Abstract
The immune system plays an important role in facilitating the spread of prion infections from the periphery to the central nervous system. CD11c+ myeloid dendritic cells (DC) could, due to their subepithelial location and their migratory capacity, be early targets for prion infection and contribute to the spread of infection. In order to analyze mechanisms by which these cells may affect prion propagation, we studied in vitro the effect of exposing such DC to scrapie-infected GT1-1 cells, which produce the scrapie prion protein PrPSc. In this system, the DC efficiently engulfed the infected GT1-1 cells. Unexpectedly, PrPSc, which is generally resistant to protease digestion, was processed and rapidly degraded. Based on this observation we speculate that CD11c+ DC may play a dual role in prion infections: on one hand they may facilitate neuroinvasion by transfer of the infectious agent as suggested from in vivo studies, but on the other hand they may protect against the infection by causing an efficient degradation of PrPSc. Thus, the migrating and highly proteolytic CD11c+ myeloid DC may affect the balance between propagation and clearance of PrPSc in the organism.
Prions are the cause of severe neurodegenerative diseases in a number of animals as well as in humans. These proteinaceous infectious particles are devoid of nucleic acids, and their only known components are modified isoforms, PrPSc, of a normal cellular glycoprotein denoted PrPC (34). A biochemical hallmark of PrPSc is that it contains a protease-resistant core, denoted PrP27-30. In contrast to most other infectious agents, it is generally believed that prions are nonimmunogenic (33, 41). Nevertheless, the immune system plays an important and paradoxical role in the transmission of prion infections from the periphery to the central nervous system. Nonspecific immunosuppression tends to decrease (31), whereas nonspecific stimulation of the immune system tends to increase the spread (7) of peripherally inoculated prions. For instance, immunodefective SCID mice are relatively resistant to peripheral inoculation of scrapie (3, 10, 16, 19, 30), but susceptibility can be restored by reconstitution with bone marrow cells from wild type mice (10).
In the immune system, migrating CD11c+ dendritic cells (DC) of myeloid origin are important in the uptake and transport of antigens from subepithelial sites to lymphatic tissues (20, 42). Recently, this type of DC has been implicated in the spread of PrPSc from the gut to lymphoid tissue (12), and, further, from lymphoid tissues to the central nervous system (1). The CD11c+ DC are distinct from the nonmigrating follicular DC (FDC), which reside in the center of lymphoid organs. FDC have previously been suggested to facilitate the spread of prion infections to the nervous system by serving as a source for their replication (3, 24, 27). Thus, PrPC, a prerequisite for prion replication, and/or PrPSc have been detected in FDC-containing regions of both human and murine lymphoid tissues (3, 16, 17, 27). Tumor necrosis factor-α-deficient mice, which fail to develop mature FDC, as well as mice treated with the soluble lymphotoxin-β receptor-immunoglobulin fusion protein, which eliminates functional FDC, show prolonged incubation time after peripheral administration of scrapie (21, 27). The mechanisms by which DC of the various phenotypes are involved in propagation of prions to the nervous system remain, however, to be clarified.
The present study was undertaken with the aim to determine how CD11c+ DC can affect PrPSc when encountering a scrapie-infected cell. In an effort to mimic the complex cell-cell interactions that take place in vivo, we used PrPSc-producing cells rather than purified PrPSc rods (35). For this purpose, we exposed primary cultures of mouse CD11c+ DC to scrapie-infected neuronal GT1-1 cells (ScGT1-1 cells). These cells produce abundant levels of both PrPC and PrPSc (37). We here report that ScGT1-1 cells underwent cell death and were phagocytosed by DC under coculture conditions. This was, surprisingly, accompanied by a gradual appearance of the three glycoforms of PrP27-30 and later by the total disappearance of PrPSc from the culture. Given the general resistance of PrPSc to degradation by a variety of physical and/or chemical means, we find this process striking.
MATERIALS AND METHODS
GT1-1 cell culture and scrapie infection.
GT1-1-cells, a subtype of murine immortalized gonadotropin-releasing hormone neurons (26), were provided as a generous gift from Pamela Mellon (University of California, San Francisco). The cells were grown in 75-cm2 cell culture flasks (Costar, Corning, N.Y.) with Dulbecco's modified Eagle's medium (4.5 g of glucose/liter) containing GLUTAMAX I supplemented with 5% heat-inactivated fetal bovine serum (FBS), 5% heat-inactivated horse serum, and penicillin-streptomycin (50 U/ml) (all from GibcoBRL, Paisley, United Kingdom). The cells were split once a week using 1× trypsin-EDTA (GibcoBRL). Infection of the GT1-1 cells with mouse-adapted scrapie was performed in 24-well cell culture clusters (Costar). The GT1-1 cells were incubated with 0.1% homogenate of mouse brains infected with the Rocky Mountain Laboratory strain of scrapie (the homogenates were obtained as a generous gift from Stanley B. Prusiner, University of California, San Francisco) at 30°C in CO2-independent medium (GibcoBRL) supplemented with 5% FBS, 5% heat-inactivated horse serum, penicillin-streptomycin (50 U/ml), and 2 mM l-glutamine (GibcoBRL). After 4 days, the medium was changed, and the ScGT1-1 cells were transferred to an incubator with 5% CO2 at 37°C. Western blotting confirmed the presence of protease-resistant PrPSc after 14 passages.
