Abstract
A new virus previously arose in BALB/c females mated repeatedly to C57BL/6 (B6) males and then injected with fixed, activated B6 male spleen cells (V. S. Ter-Grigorov, O. Krifuks, E. Liubashevsky, A. Nyska, Z. Trainin, and V. Toder, Nat. Med. 3:37-41, 1997). In the present study, BALB/cJ mice inoculated with virus-containing plasma from affected mice developed splenomegaly, which was caused by increased numbers of Sca-1+ Lin− hematopoietic stem cells (HSC) and their differentiated progeny. Biological and molecular analyses of a new virus revealed a mixture of murine leukemia viruses (MuLVs). These MuLVs comprised ecotropic and mink lung cell focus-forming (MCF) virus classes and are termed Rauscher-like MuLVs because they bear numerous similarities to the ecotropic and MCF viruses of the Rauscher MuLV complex but do not include a spleen focus-forming virus. The ecotropic virus component alone transferred some disease characteristics, while MCF virus alone did not. Thus, we have described a novel virus mixture, termed Rauscher-like MuLV, that causes an increase in hematopoiesis due to activation of pluripotent HSC. Experiments using mice and a protocol that replicated the pregnancy and immunization strategy of the original experiment demonstrated that endogenous BALB/c mouse ecotropic and xenotropic MuLVs are activated by these treatments. Emv1 was expressed in the spleens of multiparous mice but not in those of virgin mice, and Bxv1Emv1-pseudotyped MuLVs were recovered following injection of fixed, activated B6 cells. Thus, multiple pregnancies and allostimuli appear to have provided the signals required for activation of and recombination among endogenous viruses and could have resulted in generation of the Rauscher-like MuLV mixture.
The generation of highly pathogenic retroviruses from endogenous viruses has been clearly demonstrated in a number of diseases including T-cell lymphomas in AKR mice (7, 19, 26, 47, 50) and a murine AIDS termed MAIDS (11, 29). A system recently described by Ter-Grigorov et al. has also implicated endogenous viruses in the generation of new pathogenic retroviruses that induce immunodeficiency (49). In those experiments, BALB/c females mated to C57BL/6 (B6) males for 1 year and then immunized with concanavalin A-activated paternal lymphocytes developed one of two conditions: approximately 75% of treated mice developed a chronic disease resembling AIDS (AIDS-like disease), while 25% developed acute leukemia and died 2 to 4 months after alloimmunization. No BALB/c females mated to syngeneic males prior to alloimmunization developed AIDS-like disease or tumors (49).
The typical course of the AIDS-like disease reported by Ter-Grigorov progresses through four stages: (i) acute lymphoproliferation beginning 4 to 7 weeks after immunization and lasting 2 to 4 weeks; (ii) clinical latency, with a reduction of spleen size, lasting 2 to 16 weeks; (iii) progressive splenomegaly, cachexia, and skin lesions; and (iv) immunodeficiency and progressive lymphatic tissue exhaustion with secondary pulmonary or intestinal infections (49). The disease was attributed to murine leukemia viruses (MuLVs) selected in BALB/c females during the course of experiments. However, the exact composition of the population of selected viruses was not known. Thus, we sought to characterize the virus population and investigate their role in disease induction.
MATERIALS AND METHODS
Mice.
Female and male BALB/cJ and BALB/cByJ mice were purchased from The Jackson Laboratory (Bar Harbor, Maine).
Virus testing.
The disease-inducing isolate, termed here Rauscher-like MuLV (RL-MuLV), was vir6, one of eight independent isolates recovered by Ter-Grigorov and collaborators from the plasma of diseased BALB/c mice inoculated with plasma of diseased BALB/c females allostimulated with B6 lymphocytes. The virus isolate was used to infect SC-1 cells and after three passages was pelleted from tissue culture supernatants by ultracentrifugation at 95,000 × g. The pellet was tested by the Diagnostic Laboratory at The Jackson Laboratory for mouse hepatitis virus, mouse thymic virus, mouse parvovirus, pneumonia virus of the mouse, polyomavirus, mammalian orthoreovirus serotype 3, enzootic diarrhea virus of infant mice, Sendai virus, Mycoplasma pulmonis, Mycoplasma arthridis, cilium-associated respiratory bacillus, ectromelia virus, encephalitozoon cuniculi, Theiler's murine encephalomyelitis virus, Hantaan virus (Korean hemorrhagic virus), lymphocytic choriomeningitis virus, lactate dehydrogenase enzyme, minute virus of the mouse, mouse adenovirus, and mouse cytomegalovirus and was found to be negative for all the pathogens.
The RL-MuLV was propagated in BALB/cJ mice older than 5 weeks by intraperitoneal inoculation with plasma from affected mice. Samples of heparinized plasma obtained from infected BALB/cJ mice with splenomegaly (usually 3 to 4 months after infection) were passed through 0.22-μm-pore-size filters, divided into aliquots, and stored at −70°C until used.
Tests for ecotropic virus were the XC plaque assay (45), with titers expressed in PFU, and immunofluorescence of infected splenocytes and tissue culture cells with monoclonal antibody (MAb) 48 (12). Tests for mink lung cell focus-forming (MCF) virus were the SC-1 UV-Mink assay (15) and immunofluorescence of infected splenocytes and tissue culture cells with MAb 514 (12). Experimental 3- to 4-week-old BALB/cJ mice were inoculated intraperitoneally with 8,000 to 10,000 XC PFU of either the RL-MuLV mixture or the ecotropic virus component of RL-MuLV mixture and 8,000 to 10,000 UV-Mink PFU of MCF virus.
N, B, or NB tropism of the RL-MuLV ecotropic component was determined by titrating the harvest of SC-1 RL-MuLV-infected cultures in BALB 3T3 (Fv1bb) and NIH 3T3 (Fv1nn) cells as previously described (34).
CFU assay.
Single-cell suspensions of 105 cells prepared from 15 infected or 15 control mouse spleens were inoculated into BALB/cByJ male mice irradiated by 900 rad. Eight days after transfer, mice were sacrificed and splenic colonies were counted after fixation in Bouin's fixative. In some cases, cells taken from colonies in unfixed spleens were counted and equal cell numbers were subjected to a second transfer.
Fluorescence-activated cell sorting (FACS) analysis.
Single-cell suspensions from 10 infected and 10 control mouse spleens were stained after lysing erythrocytes by osmotic shock and analyzed with a FACScan cytometer by using the Cell Quest program (Becton Dickinson). The following MAbs were used to stain cells: TER119 for erythroid cells (PharMingen, San Diego, Calif.), H57-597 for T-cell receptor (TCR) α/β T cells (PharMingen), GL3 for TCR γ/δ T cells (PharMingen), RA3-6B2 anti-CD45R for B cells (PharMingen), M1/70 anti-CD11b/Mac-1 for macrophages and granulocytes (PharMingen), DX5 for NK cells (PharMingen), RB6-8C5 anti-Gr-1/Ly6G for granulocytes (PharMingen), F4/80 anti-pan macrophages (Caltag, Burlingame, Calif.), CD11b antimacrophages (PharMingen), CD11c anti-dendritic cells (PharMingen), H129.19 for CD4+ T cells (GIBCO BRL, Gaithersburg, Md.), 53-6.7 for CD8α+ T cells (Sigma, St. Louis, Mo.), anti-CD3e (PharMingen), and anti-CD5 (PharMingen). Dead cells were excluded by propidium iodide staining (Sigma).
