Abstract
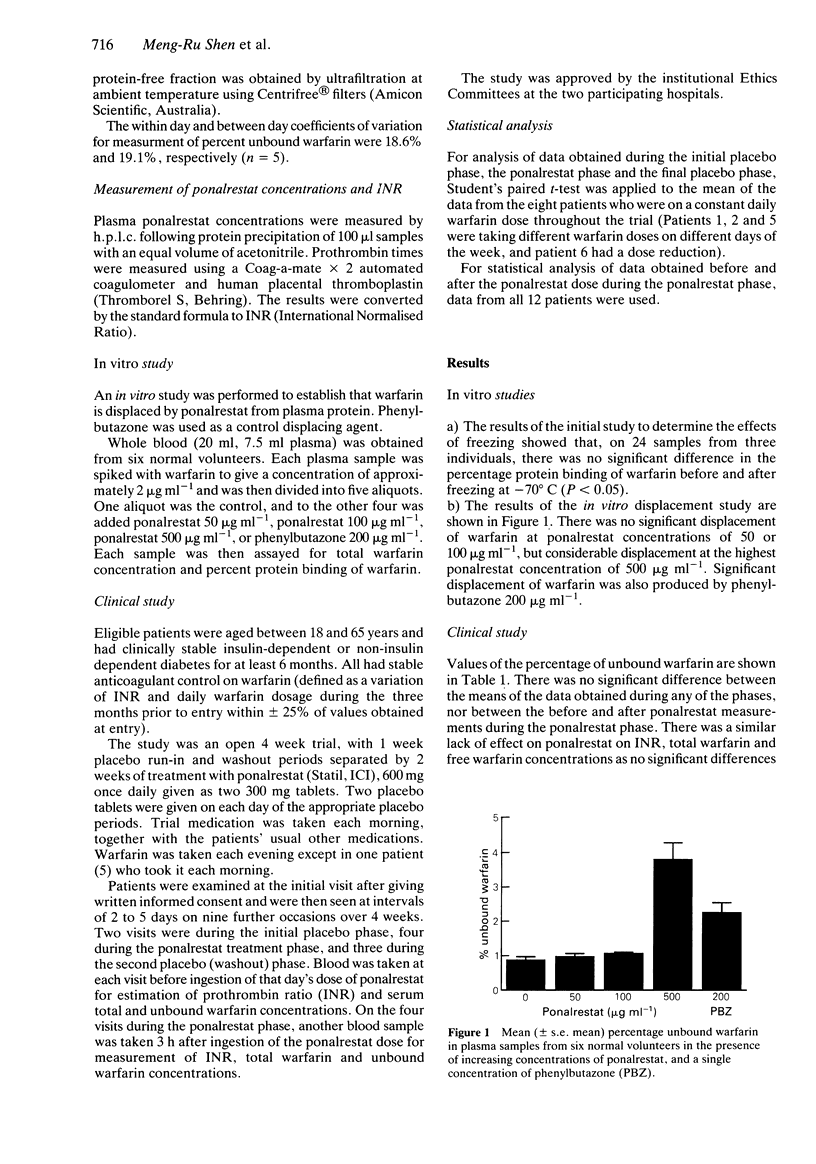
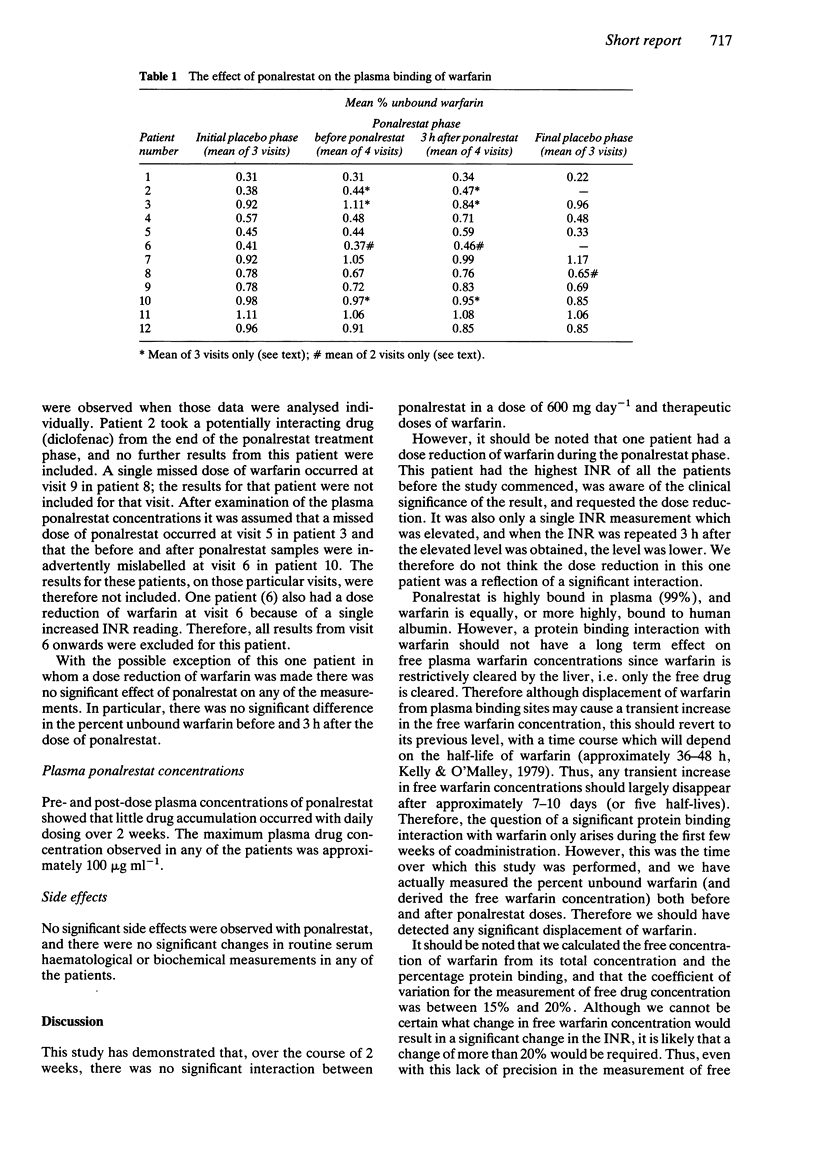
Ponalrestat (Statil, ICI; Prodiax, Merck Sharp and Dohme) is an aldose reductase inhibitor which is highly protein bound. Ponalrestat markedly displaced warfarin from its protein binding in vitro at a concentration of 500 micrograms ml-1, but not at a concentration of 50 or 100 micrograms ml-1. Twelve diabetic patients (six males), age range 38-65 years, in receipt of chronic stable warfarin therapy, were given ponalrestat (600 mg daily) for 2 weeks in an open trial. A matching placebo tablet was administered for 1 week before and after the active treatment period. Patients were seen ten times (four times during the ponalrestat phase), and during the ponalrestat phase, plasma samples were also taken before and at 3 h after the daily dose of ponalrestat. At none of the visits was there any significant change in prothrombin ratio (INR), plasma total or unbound warfarin concentrations, or percentage protein binding of warfarin. No clinical complications of combination treatment were detected. The maximum ponalrestat concentration observed in the patients was approximately 100 micrograms ml-1. We conclude that no significant interaction between these drugs occurs at the doses of ponalrestat studied.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen M. P., Dasmahapatra A., Shapiro E. Reduced glomerular sodium/potassium adenosine triphosphatase activity in acute streptozocin diabetes and its prevention by oral sorbinil. Diabetes. 1985 Nov;34(11):1071–1074. doi: 10.2337/diab.34.11.1071. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Sherman W. R., Hallcher L. M., Service F. J., O'Brien P. C., Grina L. A., Palumbo P. J., Swanson C. J. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann Neurol. 1980 Dec;8(6):590–596. doi: 10.1002/ana.410080608. [DOI] [PubMed] [Google Scholar]