Differentiation and isolation of CD11c+ DC.
Murine bone marrow-derived DC were obtained from femur and tibia bone marrow of C57BL/6 mice (obtained from B&K Universal AB, Sollentuna, Sweden, or the Microbiology and Tumor Biology Center, Karolinska Institutet) essentially as described by Inaba et al. (13). Briefly, after removal of small pieces of bone and debris, the cells were pelleted and resuspended in Dulbecco's modified Eagle's medium (4.5 g/liter of glucose) with GLUTAMAX I, 15% FBS, and penicillin-streptomycin (50 U/ml) to which recombinant murine granulocyte-macrophage colony-stimulating factor (granulocyte-macrophage CSF) (10 ng/ml) and murine interleukin-4 (10 ng/ml; PeproTech, Rocky Hill, N.Y.) were added. The cells were grown in six-well cell culture clusters (Costar) in 5% CO2 at 37°C and replated after 7 days. For activation, lipopolysaccharide (LPS) (10 μg/ml; Sigma-Aldrich Chemie, Steinhem, Germany) was added to the cells overnight. In contrast to murine bone marrow macrophages, DC differentiated from bone marrow express CD11c. Thus, to obtain pure DC, the cells from the bone marrow cultures were affinity purified using MACS CD11c+ magnetic MicroBeads (Miltenyi Biotech, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Purity of the DC was assessed, and presence of contaminating macrophages was excluded, using monoclonal antibodies recognizing CD11c (BD Pharmingen, San Diego, Calif.), major histocompatibility complex (MHC) class II, and the macrophage marker F4/80 (Serotec Ltd., Oxford, United Kingdom) for flow cytometric analysis.
Cocultivation of DC with GT1-1 cells.
Before exposure to DC, GT1-1 cells or ScGT1-1 cells were grown overnight in 10-mm-diameter tissue culture dishes (Corning) to a concentration of about 106 cells/dish, and the CD11c+-sorted murine bone marrow DC were added at a concentration of either 1 × 106 or 2 × 106 cells/dish, giving an approximate DC/GT1-1 cell ratio of 1:1 or 2:1. The cells were incubated for 24 to 96 h in AIM-V medium (GibcoBRL) supplemented with granulocyte-macrophage CSF (10 ng/ml; PeproTech). In one set of experiments, an anti-Fas ligand antibody (MFL3; BD Pharmingen) was added to the medium at a concentration of 10 μg/ml. In another set of experiments the DC were cultured with the GT1-1 or ScGT1-1 cells without cell-cell contact in a Transwell system (pore size, 0.4 μm; Costar)
Immunofluorescence.
Cells grown on 10-mm-diameter tissue culture dishes (Costar) were fixed in 10% formalin (Merck KGaA, Darmstadt, Germany) for 30 min, permeabilized with 0.1% Triton X-100 (Sigma, St Louis, Mo.) in phosphate-buffered saline (PBS) for 5 min, blocked with 5% bovine serum albumin (Sigma) in PBS for 40 min, and then incubated with the antibodies. The following primary monoclonal antibodies were used: recombinant Fab D13 (40) (provided as a generous gift from Stanley B. Prusiner), 1.4 μg/ml; anti-neuron-specific class III β-tubulin (TUJ1; BABCO, Richmond, Calif.), 2 μg/ml; and anti-MHC class II purified from supernatant of the hybridoma M5/114.15.2 (also available from BD Pharmingen), 10 μg/ml. All antibodies were diluted in PBS containing 5% bovine serum albumin. The cells were incubated with the primary antibodies overnight at 4°C followed by incubation with secondary antibodies for 40 min at room temperature. The secondary antibodies used were Alexa Fluor 488-conjugated goat anti-human immunoglobulin G (IgG) (Molecular Probes, Leiden, The Netherlands), 4 μg/ml, for detection of Fab D13; Cy2-conjugated donkey anti-mouse IgG (Jackson Immunoresearch, West Grove, Pa.), 6.5 μg/ml, for detection of TUJ1; and Texas Red-conjugated donkey anti-rat IgG (Jackson Immunoresearch), 30 μg/ml, for detection of M5/114.15.2. The cells were rinsed in PBS with 1% NH4Cl (Sigma) and mounted in antifading solution (5% n-propyl gallate, 100 mM Tris-HCl [pH 9], 70% glycerol).
Western immunoblotting.
After removal of the medium, the cells in the cultures were lysed in 100 μl of lysis buffer (10 mM Tris-Cl [pH 8], 150 mM NaCl, 0.5% sodium deoxycholate, 0.5% Triton X-100) on ice. In samples that later were not treated with proteinase K (PK) (see below) the following protease inhibitors were added to the lysis buffer: pepstatin A, leupeptin, and aprotinin (1 μg/ml each; Sigma) and phenylmethylsulfonyl fluoride (0.2 mM; Sigma). Insoluble debris was removed by centrifugation at 16,000 × g, and the protein content was determined by using the Bio-Rad Bradford protein assay (Bio-Rad) and spectroscopy (Ultrospec plus; Pharmacia LKB, Cambridge, United Kingdom) at 595 nm according to the manufacturer's instructions. The lysate was then split into two parts. One part was treated with PK (10 μg/ml; Boehringer Mannheim, Mannheim, Germany) at 37°C for 30 min and the reaction was stopped by incubation with 3 mM phenylmethylsulfonyl fluoride (Sigma). The remaining lysate was not PK-treated. The samples were boiled in sodium dodecyl sulfate (SDS) sample buffer and investigated by Western blotting. The media sampled from the cultures were spun at 1,500 × g for 10 min, and the supernatants were removed. Fifty microliters of lysis buffer was added to the pellet in each centrifuge tube, after which the lysates were boiled in SDS sample buffer. In one set of experiment these lysates were analyzed separately, but in all other experiments they were added to the lysates from the cells in the culture. The removed supernatants were precipitated in 80% ice-cold acetone, centrifuged at 4°C at 3,500 × g for 30 min, dissolved in SDS sample buffer, and boiled.
