Abstract
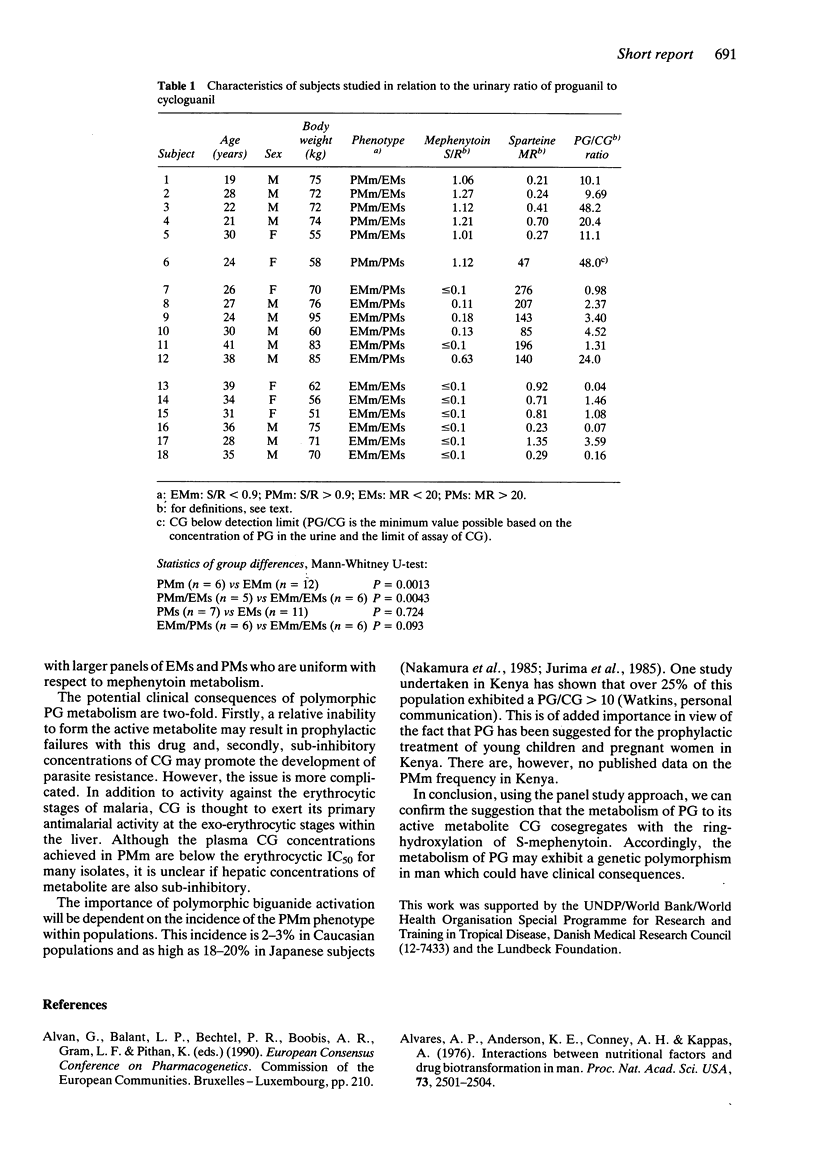
The activation of the antimalarial drug proguanil (PG) to the active metabolite cycloguanil (CG) has been evaluated in a panel of 18 subjects. These subjects had previously been screened and classified as mephenytoin poor (PMm) or extensive metabolisers (EMm) and sparteine poor (PMs) or extensive metabolisers (EMs). Five subjects had the phenotype PMm/EMs, one was PMm/PMs, six subjects were EMm/PMs and six were EMm/EMs. The PG/CG ratio in urine (8 h) was significantly higher in PMm than in EMm (P = 0.0013). This study shows that the P450-isozyme involved in the polymorphic oxidation of mephenytoin is of critical importance in the activation of PG to CG and this may explain the large intersubject variability in CG concentrations in man. PMm make up about 3% of Caucasians, but up to about 20% of Orientals. From the present study, it may be anticipated that the antimalarial effect of PG is absent or impaired in this phenotype. The sparteine polymorphism appeared not to influence the activation of PG to CG significantly.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvares A. P., Anderson K. E., Conney A. H., Kappas A. Interactions between nutritional factors and drug biotransformations in man. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2501–2504. doi: 10.1073/pnas.73.7.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brøsen K., Gram L. F. Clinical significance of the sparteine/debrisoquine oxidation polymorphism. Eur J Clin Pharmacol. 1989;36(6):537–547. doi: 10.1007/BF00637732. [DOI] [PubMed] [Google Scholar]
- Brøsen K., Otton S. V., Gram L. F. Sparteine oxidation polymorphism in Denmark. Acta Pharmacol Toxicol (Copenh) 1985 Nov;57(5):357–360. doi: 10.1111/j.1600-0773.1985.tb00058.x. [DOI] [PubMed] [Google Scholar]
- CARRINGTON H. C., CROWTHER A. F., DAVEY D. G., LEVI A. A., ROSE F. L. A metabolite of paludrine with high antimalarial activity. Nature. 1951 Dec 22;168(4288):1080–1080. doi: 10.1038/1681080a0. [DOI] [PubMed] [Google Scholar]
- Dollery C. T., Fraser H. S., Mucklow J. C., Bulpitt C. J. Contribution of environmental factors to variability in human drug metabolism. Drug Metab Rev. 1979;9(2):207–220. doi: 10.3109/03602537908993891. [DOI] [PubMed] [Google Scholar]
- Drøhse A., Bathum L., Brøsen K., Gram L. F. Mephenytoin and sparteine oxidation: genetic polymorphisms in Denmark. Br J Clin Pharmacol. 1989 May;27(5):620–625. doi: 10.1111/j.1365-2125.1989.tb03426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichelbaum M., Spannbrucker N., Steincke B., Dengler H. J. Defective N-oxidation of sparteine in man: a new pharmacogenetic defect. Eur J Clin Pharmacol. 1979 Sep;16(3):183–187. doi: 10.1007/BF00562059. [DOI] [PubMed] [Google Scholar]
- Helsby N. A., Ward S. A., Edwards G., Howells R. E., Breckenridge A. M. The pharmacokinetics and activation of proguanil in man: consequences of variability in drug metabolism. Br J Clin Pharmacol. 1990 Oct;30(4):593–598. doi: 10.1111/j.1365-2125.1990.tb03818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsby N. A., Ward S. A., Howells R. E., Breckenridge A. M. In vitro metabolism of the biguanide antimalarials in human liver microsomes: evidence for a role of the mephenytoin hydroxylase (P450 MP) enzyme. Br J Clin Pharmacol. 1990 Aug;30(2):287–291. doi: 10.1111/j.1365-2125.1990.tb03777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurima M., Inaba T., Kadar D., Kalow W. Genetic polymorphism of mephenytoin p(4')-hydroxylation: difference between Orientals and Caucasians. Br J Clin Pharmacol. 1985 Apr;19(4):483–487. doi: 10.1111/j.1365-2125.1985.tb02673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpfer A., Preisig R. Pharmacogenetics of mephenytoin: a new drug hydroxylation polymorphism in man. Eur J Clin Pharmacol. 1984;26(6):753–759. doi: 10.1007/BF00541938. [DOI] [PubMed] [Google Scholar]