For analysis of hematopoietic stem cells (HSC), bone marrow cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-Sca-1 (clone D7; PharMingen) and anti-CD3, anti-CD-5, anti-B220, anti-CD4, anti-CD8, anti-TER119, anti-Gr-1, and anti-CD11b MAbs conjugated with phycoerythrin.
Cell lines.
The following cell lines were used to propagate MuLVs and to classify them and determine their host ranges: Mus dunni mouse fibroblast (III8c), XC, BALB 3T3 (A31; Fv1bb, susceptible to B- and NB-tropic viruses), NIH 3T3 (Fv1nn, susceptible to N- and NB-tropic viruses), SC-1 (Fv100, susceptible to N-, B-, and NB-tropic viruses), and mink lung cells. All cell lines were purchased from the American Type Culture Collection (ATCC; Manassas, Va.). NRK cells transfected with spleen focus-forming virus (SFFV) alone and SFFV plus Friend MuLV (F-MuLV) 57 (21) were a generous gift from Leonard Evans.
Cloning.
RNA isolated from virions (14) pelleted from RL-MuLV-infected SC-1 cells was used to make a cDNA library with the SuperScript plasmid system for cDNA synthesis and plasmid cloning (GIBCO BRL). DNAs of clones that hybridized with ecotropic virus-specific (9) or xenotropic-polytropic-MCF virus-specific probes (7) were sequenced. A plasmid containing a full-length MuLV (pAKV) was kindly provided by W. Herr.
To clone the env gene of MuLV, produced by the endogenous ecotropic locus Emv1, splenocytes from multiparous BALB/cJ females that had been mated to B6 males and that had received multiple injections of B6 lymphocytes were cocultured with M. dunni cells. After the fifth passage, virions were pelleted from cell-free supernatants, and viral RNA was used to make a cDNA library as described above. Plasmid DNAs of clones that hybridized with the ecotropic virus env-specific probe (9) were sequenced. To clone the env gene of MuLV produced by the endogenous locus Bxv1, the same RNA was used to prepare cDNA by using SuperScript II reverse transcriptase in the buffer supplied by the manufacturer (GIBCO BRL) and a deoxythymine (15) primer (Promega Biotec, Inc., Madison, Wis.). The cDNAs were then amplified by PCR with forward primer 5′CCGAGTCGGTGACACAGTGTG3′ (nucleotides [nt] 5589 to 55609 within the pol gene according to the Rauscher MuLV numbering) (28) and reverse primer 5′CATTCCCCCCTTTTTCTGGAAACT3′ (nt 7845 to 7822 within the env gene according to the Rauscher MuLV numbering) (28). The PCR products were size fractionated on 1% agarose gels, purified, and cloned into the SrfI site of pCRScript according to the manufacturer's protocol (Stratagene, Inc., La Jolla, Calif.). At least three independent plasmids were sequenced for each virus.
Immunofluorescence analysis.
The following antibodies (Abs) were used to stain cell lines and cell suspensions from mouse spleens: MAb 48, specific for Rauscher and Friend ecotropic MuLVs (12, 13) (ATCC); MAb 514, specific for MCF MuLVs (12) (ATCC); MAb 603, specific for xenotropic MuLV (42); and goat anti-F-MuLV gp70 polyclonal serum (1). FITC-labeled anti-mouse polyvalent immunoglobulins (Sigma) were used at the second step.
Western blot analysis.
Proteins were collected from CsCl2 gradients generated from spleens of control or infected mice and generated from NRK cells infected with SFFV alone or with SFFV plus F-MuLV. To dissolve insoluble proteins, 0.2% NP-40 was added to the protein fractions. Six samples from one gradient were dialyzed against 6 liters of phosphate-buffered saline (PBS)-0.1 M EDTA (pH 8.0)-0.2% NP-40 for 4 h at 4°C. After dialysis, samples were diluted with an equal volume of PBS and stored at −70°C. Phenylmethylsulfonyl fluoride (200 μg/ml) was added prior to freezing. Proteins were electrophoresed on polyacrylamide gels under reducing conditions in the presence of 1% sodium dodecyl sulfate, transferred to nitrocellulose membranes, immunoblotted with goat anti-F-MuLV gp70 polyclonal serum (1), and detected with Western blotting detection reagents (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.).
Cocultivation assay.
SC-1 cells were plated at 2 × 105 per 60-mm-diameter dish the day before infection. On the day of infection, the medium was changed and 106 mitomycin C-treated single-cell suspensions from the spleens of infected mice were added, followed by immunofluorescence analysis on day 5 after infection.
Nucleotide sequence accession numbers.
Nucleotide sequences of the env genes have been submitted to the GenBank nucleotide sequence database and have been assigned accession no. AF288940 for ecotropic RL-MuLV, AF288942 for MCF RL-MuLV, AF288939 for the env gene of MuLV produced by the endogenous ecotropic virus locus Emv1, and AF288941 for the env gene of MuLV produced by the endogenous locus Bxv1.
RESULTS
Characteristics of disease in normal BALB/cJ mice inoculated with plasma from affected mice.
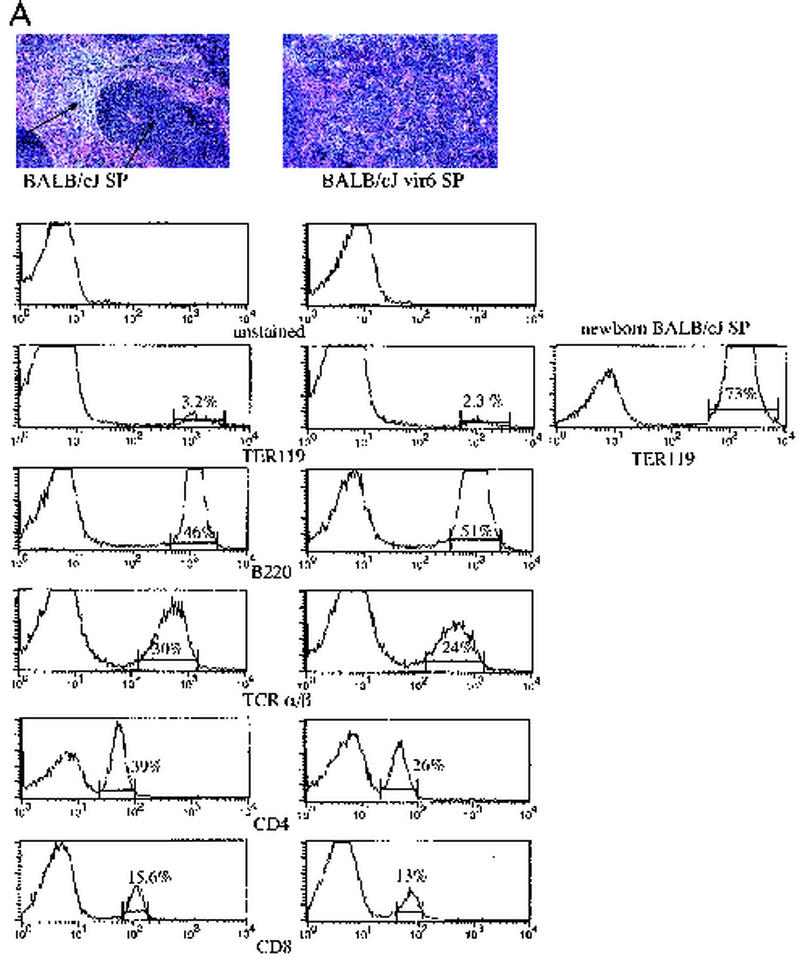
In a previous study, almost all BALB/c female mice mated repeatedly with B6 males for a year and then inoculated with fixed, activated B6 spleen cells developed splenomegaly (49). Electron-microscopic studies of spleens from infected mice showed an abundance of C-type virions, suggesting that MuLVs might be involved in pathogenesis of the disease (49). To determine whether MuLVs were indeed responsible for this effect, we studied BALB/cJ mice injected with a cell-free, in vivo-passaged vir6 isolate recovered from filtered plasma of affected mice. At 4 to 6 months after inoculation, spleen weights had increased to a mean of approximately 400 mg, compared to a mean of 120 mg in uninfected mice. Histologic studies of enlarged spleens revealed an expanded red pulp with enhanced extramedullary hematopoiesis, evidenced by increased numbers of erythroid and myeloid precursors and megakaryocytes (Fig. 1A). Hematocrit in infected mice (41% ± 5% [mean ± standard deviation] for four mice) did not differ significantly from that in uninfected mice (49% ± 5% for four mice). Cell suspensions from enlarged spleens of vir6-infected mice (3 to 5 months after infection) were stained with lineage-specific MAbs against erythroid cells, TCR α/β and TCR γ/δ T cells, B cells, macrophages, NK cells, and granulocytes and were analyzed by FACS. The proportion of each cell type was the same as those in the spleens of normal and infected mice, except for a slightly lower percentage of TCR α/β T cells in infected mice (Fig. 1A), indicating that proliferation was not restricted to a specific cell type and that all cell types contributed to splenomegaly in infected mice.