The samples were loaded on 12% Tris-glycine precast gels and electrophoresed at 125 V, according to the method of Laemmli (18) (Fig. 1B; see also Fig. 4) or NuPAGE 12% Bis-Tris gels with MOPS (morpholinepropanesulfonic acid) SDS running buffer at 200 V according to the manufacturer's instructions (Invitrogen, Paisley, United Kingdom) (see Fig. 3B through E). Proteins were transferred to Immobilon-PSQ transfer membranes (Millipore, Bedford, Mass.) at 35 V for 3 h, blocked in 5% nonfat dry milk (Bio-Rad), and incubated with recombinant Fabs D13, D18, or R1 (all obtained from Stanley B. Prusiner), 1 μg/ml each, followed by the secondary goat anti-human F(ab)2 peroxidase-conjugated antibody (Pierce, Rockford, Ill.), 0.16 μg/ml. Detection was performed with enhanced chemiluminescence (ECLplus; Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, United Kingdom). Optical densities of the bands were determined using a Gel Doc 2000 with the software Quantity One (version 4.2.2; Bio-Rad). The membranes were incubated in stripping buffer (100 mM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl [pH 6.7]) for 30 min at 50°C, rinsed, and blocked with 5% nonfat dry milk before exposure to the antibody TUJ1, 0.5 μg/ml. As secondary antibody, a peroxidase-conjugated goat anti-mouse immunoglobulin (0.2 μg/ml; DAKO, Glostrup, Denmark) was used.
FIG. 1.
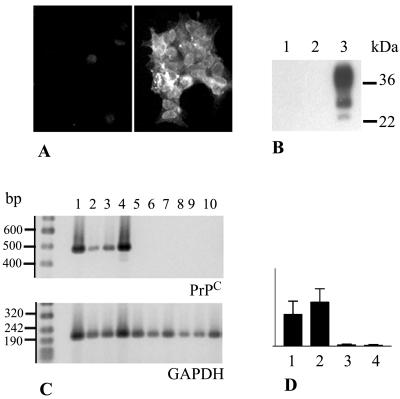
PrPC and PrPC-mRNA in GT1-1 cells and CD11c+ DC. (A) PrPC immunofluorescence in LPS-activated DC (left) (negative) and control GT1-1 cells (right) (positive) using Fab D13. (B) Western blot, using Fab D13, of lysates from DC (lane 1), DC activated with LPS (lane 2), and GT1-1 cells (lane 3). (C) RT-PCR showing transcription of mRNA encoding PrPC (upper gel) and GAPDH (lower gel) in GT1-1 cells (lanes 1 and 2), and ScGT1-1 cells (lanes 3 and 4), DC (lanes 5, 6 and 7), and DC activated with LPS (lanes 8, 9, and 10). (D) PrPC mRNA standardized to GAPDH. Bars 1 to 4 show results for GT1-1, ScGT1-1, DC, and DC activated with LPS, respectively.
FIG. 4.
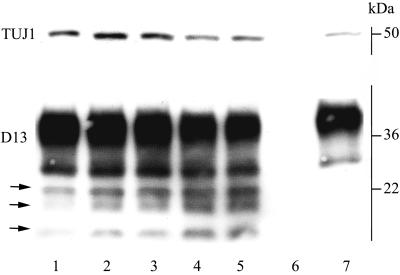
Western blot of lysates from ScGT1-1 cells exposed to various amounts of DC for 48 h. Each lane represents the amount of proteins in an individual cell culture dish. Shown are ScGT1-1 cells (lane 1); ScGT1-1 cells exposed to DC, ratio 2:1 (lanes 2 and 3); ScGT1-1 cells exposed to DC, ratio 1:1 (lanes 4 and 5); control DC (lane 6); and control uninfected GT1-1 cells (lane 7). ScGT1-1 cells and GT1-1 cells where grown at 106 cells/dish (lanes 1 to 5 and 7), and 0.5 × 106 (lane 2 and 3) or 1 × 106 (lanes 4, 5, and 6) DC were added. The blot was first developed with D13 and then stripped and rehybridized to the neuron-specific β-tubulin antibody TUJ1 (upper line). The three glycoforms of PrP27-30 (arrows) increase, while β-tubulin decreases, with increasing amounts of DC.
FIG. 3.
