Abstract
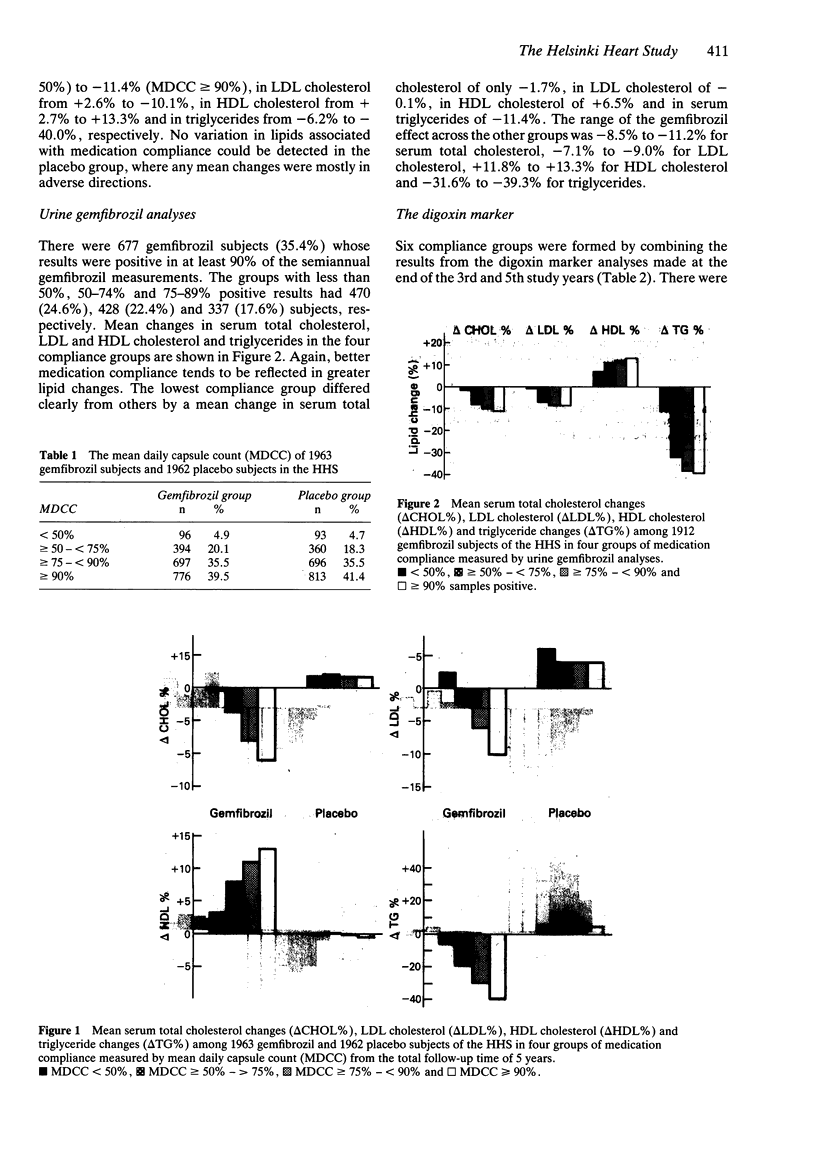
1. To control the bias caused by poor medication compliance in the Helsinki Heart Study three methods were used to measure medication compliance during the total 5 years follow up time: continuous capsule counting, semi-annual urine gemfibrozil analysis and a new method, the digoxin marker at the end of the third and fifth study years. 2. The serum lipid responses to gemfibrozil treatment varied linearly with the level of medication compliance, e.g. the mean change in serum total cholesterol was -11.4% among those whose apparent capsule consumption was greater than or equal to 90% of the scheduled dosage, -11.2% among those who had greater than or equal to 90% positive gemfibrozil analyses and -11.4% among those with good compliance according to both digoxin marker measurements. In contrast the mean serum cholesterol change was only -0.02% if the mean daily capsule count was less than 50%, -1.7% with fewer than 50% positive gemfibrozil analyses and -1.1% if the result was poor in both digoxin marker measurements. 3. Combining the different method findings revealed that the cholesterol changes tended to be small in those groups who had poor compliance classification measured by any of the methods, even if the other results showed good compliance.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cramer J. A., Scheyer R. D., Mattson R. H. Compliance declines between clinic visits. Arch Intern Med. 1990 Jul;150(7):1509–1510. [PubMed] [Google Scholar]
- Frick M. H., Elo O., Haapa K., Heinonen O. P., Heinsalmi P., Helo P., Huttunen J. K., Kaitaniemi P., Koskinen P., Manninen V. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987 Nov 12;317(20):1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- Friedewald W. T., Levy R. I., Fredrickson D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972 Jun;18(6):499–502. [PubMed] [Google Scholar]
- Manninen V., Elo M. O., Frick M. H., Haapa K., Heinonen O. P., Heinsalmi P., Helo P., Huttunen J. K., Kaitaniemi P., Koskinen P. Lipid alterations and decline in the incidence of coronary heart disease in the Helsinki Heart Study. JAMA. 1988 Aug 5;260(5):641–651. [PubMed] [Google Scholar]
- Mäenpä H., Javela K., Pikkarainen J., Mälkönen M., Heinonen O. P., Manninen V. Minimal doses of digoxin: a new marker for compliance to medication. Eur Heart J. 1987 Oct;8 (Suppl 1):31–37. doi: 10.1093/eurheartj/8.suppl_i.31. [DOI] [PubMed] [Google Scholar]
- Mäenpä H., Manninen V., Heinonen O. P. Comparison of the digoxin marker with capsule counting and compliance questionnaire methods for measuring compliance to medication in a clinical trial. Eur Heart J. 1987 Oct;8 (Suppl 1):39–43. doi: 10.1093/eurheartj/8.suppl_i.39. [DOI] [PubMed] [Google Scholar]
- Pullar T., Kumar S., Tindall H., Feely M. Time to stop counting the tablets? Clin Pharmacol Ther. 1989 Aug;46(2):163–168. doi: 10.1038/clpt.1989.121. [DOI] [PubMed] [Google Scholar]
- Roth H. P., Caron H. S., Hsi B. P. Measuring intake of a prescribed medication. A bottle count and a tracer technique compared. Clin Pharmacol Ther. 1970 Mar-Apr;11(2):228–237. doi: 10.1002/cpt1970112228. [DOI] [PubMed] [Google Scholar]