FIG. 1.
Selective proliferation of Sca-1+ cells results in increased extramedullary hematopoiesis and splenomegaly in vir6-infected mice. (A) (Top) Hematoxylin- and eosin-stained sections from spleens of a normal 4.5-month-old BALB/cJ male and an age-matched vir6-infected BALB/cJ male. Arrows, white and red pulps. (Bottom) Flow-cytometric analysis of splenic cells of the same mice. Nucleated spleen cells were stained with anti-Ter119, anti-B220/CD45R, anti-TCR α/β, anti-CD4, and anti-CD8 and analyzed by FACS. Mice infected more than 4 months had enlarged spleens with 160 × 107 to 180 × 107 total cells compared to 6.8 × 107 to 7.6 × 107 total cells in the spleens of uninfected mice. The percentages of cells with surface markers for other cell lineages were also determined, and no differences between infected and control mice were observed (data not shown). Analyzed markers included TCR γ/δ T cells, CD11b/Mac-1 and F4/80 (macrophage specific), NK cells (MAb DX5), and Gr-1/Ly6G (granulocyte specific). To ensure that the osmotic shock used to lyse red blood cells did not affect TER119-positive cells, splenocytes from newborn BALB/cJ mice were included. Data from one of eight experiments are shown. (B) Flow-cytometric analysis of the same splenic cells with anti-Sca-1 MAbs and antibodies against lineage-specific markers. Fifteen infected and 10 uninfected BALB/cJ mice were used. Values are averages ± standard deviations.
Since all hematopoietic lineages were present in the enlarged spleens of infected mice, it is possible that these lineages resulted from the expansion of an HSC population. To determine if the numbers of HSC in enlarged spleens were also increased, 105 cells from the spleens of normal and infected mice were tested in a CFU assay of irradiated recipients. In three independent experiments, 105 cells isolated from the spleens of infected or normal BALB/cJ mice were intravenously injected into irradiated 3- to 4-week-old BALB/cByJ male mice. Eight days later, the mice were killed, spleens were fixed, colonies were counted, and spleen sections were stained. Transfer of normal spleen cells to irradiated recipients (n = 5) resulted in an average of 5 ± 1 colonies per spleen, while transfer of spleen cells from infected mice to the recipients (n = 5) resulted in an average of 25 ± 3 colonies per spleen. To ensure that these CFU were indeed produced by stem cells, we performed a secondary transfer. In this transfer, uninfected cells (105) from primary colonies did not produce any colonies in recipient mice (n = 4), as expected, while the same number of cells from infected primary colonies produced 4 ± 1 colonies/spleen upon transfer into recipient mice (n = 4). The colonies formed from transfers of infected splenocytes also comprised a mixture of hematopoietic cell types (not shown). These data suggest that virus infection leads to expansion of HSC as measured by in vivo CFU assay.
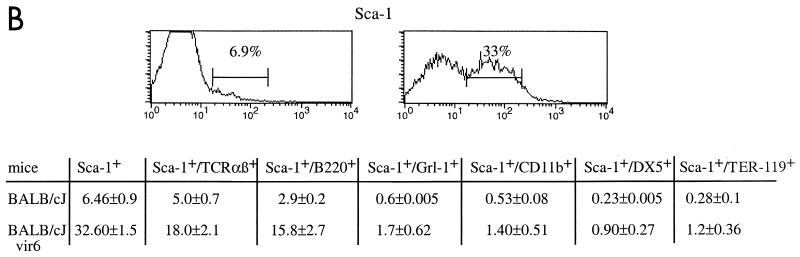
In the mouse, HSC are lineage marker negative (Lin−) and Sca-1+ (37, 38). As these cells differentiate, they acquire lineage-specific markers and gradually lose stem cell markers. To determine whether splenomegaly in vir6-infected BALB/cJ mice might be due to the proliferation of the HSC, splenocytes of vir6-infected and uninfected BALB/cJ mice were stained with antibodies against lineage-specific markers and against Sca-1. Spleens of uninfected BALB/cJ mice contained an average of 6.9% Sca-1+ cells, whereas spleens of vir6-infected BALB/cJ mice contained an average of 33% Sca-1+ cells (Fig. 1B). For all lineages, spleens of infected mice contained approximately three to five times more cells expressing the Sca-1+ marker than spleens of uninfected mice (Fig. 1B). We then used the same antibody-staining protocol to compare HSC populations in the bone marrow of infected and uninfected BALB/cJ mice. In uninfected BALB/cJ mice, the Sca-1 fraction that did not express lineage-specific markers made up approximately 0.02% ± 0.005% (n = 5) of the bone marrow cell population. In contrast, the same cell fraction comprised 0.26% ± 0.02% (n = 5) of the bone marrow cell population of vir6-infected age- and sex-matched BALB/cJ mice. Thus, in both the spleens and bone marrow, the proportion of HSC in vir6-infected mice was 5- to 10-fold greater than that in uninfected mice.
Characteristics of MuLVs in the pathogenic inoculum.