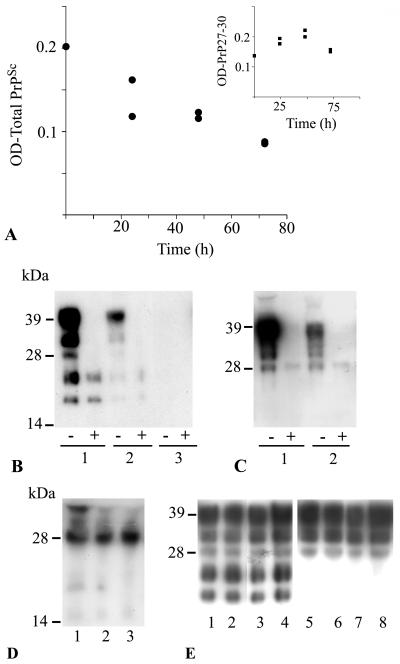
Effect of DC on PrPSc derived from ScGT1-1 cells. (A) Intensity of protease-resistant prion protein in PK-treated (•) lysates at different times after exposure of ScGT1-1 cells to DC, ratio 1:1. The inset shows the intensity of the low-molecular-mass bands (sizes between 19 and 27 kDa) in non-PK-treated lysates, standardized to the intensity of β-tubulin, sampled at different time points after exposure of ScGT1-1 cells to DC; ratio 1:1. (B) Western blot, using Fab D13, of cell culture lysates from ScGT1-1 cells (lane 1); ScGT1-1 cells exposed to DC, ratio 1:1, for 72 h (lane 2); and ScGT1-1 cells exposed to DC, ratio 1:2, for 72 h (lane 3). (C) GT1-1 cells (lane 1) and GT1-1 cells exposed to DC, ratio 1:1, for 72 h (lane 2). Symbols: −, non-PK-treated lysates; +, PK-treated lysates. (D) precipitated proteins from the medium collected from cultures of ScGT1-1 cells (lane 1); ScGT1-1 cells exposed to DC, ratio 1:1, for 72 h (lane 2); or ScGT1-1 cells exposed to DC, ratio 1:2, for 72 h (lane 3). (E) Transwell experiment showing no difference in PrP expression between ScGT1-1 cells unexposed (lanes 1 and 2) and exposed (lanes 3 and 4) to DC, ratio 1:1, for 48 h. Exposure to DC did not affect PrPC expression in uninfected GT1-1 cells (lanes 5 and 6 show unexposed GT1-1 cells, and lanes 7 and 8 show GT1-1 cells exposed to DC).
RNA analysis of PrPC.
Total RNA was extracted in duplicates or triplicates from the cells using an RNeasy Mini kit (Qiagen GmbH, Hilden, Germany). RNA content was determined by UV spectroscopy (Ultrospec plus). RNA (1 μg) was treated with 1.0 U of DNase I, amplification grade (DNase; GibcoBRL) according to the manufacturer's instructions. Total DNase-treated RNA was reverse transcribed to cDNA by random priming using Superscript II (GibcoBRL) in a 20-μl reaction mixture according to the manufacturer's instructions.
Specific amplification of PrPC and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts was subsequently performed according to the following protocol. Each PCR mixture contained 1 μl of template; 100 ng of each primer; 1× Titanium Taq DNA polymerase (Clontech Laboratories Inc, Palo Alto, Calif.); 1× Titanium Taq PCR buffer (Clontech); and a 10 mM concentration (each) of dGTP, dATP, dTTP, and dCTP (GibcoBRL). With the use of a GeneAmp PCR system 9700 (P-E Biosystems, Foster City, Calif.) the PrPC-PCR was run for 40 cycles: denaturation at 94°C for 30 s and annealing and extension at 70°C for 1 30 mins. The GAPDH-PCR was run for 20 or 25 cycles: denaturation at 94°C for 30 s, annealing at 56°C for 30 s and extension at 72°C for 30 s. The primer sets used for these assays were as follows: PrP (forward), 5′-AATCAGTGGAACAAGCCCAG-3′; PrP (reverse), 5′-ATCCCACGATCAGGAAGATG-3′; GAPDH (forward), 5′-CCATGGAGAAGGCCGGGG-3′; GAPDH (reverse), 5′-CAAAGTTGTCATGGATGACC-3′ (GibcoBRL). The primers for PrPC and GAPDH were designed to generate fragments of 478 and 195 bp, respectively; GAPDH primer sequences were taken from the work of Der et al. (6). The PCR products were electrophoresed in 2% agarose (Sigma) gels in 1× TAE buffer (40 mM Tris, 20 mM acetic acid, 1 mM EDTA [pH 8.3]) (Bio-Rad, Hercules, Calif.) followed by staining with SYBR gold nucleic acid gel stain (Molecular Probes) and were visualized with a UV transilluminator (Gel Doc 2000; Bio-Rad).
RESULTS
Expression of PrPC in DC versus GT1-1 cells.
In order to study the effect of DC on PrP molecules derived from GT1-1 cells, it was important to determine whether the former cells express any endogenous PrPC. To analyze the presence of PrPC in DC, we first used immunofluorescence microscopy with the recombinant PrP Fab D13 (Fig. 1A). The present CD11c+ antibody affinity-purified DC, which displayed all morphological and phenotypic characteristics of DC, contained no PrPC either before (Fig. 1B) or after (Fig. 1A and B) 12 h of activation with LPS. In contrast, control GT1-1 cells showed marked PrPC immunolabeling at the cell surface (Fig. 1A). This result was confirmed by Western blots developed with the Fab D13. Lysates from the GT1-1 cells contained, as expected (37), large amounts of PrPC, while no PrPC could be detected in lysates from either immature or LPS-activated DC (Fig. 1B). To further investigate the level at which PrP expression is repressed in DC, we studied PrP mRNA levels using reverse transcription (RT)-PCR. An amplified product of 478 bp was evident in the GT1-1 cells after RT-PCR, but was not seen in the DC (Fig. 1C and D). Thus, neither PrPC nor PrP mRNA is expressed in mouse CD11c+ DC in vitro, even after activation with LPS. This result facilitates analyses of the fate of GT1-1 cell-derived PrPC and PrPSc delivered to the DC.
