PubMed] [Google Scholar]