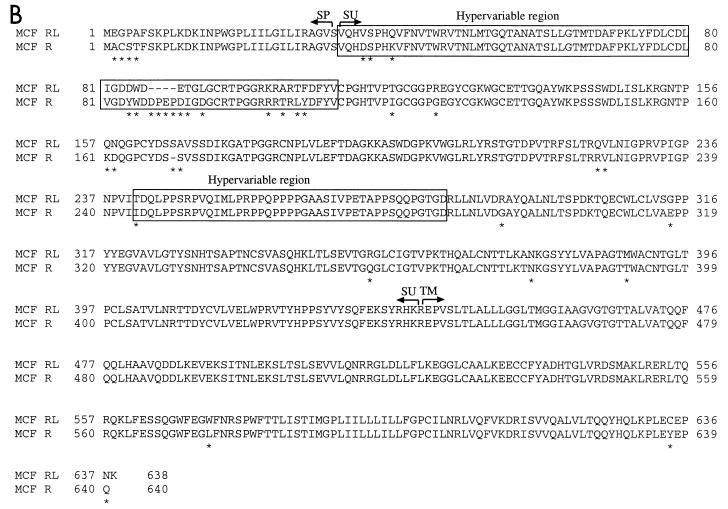
To molecularly clone the disease-inducing viruses, virions from vir6-infected SC-1 cells were pelleted and viral RNA was used to produce a cDNA library; clones that hybridized with MuLV-specific probes were sequenced. Sequence analysis demonstrated a mixture of two viruses: a replication-competent ecotropic MuLV and an MCF virus. Defective virus genomes were not encountered. To determine the similarities between these MuLVs and other known MuLVs, we compared their Env amino acid sequences. Amino acid alignments of the env gene products indicated that both components were highly similar, but not identical, to the corresponding components of the Rauscher MuLV complex (Fig. 2). Therefore, the virus mixture in the vir6 isolate is referred to as RL-MuLV.
FIG. 2.
Alignment of env gene products. (A) Alignment of predicted amino acid sequences of the ecotropic virus component of the R-MuLV complex (eco R) (28) and the ecotropic virus component of the RL-MuLV mixture (eco RL). (B) Alignment of predicted amino acid sequences of the env gene product of the MCF virus component of the R-MuLV complex (MCF R) (52) and the MCF virus component of the RL-MuLV mixture (MCF RL). Unlike the Rauscher MCF virus, the Friend MCF virus lacks aa 87 to 90 (41). The two regions with the most marked differences between xenotropic and polytropic amino acid sequences are boxed (hypervariable regions); another boxed region is the proline-rich region (40). ∗, different amino acids; -, deleted amino acids. SP, signal peptide; SU, surface protein; TM, transmembrane protein.
To verify the similarity between the components of the RL-MuLV and those in the Rauscher virus complex, splenocytes from infected BALB/cJ mice were stained with MAb 48, which binds to gp70Env of the ecotropic virus component of the Friend and Rauscher MuLV complexes, and analyzed by FACS. Splenocytes from infected animals were stained by MAb 48, suggesting a close similarity between surface glycoproteins produced by these viruses (data not shown). In addition, infected splenocytes were positive for staining with MAb 514, which binds to Env of most MCF MuLVs (data not shown).
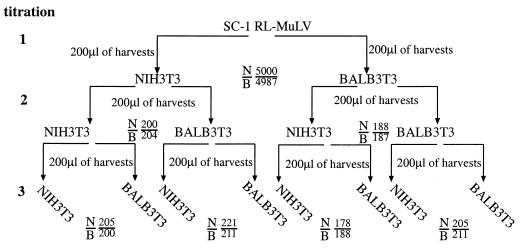
Ecotropic MuLVs are classified according to their host range as either N-tropic, B-tropic, or NB-tropic depending upon whether they grow preferentially on cells from NIH Swiss mice, cells from BALB/c mice, or on both cell types, respectively (26). This phenotype is determined by alleles of the Fv1 gene (3); BALB/c mice have the b allele of the gene (Fv1bb) and are resistant to the N-tropic virus (25). To determine whether the ecotropic component of the RL-MuLV mixture is N-, B-, or NB-tropic, we subjected supernatants from RL-MuLV-infected SC-1 cells to a quantitative assay at a low multiplicity of infection of Fv1nn and Fv1bb cells (34). The ecotropic virus component of the RL-MuLV mixture was equally infectious on either cell type (Fig. 3), indicating that the ecotropic virus component of the RL-MuLV mixture is NB-tropic.
FIG. 3.
The ecotropic virus component of the RL-MuLV mixture is NB-tropic. Supernatants from RL-MuLV-infected SC-1 cells were titrated simultaneously in Fv1nn (NIH 3T3) and Fv1bb (BALB 3T3) cells (titration 1). The XC plaque assay was used for quantitation, and extra plates of BALB 3T3 and NIH 3T3 cells were infected at the titration end point (10−3) to reserve for harvesting. Titers of the virus on both NIH 3T3 and BALB 3T3 cells were approximately equal. To increase the virus titers, the plates reserved at titration end points were passaged once and their supernatants were collected. These supernatants were titrated again on NIH 3T3 and BALB 3T3 cells, and extra plates of BALB 3T3 and NIH 3T3 cells infected at the titration end point (10−3) were reserved for harvesting (titration 2). Cells at titration end points were passaged once, and their supernatants were collected and used for titration in NIH 3T3 and BALB 3T3 cells again, with extra plates at the titration end point (10−3) saved for harvesting (titration 3). Titers are expressed as PFU per 0.2 ml. N/B, number of PFU in NIH 3T3 cells/number of PFU in BALB 3T3 cells.
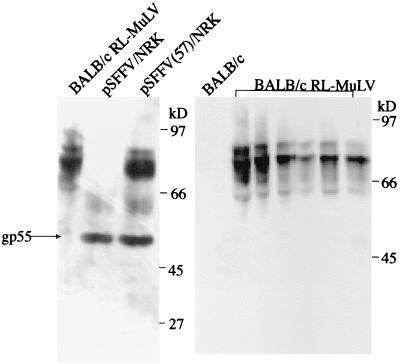
Both the Rauscher and Friend viral complexes contain replication-defective SFFVs, which encode an abnormal gp55 envelope glycoprotein that binds to the erythropoietin receptor (31). To determine whether the RL-MuLV mixture contains an SFFV, protein extracts from the spleens of infected and uninfected BALB/cJ mice were analyzed by immunoblotting with goat anti-F-MuLV gp70 polyclonal Abs (Fig. 4). Even though the Abs were capable of detecting the gp55 of SFFV, no gp55 protein was present in the extracts from spleens of RL MuLV-infected mice (Fig. 4). To confirm these results, Hirt DNA from SC-1 cells infected with the mixed-virus stocks was examined by Southern blot hybridization using probes reactive with env sequences specific for ecotropic (9) and MCF xenotropic-polytropic MuLVs (7) as well as a full-length MuLV. The Hirt DNA exhibited only 8.8-kb hybridizing species, consistent with full-length MuLV genomes (data not shown). Thus, these experiment confirmed the absence of SFFV in the RL-MuLV mixture.
FIG. 4.
Pathogenic RL-MuLV mixture does not contain SFFV. Protein extracts isolated from spleens of infected and uninfected BALB/cJ mice and cell lines expressing gp55 SFFV were separated on sodium dodecyl sulfate-acrylamide gels, blotted, and probed with goat anti-F-MuLV gp70 polyclonal antibodies. pSFFV/NRK, protein extract from the NRK cells transfected with SFFV; pSFFV(57)/NRK, protein extract from the cells transfected with SFFV and full-length competent ecotropic MuLV.