Processing of PrPC and PrPSc by DC in culture.
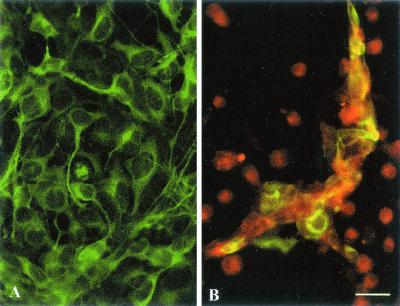
We next seeded immature CD11c+ DC onto ScGT1-1 cells growing in 10-mm-diameter dishes. Within 24 to 48 h, the DC adhered to and caused shrinkage of the ScGT1-1 cells as observed by phase-contrast microscopy and immunofluorescence (Fig. 2). To differentiate between the two cell types in the coculture, we used two cell-specific markers: MHC class II, which is expressed in DC, and neuron specific β-tubulin (TUJ1) that is expressed in GT1-1 cells. MHC class II immunopositive DC surrounded clusters of small shrunken β-tubulin immunopositive ScGT1-1 cells (Fig. 2B). The degenerated cells were subsequently engulfed by the DC in a manner similar to what has been described for the capture of various killed tumor cells by DC (28). DC were the only cell type to remain after 4 days of cocultivation in our system. Since the ScGT1-1 cells contain both isoforms of PrP, the present system enabled studies of potential interactions between the PrP isoforms during degradation of apoptotic ScGT1-1 cells in the PrPC-negative DC. Surprisingly, Western blot analysis of the cell lysates revealed a progressive decrease and subsequent loss of the PK-resistant PrP27-30 bands, which correspond to N-terminally trimmed products of the full-length PrPSc (39) (Fig. 3A and B). With an increased DC/ScGT1-1 ratio the rate of disappearance of PK-resistant bands was increased (Fig. 3B). The disappearance of the PK-resistant bands was not accompanied by an increase in PrPSc in either the pellets or acetone-precipitated fractions (Fig. 3D) of cell medium removed after cocultivation, which indicates that the disappearance of PrPSc from the cells was not caused by its excretion to the extracellular medium. As expected, no PK-resistant bands were found in lysates from cocultures of DC and uninfected GT1-1 cells, which were also engulfed by the DC and displayed a progressive loss of PrPC (Fig. 3C). An anti-Fas ligand antibody added to the medium during the coincubation had no effect on the progressive decrease of the PK-resistant PrP27-30 bands or cell death (data not shown) indicating that the DC-induced killing of the ScGT1-1 cells was mediated by other mechanisms than the Fas/FasL pathway. However, experiments using a Transwell system showed that the effects was not due to factors released from the DC into the medium, since no effects on either the expression of PrPC or the occurrence of PrPSc was seen in this system (Fig. 3E).
FIG. 2.
Coculture of ScGT1-1 cells and DC. (A) ScGT1-1 cells, immunolabeled with anti-β-tubulin antibodies (green); (B) ScGT1-1 cells (green to yellowish) exposed to DC, ratio 1:1, immunolabeled with anti-MHC class II antibodies (red), for 48 h. Note the extensive loss of GT1-1 cells. Remnants are surrounded by clusters of DC. Scale bar, 25 μm.
Taken together, these results suggest that DC degraded the PrPSc derived from ScGT1-1 cells. To analyze this degradation in further detail, we also examined non-PK-treated lysates of ScGT1-1 cells cocultured with DC (Fig. 4). Western blot analysis indicated an increase in a set of small PrP bands, identified as the three PrP27-30 species (arrows in Fig. 4), during the first days after the cells were mixed. Indeed, these low-molecular-weight PrP bands were electrophoretically indistinguishable from the three protease-resistant species that appear after proteolysis of PrPSc in the mouse system (compare lanes 1 in Fig. 3B). Exposing lysates to PK could mask this process by digesting the total amount of the full-length PrPSc into PrP27-30. In lysates from cocultures of ScGT1-1 cells and DC, low molecular weight PrP bands could be clearly visualized (the three lower bands in Fig. 3B), and their final loss was preceded by an increase in their amount (Fig. 3A [inset] and 4). These PrP bands appeared in lysates containing protease inhibitors to the same extent as in lysates without such inhibitors (data not shown). The proportions in intensity between the three bands did not undergo any changes. Since PrP27-30 products increased in parallel with a decrease in the marker for the GT1-1 cells, neuron-specific β-tubulin (Fig. 4), these data indicate an ongoing N-terminal trimming of the full-length PrPSc together with the ScGT1-1 cells induced by the DC. Exposure of the ScGT1-1 cells to LPS-activated DC, which have a lower phagocytic but a higher proteolytic capacity (14), had similar effects. As expected, lysates from cocultures of DC and control uninfected GT1-1 cells contained no PrP27-30 bands (Fig. 3C). The loss of PrPC and PrPSc observed using the PrP Fab D13 (raised against PrP amino acids [aa] 96 to 106) was also found using Fabs D18 (raised against PrP aa 133 to 157) and R1 (raised against PrP aa 225 to 231) (data not shown). Interestingly, the fact that no PrPC was detected in the lysates from the DC sampled 4 days after their coculture with ScGT1-1 cells shows that, similar to LPS, scrapie-infected cells do not induce PrPC in the present DC.