- Kelly J. G., O'Malley K. Clinical pharmacokinetics of oral anticoagulants. Clin Pharmacokinet. 1979 Jan-Feb;4(1):1–15. doi: 10.2165/00003088-197904010-00001. [DOI] [PubMed] [Google Scholar]
- MacKichan J. J. Pharmacokinetic consequences of drug displacement from blood and tissue proteins. Clin Pharmacokinet. 1984 Jan;9 (Suppl 1):32–41. doi: 10.2165/00003088-198400091-00005. [DOI] [PubMed] [Google Scholar]
- Mayer J. H., Tomlinson D. R. Prevention of defects of axonal transport and nerve conduction velocity by oral administration of myo-inositol or an aldose reductase inhibitor in streptozotocin-diabetic rats. Diabetologia. 1983 Nov;25(5):433–438. doi: 10.1007/BF00282524. [DOI] [PubMed] [Google Scholar]
- Mungall D., Wong Y. Y., Talbert R. L., Crawford M. H., Marshall J., Hawkins D. W., Ludden T. M. Plasma protein binding of warfarin: methodological considerations. J Pharm Sci. 1984 Jul;73(7):1000–1001. doi: 10.1002/jps.2600730738. [DOI] [PubMed] [Google Scholar]
- Pirart J. Diabète et complications dégénératives présentation d'une étude prospective portant sur 4400 cas observés entre 1947 et 1973 (deuxième partie). Diabete Metab. 1977 Sep;3(3):173–182. [PubMed] [Google Scholar]
- Pirart J. Diabète et complications dégénératives. Présentation d'une étude prospective portant sur 4400 cas observés entre 1947 et 1973 (troisième et dernière partie). Diabete Metab. 1977 Dec;3(4):245–256. [PubMed] [Google Scholar]
- Stribling D., Mirrlees D. J., Harrison H. E., Earl D. C. Properties of ICI 128,436, a novel aldose reductase inhibitor, and its effects on diabetic complications in the rat. Metabolism. 1985 Apr;34(4):336–344. doi: 10.1016/0026-0495(85)90223-9. [DOI] [PubMed] [Google Scholar]
- Ueland P. M., Kvalheim G., Lønning P. E., Kvinnsland S. Determination of warfarin in human plasma by high performance liquid chromatography and photodiode array detector. Ther Drug Monit. 1985;7(3):329–335. doi: 10.1097/00007691-198507030-00018. [DOI] [PubMed] [Google Scholar]