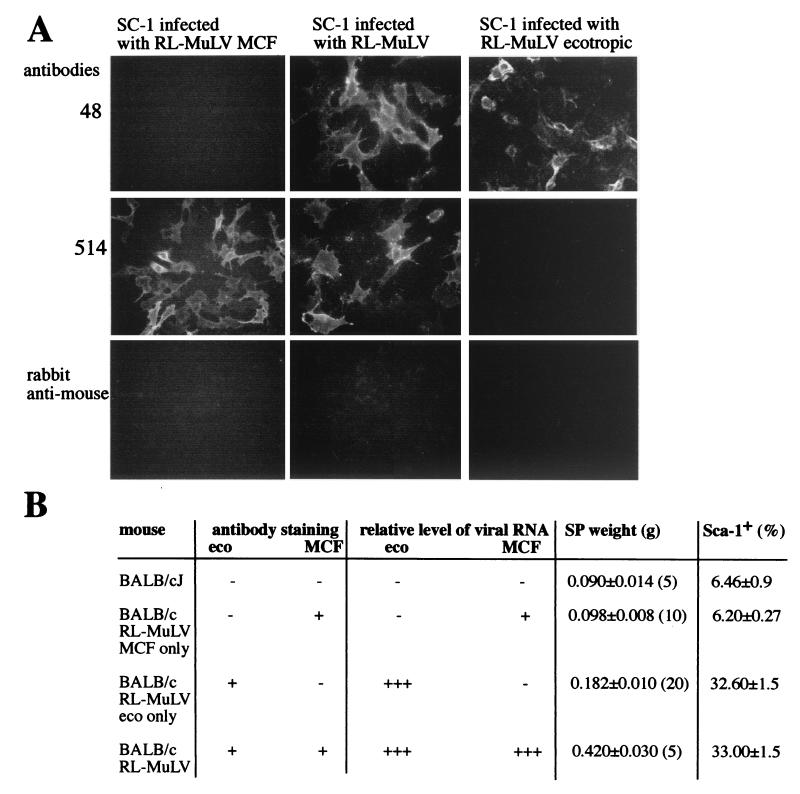
To determine whether the MCF and ecotropic viruses of the RL-MuLV mixture must both be present to induce disease, we produced SC-1 cells infected with the ecotropic virus component alone and mink lung cells infected with MCF virus component alone. Virus pools were first passaged at limiting dilutions either in mink lung cells to eliminate ecotropic virus or in XC cells to eliminate MCF virus. After six passages, cell harvests were used to infect SC-1 cells, which were subsequently passaged five additional times. To confirm that the cells were infected with MCF or ecotropic virus and were free of other components, they were stained with MAbs 514 and 48 (Fig. 5A). While SC-1 cells infected with the RL-MuLV mixture were positive for both MAbs, SC-1 cells infected with MCF virus were stained only with MAb 514 (Fig. 5A). Similarly, SC-1 cells infected with ecotropic virus were stained only with MAb 48 (Fig. 5A).
FIG. 5.
The ecotropic virus, but not the MCF virus, component of the RL-MuLV mixture causes splenomegaly. (A) Harvests from RL-MuLV-infected SC-1 cells were passaged six times in mink lung cells. After the sixth passage, harvests were used to infect SC-1 cells. Infected SC-1 cells were passaged and then stained with MAbs 48 and 514 followed by FITC-labeled polyclonal rabbit anti-mouse immunoglobulins. Similarly, harvests from RL-MuLV-infected SC-1 cells were passaged five times in XC cells, followed by infection of SC-1 cells. Also shown is the staining of infected RL-MuLV-infected SC-1 cells. (B) Harvests of the RL-MuLV ecotropic virus and MCF virus components were tested for the ability to cause disease in BALB/cJ mice. Harvest of SC-1 vir6-infected cells was used as a control. Four-week-old mice were injected with harvests from vir6-infected, MCF virus-infected, or ecotropic virus-infected SC-1 cells. Three months after infection, mice were sacrificed, spleen weights were recorded, and RNA isolated from a portion of the spleen was subjected to Northern blot analysis with ecotropic virus- and MCF virus-specific probes. +++, +, and −, relative levels of viral RNA detected in spleens by Northern blot analysis. A single-cell suspension isolated from another portion of the spleen was stained with anti-Sca-1 MAb and analyzed by FACS. Splenic cells were also cocultured with SC-1 cells. On day 5, infected SC-1 cells were stained with MAbs 514 and 48. + and −, positive and negative staining with MAbs. Numbers in parentheses, numbers of mice used. For the infection studies, 8,000 XC PFU of vir6 and ecotropic virus and 8,000 UV-mink PFU of MCF virus were used.
Harvests of cells infected with MCF virus, ecotropic virus, or vir6 were then tested for their ability to induce disease in adult BALB/cJ mice. Patterns of virus recovery based on Northern blot analysis of splenic RNA hybridized with ecotropic virus- and xenotropic-polytropic MCF virus-specific probes, induction of splenomegaly, and frequencies of Sca-1+cells are shown in Fig. 5B. All animals became infected with injected viruses. No splenomegaly developed in 3 months of observations of mice infected with MCF virus alone. In contrast, all 20 mice infected with ecotropic virus alone developed splenomegaly. In addition, all mice infected with ecotropic virus demonstrated increased frequencies of Sca-1+ cells (Fig. 5B). However, the MCF virus inoculum did not infect the mice to same extent as ecotropic virus (Fig. 5B). This is not surprising because MCF viruses can only rarely replicate well in the absence of ecotropic virus (6). These results indicate that, although splenomegaly is induced by the ecotropic virus component of the virus mixture alone, a synergy between MCF virus and ecotropic virus is required to cause full-scale disease.
Roles for multiple pregnancies and allostimulation in endogenous MuLV expression.
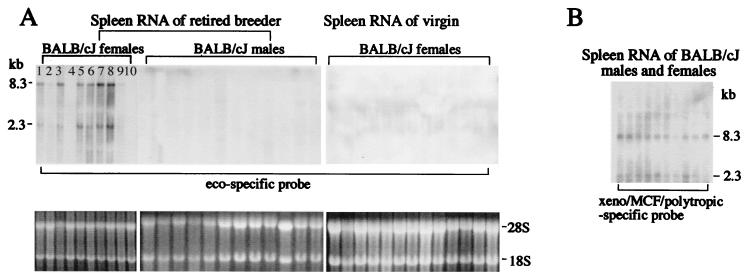
Some endogenous proviruses become expressed at high levels spontaneously or following exogenous stimuli. When this occurs, endogenous viruses may generate virions and thus may be progenitors of exogenous viruses. In the experiments of Ter-Grigorov et al. (49), two conditions may have contributed to the production of the pathogenic virus: multiple pregnancies and allostimulation. To determine the importance of multiple pregnancies in the induction of virus expression, we compared levels of endogenous ecotropic and xenotropic-polytropic virus expression in spleens from virgin and multiparous females and retired breeder males (Fig. 6). Xenotropic-polytropic viruses were constitutively expressed at low levels in all animals regardless of age and gender (Fig. 6B). However, high levels of ecotropic virus expression were seen in multiparous females but not in age-matched virgin BALB/cJ females or retired breeder males (Fig. 6A). SC-1 cells cocultured with spleen cells from BALB/cJ retired breeder females were positive for ecotropic MuLV based on Northern blot analysis with the ecotropic virus-specific probe (10 of 10 mice tested; not shown) but only infrequently positive for MuLV reactive with the xenotropic-polytropic probe (2 of 15 mice tested; not shown). Therefore, these results suggest that multiple pregnancies up-regulate expression of the endogenous ecotropic provirus in BALB/cJ mice.
FIG. 6.
Multiple pregnancies up-regulate expression of Emv1. RNA was isolated from the spleens of retired breeder BALB/cJ females and males and of virgin BALB/cJ females. Twenty micrograms of RNA was subjected to Northern blot analysis with ecotropic virus-specific (A) and xenotropic-polytropic-MCF virus-specific (B) probes. Full-genome (8.3-kb) and subgenomic (2.3-kb) viral transcripts are shown.