Taken together, the results show that mouse bone marrow-derived CD11c+ DC cause degradation of PrPSc in vitro. Since neuron specific β-tubulin was degraded concomitantly with an increase in the partially degraded PrPSc, it is suggested that the degradation occurs subsequent to killing and digestion of the ScGT1-1 cells by the DC.
DISCUSSION
The possibility that certain cells may degrade PrPSc has recently come into focus. For instance, prion-infected N2a cells can be cleared from PrPSc and prion infectivity by treatment with PrPC antibodies, and this clearance was suggested to be the result of an intracellular degradation of PrPSc in combination with hindered formation of new PrPSc (9, 32). Studies have also suggested that degradation of scrapie can be caused by macrophages (2, 5). Furthermore, vaccination with self-PrP peptides can reduce the amount of PrPSc in subcutaneous neuroblastoma produced by the transplantation of ScN2a cells in syngeneic A/J mice (38). Our study directly shows that postmitotic cells with strong phagocytic and proteolytic capacities, such as DC, can efficiently degrade PrPSc. Taken together, our results suggest that exogenous PrPSc is digested in two steps. During the first days of coculture, full-length PrPSc is trimmed to PrP27-30 (Fig. 4). In analogy to other cultured cells, this trimming may occur in acidic organelles such as endosomes. In a second step, these PrP27-30 species are rendered progressively more sensitive to proteolysis, as shown by their complete degradation by proteinase K (Fig. 3B). The molecular mechanisms responsible for such a change remain to be determined, but we surmise that they may include the disaggregation of PrPSc. Existence of protease-sensitive PrPSc species has been previously demonstrated by Safar et al. (36). Thus, a balance could be envisioned between the formation and degradation of PrPSc at early time points after infection in a host, and DC may play a crucial role in this balance to regulate the outcome of the infection.
Although it has been reported that neither FDC nor CD11c+ DC may be absolutely required for neuroinvasion after inoculation of high doses of scrapie (29), and we now find that the latter cells can efficiently degrade PrPSc, CD11c+ DC may still have the capacity to spread prion infectivity in an organism. The time scale for degradation of PrPSc, observed in the present study, would allow PrPSc-containing DC to migrate from the periphery, where they may encounter the scrapie agent, to lymphoid organs. PrPSc has been found in lymphatic CD11c+ DC after intestinal scrapie inoculation in rats (12), and CD11c+ DC isolated from the spleen of scrapie-infected mice are able to transmit prions to the central nervous system (1).
Several possible mechanisms for involvement of CD11c+ DC in spread of scrapie to the nervous system exist. (i) Transport of scrapie to the spleen and lymph nodes may occur, thereby exposing nerve endings and/or FDC to the infectious agent (11). (ii) Processing of scrapie to smaller, more infectious particles may occur. We are now starting bioassays in mice to the establish the rate at which prion infectivity is cleared from the cocultures and to see if infectivity increases transiently while PrP27-30 becomes progressively sensitive to proteases. Given the very small infectious titers found in cultured cells (4), these bioassays will require very long periods of time. CD11c+ DC could also assist the spread of prions by (iii) rapid induction of PrP conformational changes in the acidic endosomes in DC or (iv) release of so-called exosomes containing infectious material to surrounding cells. Considering the close association between axon terminals and DC described in the skin and liver (8, 22), and the finding of DC situated at an interface between the brain and the blood circulation in the choroid plexus and leptomeninges (23, 25), CD11c+ DC may also be in a position to present prion infectivity directly to the nervous system. Thus, it is speculated that CD11c+ DC may on the one hand facilitate neuroinvasion, as suggested by the previous in vivo studies, but on the other hand be involved in an efficient degradation of PrPSc, as shown by our in vitro study. Such a dual function of phagocytic cells may also occur in the processing of other pathogens. For instance, CSF-1 dependent cells, which include populations of macrophages and DC, can protect against systemic infection with L. monocytogenes but facilitate their neuroinvasion (15).
In conclusion, the present study shows that in vitro the murine bone marrow-derived CD11c+ DC express neither PrPC nor PrPC mRNA either before or after exposure to LPS or PrPSc. On the other hand, we obtained direct evidence that these DC can cause a progressive and efficient degradation of PrPSc derived from scrapie-infected GT1-1 cells. Since CD11c+ DC may be exposed to prions early during an infection, the observed processing of PrPSc may play an important role in the balance between clearance and propagation of the prions in a host.
Acknowledgments
This study was supported by a grant from the EU Commission, Biomed 2 contract QLK2-CT2002-81628, and by the Swedish Research Council (grant 4480).