- Mahgoub A., Idle J. R., Dring L. G., Lancaster R., Smith R. L. Polymorphic hydroxylation of Debrisoquine in man. Lancet. 1977 Sep 17;2(8038):584–586. doi: 10.1016/s0140-6736(77)91430-1. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Goto F., Ray W. A., McAllister C. B., Jacqz E., Wilkinson G. R., Branch R. A. Interethnic differences in genetic polymorphism of debrisoquin and mephenytoin hydroxylation between Japanese and Caucasian populations. Clin Pharmacol Ther. 1985 Oct;38(4):402–408. doi: 10.1038/clpt.1985.194. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Adesnik M., Coon M. J., Estabrook R. W., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F., Kemper B. The P450 superfamily: updated listing of all genes and recommended nomenclature for the chromosomal loci. DNA. 1989 Jan-Feb;8(1):1–13. doi: 10.1089/dna.1.1989.8.1. [DOI] [PubMed] [Google Scholar]
- Sanz E. J., Villén T., Alm C., Bertilsson L. S-mephenytoin hydroxylation phenotypes in a Swedish population determined after coadministration with debrisoquin. Clin Pharmacol Ther. 1989 May;45(5):495–499. doi: 10.1038/clpt.1989.63. [DOI] [PubMed] [Google Scholar]
- Taylor R. B., Moody R. R., Ochekpe N. A. Determination of proguanil and its metabolites cycloguanil and 4-chlorophenylbiguanide in plasma, whole blood and urine by high-performance liquid chromatography. J Chromatogr. 1987 May 15;416(2):394–399. doi: 10.1016/0378-4347(87)80526-1. [DOI] [PubMed] [Google Scholar]
- Vesell E. S. Genetic and environmental factors affecting drug disposition in man. Clin Pharmacol Ther. 1977 Nov;22(5 Pt 2):659–679. doi: 10.1002/cpt1977225part2659. [DOI] [PubMed] [Google Scholar]
- Vinks A., Inaba T., Otton S. V., Kalow W. Sparteine metabolism in Canadian Caucasians. Clin Pharmacol Ther. 1982 Jan;31(1):23–29. doi: 10.1038/clpt.1982.4. [DOI] [PubMed] [Google Scholar]
- Ward S. A., Goto F., Nakamura K., Jacqz E., Wilkinson G. R., Branch R. A. S-mephenytoin 4-hydroxylase is inherited as an autosomal-recessive trait in Japanese families. Clin Pharmacol Ther. 1987 Jul;42(1):96–99. doi: 10.1038/clpt.1987.114. [DOI] [PubMed] [Google Scholar]
- Ward S. A., Watkins W. M., Mberu E., Saunders J. E., Koech D. K., Gilles H. M., Howells R. E., Breckenridge A. M. Inter-subject variability in the metabolism of proguanil to the active metabolite cycloguanil in man. Br J Clin Pharmacol. 1989 Jun;27(6):781–787. doi: 10.1111/j.1365-2125.1989.tb03440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins W. M., Chulay J. D., Sixsmith D. G., Spencer H. C., Howells R. E. A preliminary pharmacokinetic study of the antimalarial drugs, proguanil and chlorproguanil. J Pharm Pharmacol. 1987 Apr;39(4):261–265. doi: 10.1111/j.2042-7158.1987.tb06263.x. [DOI] [PubMed] [Google Scholar]
- Wedlund P. J., Aslanian W. S., McAllister C. B., Wilkinson G. R., Branch R. A. Mephenytoin hydroxylation deficiency in Caucasians: frequency of a new oxidative drug metabolism polymorphism. Clin Pharmacol Ther. 1984 Dec;36(6):773–780. doi: 10.1038/clpt.1984.256. [DOI] [PubMed] [Google Scholar]
- Wilkinson G. R., Guengerich F. P., Branch R. A. Genetic polymorphism of S-mephenytoin hydroxylation. Pharmacol Ther. 1989;43(1):53–76. doi: 10.1016/0163-7258(89)90047-8. [DOI] [PubMed] [Google Scholar]