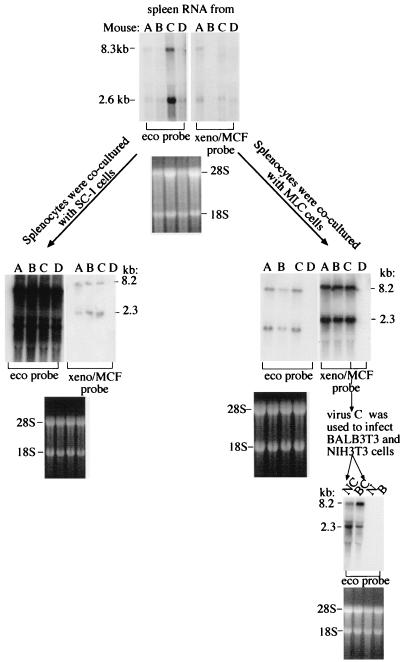
To investigate the importance of allostimulation in endogenous MuLV expression, multiparous BALB/cJ females that had been mated to B6 males were injected with either B6 lymphocytes (Fig. 7, mice of groups A to C) or with PBS (Fig. 7, mice of group D) as previously described (49). Six months later, spleen cells were tested for ecotropic and xenotropic and polytropic MuLVs by coculturing with SC-1 and mink lung cells (BALB 3T3 and NIH 3T3 cells were used in subsequent steps). Both ecotropic and xenotropic-polytropic viral RNAs were present in cells cocultured with splenocytes of alloimmunized, multiparous BALB/cJ females (Fig. 7). The xenotropism of viruses harvested from mink lung cells was confirmed by positive staining with MAb 603, which binds to Env of xenotropic viruses, and the absence of staining with MAb 514, which binds to Env of MCF viruses (not shown).
FIG. 7.
Allostimulation results in copackaging of ecotropic and xenotropic MuLV RNAs. Multiparous BALB/cJ females that had been mated to B6 males were injected with either B6 lymphocytes (mice of groups A to C, where each group consists of five mice) or with PBS (mice of group D, five mice per group). Six months later, mice were sacrificed and their spleens were divided into three parts; one part was used to isolate RNA, which was subjected to Northern blot analysis, and cells isolated from the two residual parts were cocultured with SC-1 and mink lung cells. RNA was isolated from these SC-1 and mink lung cells and was subjected to Northern blot analysis. Harvests (100 μl) from infected mink lung cells (virus C) after a sixth passage were used to infect BALB 3T3 and NIH 3T3 cells. RNA isolated from these infected cells after a third passage was also subjected to Northern blot analysis with the indicated probes.
To determine whether ecotropic virus was copackaged with xenotropic virus in allostimulated multiparous BALB/cJ females, mink lung cells cocultured with splenocytes from group C mice were passaged six times and their supernatants were used to infect NIH 3T3 and BALB 3T3 cells (Fig. 7). Harvests from infected mink lung cells (virus C) contained ecotropic viruses able to infect BALB 3T3 and NIH 3T3 cells (Fig. 7). Since mink lung cells are not permissive for ecotropic virus, ecotropic virus recovered from these cells must have been copackaged with xenotropic virus.
To determine the origin of the induced ecotropic MuLV, its env gene was cloned as described in Materials and Methods and its nucleotide and deduced amino acid sequences were compared with the corresponding published sequences of the env gene and its product for the endogenous ecotropic Emv1 provirus of BALB/c mice (27). The deduced amino acid sequence of the product of the ecotropic virus env gene induced by multiple pregnancies and alloimmune stimuli has 99% identity with the env gene product of the endogenous Emv1 provirus (not shown).
To determine the origin of the induced xenotropic virus, its env gene was cloned and its nucleotide and deduced amino acid sequences were compared with the corresponding published sequences of the env gene and its product for the endogenous xenotropic Bxv1 virus present in the genomes of BALB/c mice (33). The deduced amino acid sequence of the product of this virus env gene has 99% identity with the corresponding sequence of the env gene product of the endogenous xenotropic Bxv1 virus (not shown).
Thus, the combination of multiple pregnancies and allostimulation appears to create conditions that lead to enhanced induction of both ecotropic and xenotropic viruses that can form pseudotypes.
DISCUSSION
The data presented here identify a novel retrovirus mixture that induces splenomegaly via enhanced extramedullary hematopoiesis accompanied by proliferation of Sca-1+ Lin− HSC precursors. The mixture contains previously undescribed MCF and ecotropic virus components that have many similarities to the members of these virus classes in the Rauscher MuLV complex. Unlike the Rauscher complex, however, the novel RL-MuLV mixture does not contain a replication-defective SFFV (Fig. 4) and there was no evidence of another subgenomic component.
A number of highly pathogenic murine retroviruses have been isolated over the last 60 years by the passage of leukemia and sarcoma cells or cell-free supernatants in mice. Examples include Duplan-Laterjet (LP-BM5 or MAIDS virus) (30); Friend (22), Rauscher (43), Moloney MuLVs (35); and Moloney murine sarcoma virus (36). All of these mouse-passaged virus mixtures were found to be mixtures of ecotropic and polytropic viruses, and all except Moloney MuLV contain a replication-defective component (44). These viruses cause a wide range of diseases in mice, including T- and B-cell lymphomas; lymphoid, myeloid, and erythroid leukemias; rhabdomyosarcoma; neurological disorders; and immunosuppression (44). At least two Friend SFFV-MuLV complexes, distinguishable on the basis of their biological and molecular properties, have been identified; these isolates induce either anemia or polycythemia in susceptible mice (48).
The disease induced by the RL-MuLV mixture differs in many respects from the diseases produced by previously isolated viruses. Infection of susceptible mice, such as BALB/c, with a previously isolated Rauscher or anemia strain of Friend MuLV complexes results in a rapid persistent viremia, proliferation of erythroid progenitor cells, anemia, and splenomegaly (48). Infected mice die 4 to 6 weeks after infection, usually due to rupture of the grossly enlarged spleen. In marked contrast, infection with the RL-MuLV mixture results in proliferation of HSC progenitors in both bone marrow and spleen. Proliferation and differentiation of HSC are indicated by enhanced extramedullary hematopoiesis, evidenced by increased numbers of erythroid and myeloid precursors and megakaryocytes in spleens of infected mice (Fig. 1 and 2). The disease has some similarities with preleukemic hematopoietic hyperplasia induced by ecotropic Moloney MuLV (M-MuLV) and some strains of ecotropic F-MuLV. This hyperplasia is characterized by splenomegaly and increased numbers of hematopoietic progenitors of erythroid, myeloid, and lymphoid origin (4, 18, 46). However, the generalized hematopoietic hyperplasia is normally followed by the development of erythroid leukemia in the F-MuLV system or T-cell leukemia in the M-MuLV system (32, 51), while infection with the RL-MuLV mixture does not result in erythroleukemia or T-cell leukemia in susceptible mice observed over a 4-month period (injected either as newborns or as adults).
The disease induced by RL-MuLV mixture is also clearly different from that produced by the MAIDS virus. Like the Friend and Rauscher complex viruses, the MAIDS virus is a mixture that includes nonpathogenic, replication-competent, B-tropic ecotropic, and MCF MuLVs (23, 24) and a disease-inducing defective genome (2, 10). The defective virus encodes only a mutant gag gene. MAIDS features massive lymphoproliferation with spleen weights sometimes over 1 g and severe immune defects detectable with unseparated or separated lymphoid subsets (39).