REFERENCES
- 1.Aucouturier, P., F. Geissmann, D. Damotte, G. P. Saborio, H. C. Meeker, R. Kascsak, R. I. Carp, and T. Wisniewski. 2001. Infected splenic dendritic cells are sufficient for prion transmission to the CNS in mouse scrapie. J. Clin. Investig. 108:703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beringue, V., M. Demoy, C. I. Lasmezas, B. Gouritin, C. Weingarten, J. P. Deslys, J. P. Andreux, P. Couvreur, and D. Dormont. 2000. Role of spleen macrophages in the clearance of scrapie agent early in pathogenesis. J. Pathol. 190:495-502. [DOI] [PubMed] [Google Scholar]
- 3.Brown, K. L., K. Stewart, D. L. Ritchie, N. A. Mabbott, A. Williams, H. Fraser, W. I. Morrison, and M. E. Bruce. 1999. Scrapie replication in lymphoid tissues depends on prion protein-expressing follicular dendritic cells. Nat. Med. 5:1308-1312. [DOI] [PubMed] [Google Scholar]
- 4.Butler, D. A., M. R. Scott, J. M. Bockman, D. R. Borchelt, A. Taraboulos, K. K. Hsiao, D. T. Kingsbury, and S. B. Prusiner. 1988. Scrapie-infected murine neuroblastoma cells produce protease-resistant prion proteins. J. Virol. 62:1558-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carp, R. I., and S. M. Callahan. 1981. In vitro interaction of scrapie agent and mouse peritoneal macrophages. Intervirology 16:8-13. [DOI] [PubMed] [Google Scholar]
- 6.Der, S. D., Y. L. Yang, C. Weissmann, and B. R. Williams. 1997. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc. Natl. Acad. Sci. USA 94:3279-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickinson, A. G., H. Fraser, I. McConnell, and G. W. Outram. 1978. Mitogenic stimulation of the host enhances susceptibility to scrapie. Nature 272:54-55. [DOI] [PubMed] [Google Scholar]
- 8.Egan, C. L., M. J. Viglione-Schneck, L. J. Walsh, B. Green, J. Q. Trojanowski, D. Whitaker-Menezes, and G. F. Murphy. 1998. Characterization of unmyelinated axons uniting epidermal and dermal immune cells in primate and murine skin. J. Cutan. Pathol. 25:20-29. [DOI] [PubMed] [Google Scholar]
- 9.Enari, M., E. Flechsig, and C. Weissmann. 2001. Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc. Natl. Acad. Sci. USA 98:9295-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser, H., K. L. Brown, K. Stewart, I. McConnell, P. McBride, and A. Williams. 1996. Replication of scrapie in spleens of SCID mice follows reconstitution with wild-type mouse bone marrow. J. Gen. Virol. 77:1935-1940. [DOI] [PubMed] [Google Scholar]
- 11.Glatzel, M., F. L. Heppner, K. M. Albers, and A. Aguzzi. 2001. Sympathetic innervation of lymphoreticular organs is rate limiting for prion neuroinvasion. Neuron 31:25-34. [DOI] [PubMed] [Google Scholar]
- 12.Huang, F. P., C. F. Farquhar, N. A. Mabbott, M. E. Bruce, and G. G. MacPherson. 2002. Migrating intestinal dendritic cells transport PrPSc from the gut. J. Gen. Virol. 83:267-271. [DOI] [PubMed] [Google Scholar]
- 13.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inaba, K., S. Turley, T. Iyoda, F. Yamaide, S. Shimoyama, C. Reis e Sousa, R. N. Germain, I. Mellman, and R. M. Steinman. 2000. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J. Exp. Med. 191:927-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin, Y., L. Dons, K. Kristensson, and M. E. Rottenberg. 2002. Colony-stimulating factor 1-dependent cells protect against systemic infection with Listeria monocytogenes but facilitate neuroinvasion. Infect. Immun. 70:4682-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitamoto, T., T. Muramoto, S. Mohri, K. Doh-Ura, and J. Tateishi. 1991. Abnormal isoform of prion protein accumulates in follicular dendritic cells in mice with Creutzfeldt-Jakob disease. J. Virol. 65:6292-6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein, M. A., P. S. Kaeser, P. Schwarz, H. Weyd, I. Xenarios, R. M. Zinkernagel, M. C. Carroll, J. S. Verbeek, M. Botto, M. J. Walport, H. Molina, U. Kalinke, H. Acha-Orbea, and A. Aguzzi. 2001. Complement facilitates early prion pathogenesis. Nat. Med. 7:488-492. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Lasmezas, C. I., J. Y. Cesbron, J. P. Deslys, R. Demaimay, K. T. Adjou, R. Rioux, C. Lemaire, C. Locht, and D. Dormont. 1996. Immune system-dependent and -independent replication of the scrapie agent. J. Virol. 70:1292-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu, Y. J. 2001. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 106:259-262. [DOI] [PubMed] [Google Scholar]
- 21.Mabbott, N. A., A. Williams, C. F. Farquhar, M. Pasparakis, G. Kollias, and M. E. Bruce. 2000. Tumor necrosis factor alpha-deficient, but not interleukin-6-deficient, mice resist peripheral infection with scrapie. J. Virol. 74:3338-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markus, A. M., F. Kockerling, and W. L. Neuhuber. 1998. Close anatomical relationships between nerve fibers and MHC class II-expressing dendritic cells in the rat liver and extrahepatic bile duct. Histochem. Cell Biol. 109:409-415. [DOI] [PubMed] [Google Scholar]