The ecotropic virus component of RL-MuLV alone was capable of causing the disease with splenomegaly and HSC proliferation (Fig. 5). However, the RL-MuLV mixture containing both the ecotropic and MCF virus components induced more-profound splenomegaly (0.18 versus 0.4 g). It is possible that the MCF virus helps the ecotropic virus component to bind to receptors or to spread more efficiently. However, a direct pathogenic role for an MCF virus component might be obscured in testing MCF virus alone, as most lymphomagenic MCF MuLVs, although replication competent, require ecotropic MuLV to replicate efficiently and elicit disease (6).
There are few obvious differences in the env coding region between ecotropic Rauscher-MuLV and RL-MuLV that might explain the distinct phenotypes produced by these two related viruses (Fig. 2A). It is possible, however, that differences may lie in other segments of the genome. Alternatively, Env expressed on the surface of RL-MuLV-infected cells might interact with an unknown ligand(s) resulting in activation of downstream signaling events that lead to cell proliferation. In this respect, two nonconservative amino acid changes, H to Y at amino acid (aa) 666 and Y to C at aa 673, within the transmembrane domain are of special interest (Fig. 2A).
Events that are required for the generation of novel viruses include the inheritance of parental endogenous proviruses and their expression, copackaging, recombination, and infection of the ultimate targets (16, 17). The origins of the disease-inducing defective component of MAIDS virus as well as MCF virus genomes from endogenous viruses have been well documented (5, 7, 8, 20, 29, 47). What is the possible origin of the RL-MuLV mixture? The sequences of the ecotropic and MCF viruses in the MAIDS mixture (10, 11) differ from those described here, indicating clearly separate origins. We showed that multiple pregnancies and allostimuli result in up-regulation of expression of endogenous ecotropic Emv1 provirus (Fig. 6) and in its copackaging with endogenous xenotropic Bxv1 virus in BALB/cJ mice (Fig. 7). Since both events are prerequisites for retrovirus recombination, we hypothesize that the new retrovirus mixture may arise from endogenous MuLVs of BALB/cJ mice.
Acknowledgments
This work was supported by PHS grant CA65795 to T.V.G. and by a grant from The Jackson Laboratory to T.V.G. This work was also supported by a grant (CA34196) from the National Cancer Institute to The Jackson Laboratory.
We are thankful to J. Portis for anti-F-MuLV gp70 polyclonal antibodies and monoclonal antibody 603, to L. Evans for NRK cells transfected with SFFV alone and SFFV plus F-MuLV 57, and to S. Chattopadhyay for specific probes for ecotropic and xenotropic/MCF/polytropic viruses. We also thank Stan Short for photography work.
REFERENCES
- 1.Askovic, S., F. J. McAtee, C. Favara, and J. L. Portis. 2000. Brain infection by neuroinvasive but avirulent murine oncornaviruses. J. Virol. 74:465-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aziz, D. C., Z. Hanna, and P. Jolicoeur. 1989. Severe immunodeficiency disease induced by a defective murine leukaemia virus. Nature 338:505-508. [DOI] [PubMed] [Google Scholar]
- 3.Best, S., P. Le Tissier, G. Towers, and J. P. Stoye. 1996. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature 382:826-829. [DOI] [PubMed] [Google Scholar]
- 4.Brightman, B. K., B. R. Davis, and H. Fan. 1990. Preleukemic hematopoietic hyperplasia induced by Moloney murine leukemia virus is an indirect consequence of viral infection. J. Virol. 64:4582-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brightman, B. K., A. Rein, D. J. Trepp, and H. Fan. 1991. An enhancer variant of Moloney murine leukemia virus defective in leukemogenesis does not generate detectable mink cell focus-inducing virus in vivo. Proc. Natl. Acad. Sci. USA 88:2264-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chattopadhyay, S. K., B. M. Baroudy, K. L. Holmes, T. N. Fredrickson, M. R. Lander, H. C. Morse III, and J. W. Hartley. 1989. Biologic and molecular genetic characteristics of a unique MCF virus that is highly leukemogenic in ecotropic virus-negative mice. Virology 168:90-100. [DOI] [PubMed] [Google Scholar]
- 7.Chattopadhyay, S. K., M. W. Cloyd, D. L. Linemeyer, M. R. Lander, E. Rands, and D. R. Lowy. 1982. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature 295:25-31. [DOI] [PubMed] [Google Scholar]
- 8.Chattopadhyay, S. K., M. R. Lander, S. Gupta, E. Rands, and D. R. Lowy. 1981. Origin of mink cytopathic focus-forming (MCF) viruses: comparison with ecotropic and xenotropic murine leukemia virus genomes. Virology 113:465-483. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyay, S. K., M. R. Lander, E. Rands, and D. R. Lowy. 1980. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc. Natl. Acad. Sci. USA 77:5774-5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chattopadhyay, S. K., H. C. Morse III, M. Makino, S. K. Ruscetti, and J. W. Hartley. 1989. Defective virus is associated with induction of murine retrovirus-induced immunodeficiency syndrome. Proc. Natl. Acad. Sci. USA 86:3862-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chattopadhyay, S. K., D. N. Sengupta, T. N. Fredrickson, H. C. Morse III, and J. W. Hartley. 1991. Characteristics and contributions of defective, ecotropic, and mink cell focus-inducing viruses involved in a retrovirus-induced immunodeficiency syndrome of mice. J. Virol. 65:4232-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chesebro, B., W. Britt, L. Evans, K. Wehrly, J. Nishio, and M. Cloyd. 1983. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of friend MCF and Friend ecotropic murine leukemia virus. Virology 127:134-148. [DOI] [PubMed] [Google Scholar]
- 13.Chesebro, B., K. Wehrly, M. Cloyd, W. Britt, J. Portis, J. Collins, and J. Nishio. 1981. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: Friend-specific and FMR-specific antigens. Virology 112:131-144. [DOI] [PubMed] [Google Scholar]
- 14.Chirgwin, J. M., A. E. Prxybyla, R. J. MacDonald, and W. J. Rutter. 1979. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18:5294-5299. [DOI] [PubMed] [Google Scholar]
- 15.Cloyd, M. W., J. W. Hartley, and W. P. Rowe. 1981. Genetic study of lymphoma induction by AKR mink cell focus-inducing virus in AKR × NFS crosses. J. Exp. Med. 154:450-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coffin, J. M. 1990. Retroviridae and their replication, p. 1437-1500. In B. N. Fields and D. M. Knipe (ed.), Virology. Raven Press, New York, N.Y.