- 23.Matyszak, M. K., L. J. Lawson, V. H. Perry, and S. Gordon. 1992. Stromal macrophages of the choroid plexus situated at an interface between the brain and peripheral immune system constitutively express major histocompatibility class II antigens. J. Neuroimmunol. 40:173-181. [DOI] [PubMed] [Google Scholar]
- 24.McBride, P. A., P. Eikelenboom, G. Kraal, H. Fraser, and M. E. Bruce. 1992. PrP protein is associated with follicular dendritic cells of spleens and lymph nodes in uninfected and scrapie-infected mice. J. Pathol. 168:413-418. [DOI] [PubMed] [Google Scholar]
- 25.McMenamin, P. G. 1999. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J. Comp. Neurol. 405:553-562. [PubMed] [Google Scholar]
- 26.Mellon, P. L., J. J. Windle, P. C. Goldsmith, C. A. Padula, J. L. Roberts, and R. I. Weiner. 1990. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 5:1-10. [DOI] [PubMed] [Google Scholar]
- 27.Montrasio, F., R. Frigg, M. Glatzel, M. A. Klein, F. Mackay, A. Aguzzi, and C. Weissmann. 2000. Impaired prion replication in spleens of mice lacking functional follicular dendritic cells. Science 288:1257-1259. [DOI] [PubMed] [Google Scholar]
- 28.Nouri-Shirazi, M., J. Banchereau, D. Bell, S. Burkeholder, E. T. Kraus, J. Davoust, and K. A. Palucka. 2000. Dendritic cells capture killed tumor cells and present their antigens to elicit tumor-specific immune responses. J. Immunol. 165:3797-3803. [DOI] [PubMed] [Google Scholar]
- 29.Oldstone, M. B., R. Race, D. Thomas, H. Lewicki, D. Homann, S. Smelt, A. Holz, P. Koni, D. Lo, B. Chesebro, and R. Flavell. 2002. Lymphotoxin-alpha- and lymphotoxin-beta-deficient mice differ in susceptibility to scrapie: evidence against dendritic cell involvement in neuroinvasion. J. Virol. 76:4357-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Rourke, K. I., T. P. Huff, C. W. Leathers, M. M. Robinson, and J. R. Gorham. 1994. SCID mouse spleen does not support scrapie agent replication. J. Gen. Virol. 75:1511-1514. [DOI] [PubMed] [Google Scholar]
- 31.Outram, G. W., A. G. Dickinson, and H. Fraser. 1974. Reduced susceptibility to scrapie in mice after steroid administration. Nature 249:855-856. [DOI] [PubMed] [Google Scholar]
- 32.Peretz, D., R. A. Williamson, K. Kaneko, J. Vergara, E. Leclerc, G. Schmitt-Ulms, I. R. Mehlhorn, G. Legname, M. R. Wormald, P. M. Rudd, R. A. Dwek, D. R. Burton, and S. B. Prusiner. 2001. Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature 412:739-743. [DOI] [PubMed] [Google Scholar]
- 33.Porter, D. D., H. G. Porter, and N. A. Cox. 1973. Failure to demonstrate a humoral immune response to scrapie infection in mice. J. Immunol. 111:1407-1410. [PubMed] [Google Scholar]
- 34.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. USA 95:13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prusiner, S. B., M. P. McKinley, K. A. Bowman, D. C. Bolton, P. E. Bendheim, D. F. Groth, and G. G. Glenner. 1983. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell 35:349-358. [DOI] [PubMed] [Google Scholar]
- 36.Safar, J., H. Wille, V. Itri, D. Groth, H. Serban, M. Torchia, F. E. Cohen, and S. B. Prusiner. 1998. Eight prion strains have PrPSc molecules with different conformations. Nat. Med. 4:1157-1165. [DOI] [PubMed] [Google Scholar]
- 37.Schatzl, H. M., L. Laszlo, D. M. Holtzman, J. Tatzelt, S. J. DeArmond, R. I. Weiner, W. C. Mobley, and S. B. Prusiner. 1997. A hypothalamic neuronal cell line persistently infected with scrapie prions exhibits apoptosis. J. Virol. 71:8821-8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Souan, L., Y. Tal, Y. Felling, I. R. Cohen, A. Taraboulos, and F. Mor. 2001. Modulation of proteinase-K resistant prion protein by prion peptide immunization. Eur. J. Immunol. 31:2338-2346. [DOI] [PubMed] [Google Scholar]
- 39.Taraboulos, A., A. J. Raeber, D. R. Borchelt, D. Serban, and S. B. Prusiner. 1992. Synthesis and trafficking of prion proteins in cultured cells. Mol. Biol. Cell. 3:851-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williamson, R. A., D. Peretz, C. Pinilla, H. Ball, R. B. Bastidas, R. Rozenshteyn, R. A. Houghten, S. B. Prusiner, and D. R. Burton. 1998. Mapping the prion protein using recombinant antibodies. J. Virol. 72:9413-9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williamson, R. A., D. Peretz, N. Smorodinsky, R. Bastidas, H. Serban, I. Mehlhorn, S. J. DeArmond, S. B. Prusiner, and D. R. Burton. 1996. Circumventing tolerance to generate autologous monoclonal antibodies to the prion protein. Proc. Natl. Acad. Sci. USA 93:7279-7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vremec, D., and K. Shortman. 1997. Dendritic cell subtypes in mouse lymphoid organs: cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J. Immunol. 159:565-573. [PubMed] [Google Scholar]