- 17.Coffin, J. M. 1979. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J. Gen. Virol. 42:1-26. [DOI] [PubMed] [Google Scholar]
- 18.Davis, B. R., B. K. Brightman, K. G. Chandy, and H. Fan. 1987. Characterization of a preleukemic state induced by Moloney murine leukemia virus: evidence for two infection events during leukemogenesis. Proc. Natl. Acad. Sci. USA 84:4875-4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elder, J. H., J. W. Gautsch, F. C. Jensen, R. A. Lerner, J. W. Hartley, and W. P. Rowe. 1977. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc. Natl. Acad. Sci. USA 74:4676-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans, L. H., and M. W. Cloyd. 1984. Generation of mink cell focus-forming viruses by Friend murine leukemia virus: recombination with specific endogenous proviral sequences. J. Virol. 49:772-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans, L. H., P. H. Duesberg, D. H. Troxler, and E. M. Scolnick. 1979. Spleen focus-forming Friend virus: identification of genomic RNA and its relationship to helper virus RNA. J. Virol. 31:133-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friend, C. 1957. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J. Exp. Med. 105:307-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas, M., and T. Reshef. 1980. Non-thymic malignant lymphomas induced in C57BL/6 mice by cloned dualtropic viruses isolated from hematopoietic stromal cell lines. Eur. J. Cancer 16:909-917. [DOI] [PubMed] [Google Scholar]
- 24.Hartley, J. W., T. N. Fredrickson, R. A. Yetter, M. Makino, and H. C. Morse III. 1989. Retrovirus-induced murine acquired immunodeficiency syndrome: natural history of infection and differing susceptibility of inbred mouse strains. J. Virol. 63:1223-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartley, J. W., W. P. Rowe, and R. J. Huebner. 1970. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J. Virol. 5:221-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartley, J. W., N. K. Wolford, L. J. Old, and W. P. Rowe. 1977. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc. Natl. Acad. Sci. USA 74:789-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horowitz, J. M., and R. Risser. 1985. Molecular and biological characterization of the endogenous ecotropic provirus of BALB/c mice. J. Virol. 56:798-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khimani, A. H., M. Lim, T. G. Graf, T. F. Smith, and R. M. Ruprecht. 1997. Phylogenetic relationship of the complete Rauscher murine leukemia virus genome with other murine leukemia virus genomes. Virology 238:64-67. [DOI] [PubMed] [Google Scholar]
- 29.Kubo, Y., K. Kakimi, K. Higo, H. Kobayashi, T. Ono, Y. Iwama, K. Kuribayashi, H. Hiai, A. Adachi, and A. Ishimoto. 1996. Possible origin of murine AIDS (MAIDS) virus: conversion of an endogenous retroviral p12gag sequence to a MAIDS-inducing sequence by frameshift mutations. J. Virol. 70:6405-6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Latarjet, R., and J.-F. Duplan. 1962. Experiment and discussion on leukaemogenesis by cell-free extracts of radiation-induced leukaemia in mice. Int. J. Radiat. Biol. 5:339-344. [DOI] [PubMed] [Google Scholar]
- 31.Li, J. P., A. D. D'Andrea, H. F. Lodish, and D. Baltimore. 1990. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature 343:762-764. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald, M. E., T. W. Mak, and A. Bernstein. 1980. Erythroleukemia induction by replication-competent type C viruses cloned from the anemia- and polycythemia-inducing isolates of Friend leukemia virus. J. Exp. Med. 151:1493-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massey, A. C., M. A. Coppola, and C. Y. Thomas. 1990. Origin of pathogenic determinants of recombinant murine leukemia viruses: analysis of Bxv-1-related xenotropic viruses from CWD mice. J. Virol. 64:5491-5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moll, B., J. W. Hartley, and W. P. Rowe. 1979. Induction of B-tropic and N-tropic murine leukemia virus from B10.BR/SgLi mouse embryo cell lines by 5-iodo-2′-deoxyuridine. JNCI 63:213-217. [PubMed] [Google Scholar]
- 35.Moloney, J. B. 1959. Preliminary studies on a mouse lymphoid leukemia virus extracted from sarcoma 37. Proc. Am. Assoc. Cancer Res. 3:44. [Google Scholar]
- 36.Moloney, J. B. 1966. A virus-induced rhabdomyosarcoma of mice. Natl. Cancer Inst. Monogr. 22:139-142. [PubMed] [Google Scholar]
- 37.Morrison, S. J., and I. L. Weissman. 1994. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity 1:661-673. [DOI] [PubMed] [Google Scholar]
- 38.Morrison, S. J., D. E. Wright, S. H. Cheshier, and I. L. Weissman. 1997. Hematopoietic stem cells: challenges to expectations. Curr. Opin. Immunol. 9:216-221. [DOI] [PubMed] [Google Scholar]
- 39.Mosier, D. E., R. A. Yetter, and H. C. Morse III. 1985. Retroviral induction of acute lymphoproliferative disease and profound immunosuppression in adult C57BL/6 mice. J. Exp. Med. 161:766-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ott, D., R. Friedrich, and A. Rein. 1990. Sequence analysis of amphotropic and 10A1 murine leukemia viruses: close relationship to mink cell focus-inducing viruses. J. Virol. 64:757-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Portis, J. L., S. Czub, S. Robertson, F. McAtee, and B. Chesebro. 1995. Characterization of a neurologic disease induced by a polytropic murine retrovirus: evidence for differential targeting of ecotropic and polytropic viruses in the brain. J. Virol. 69:8070-8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Portis, J. L., and F. J. McAtee. 1983. Monoclonal antibodies derived during graft-versus-host reaction. II. Antibodies detect unique determinants common to many MCF viruses. Virology 126:96-105. [DOI] [PubMed] [Google Scholar]
- 43.Rauscher, F. J. 1962. A virus-induced disease of mice characterized by erythrocytopoiesis and lymphoid leukemia. J. Natl. Cancer Inst. 29:515-532. [PubMed] [Google Scholar]
- 44.Rosenberg, N., and P. Jolicoeur. 1997. Retroviral pathogenesis, p. 475-586. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 45.Rowe, W. P., W. E. Pugh, and J. W. Hartley. 1970. Plaque assay techniques for murine leukemia viruses. Virology 42:1136-1139. [DOI] [PubMed] [Google Scholar]
- 46.Sitbon, M., L. Evans, J. Nishio, K. Wehrly, and B. Chesebro. 1986. Analysis of two strains of Friend murine leukemia viruses differing in ability to induce early splenomegaly: lack of relationship with generation of recombinant mink cell focus-forming viruses. J. Virol. 57:389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoye, J. P., C. Moroni, and J. M. Coffin. 1991. Virological events leading to spontaneous AKR thymomas. J. Virol. 65:1273-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teich, N., J. Wyke, T. Mak, A. Bernstein, and W. Hardy. 1984. Pathogenesis of retrovirus-induced disease, p. 785-998. In R. Weiss, N. Teich, H. Varmus, and J. Coffin (ed.), RNA tumor viruses: molecular biology of tumor viruses. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 49.Ter-Grigorov, V. S., O. Krifuks, E. Liubashevsky, A. Nyska, Z. Trainin, and V. Toder. 1997. A new transmissible AIDS-like disease in mice induced by alloimmune stimuli. Nat. Med. 3:37-41. [DOI] [PubMed] [Google Scholar]
- 50.Thomas, C. Y., and J. M. Coffin. 1982. Genetic alterations of RNA leukemia viruses associated with the development of spontaneous thymic leukemia in AKR/J mice. J. Virol 43:416-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Troxler, D. H., and E. M. Scolnick. 1978. Rapid leukemia induced by cloned Friend strain of replicating murine type-C virus. Association with induction of xenotropic-related RNA sequences contained in spleen focus-forming virus. Virology 85:17-27. [DOI] [PubMed] [Google Scholar]
- 52.Vogt, M., C. Haggblom, S. Swift, and M. Haas. 1985. Envelope gene and long terminal repeat determine the different biological properties of Rauscher, Friend, and Moloney mink cell focus-inducing viruses. J. Virol. 55:184-192. [DOI] [PMC free article] [PubMed] [Google Scholar]