Abstract
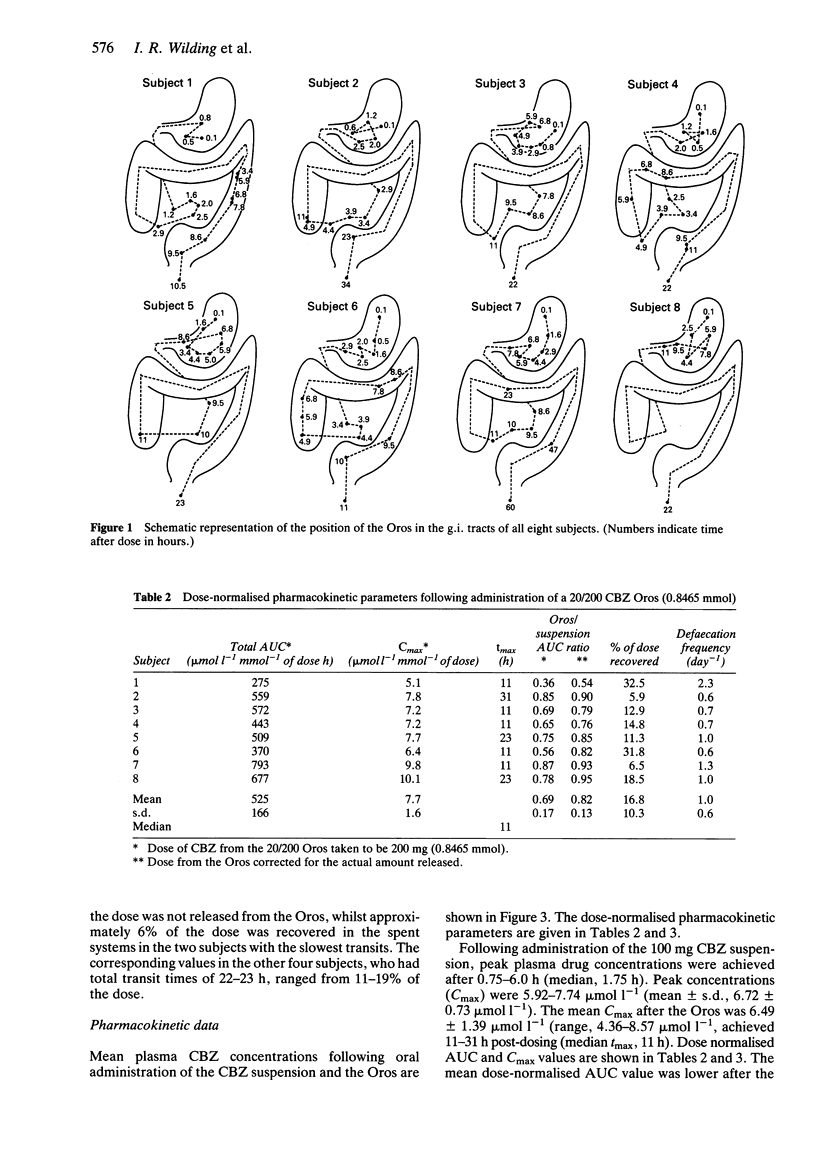
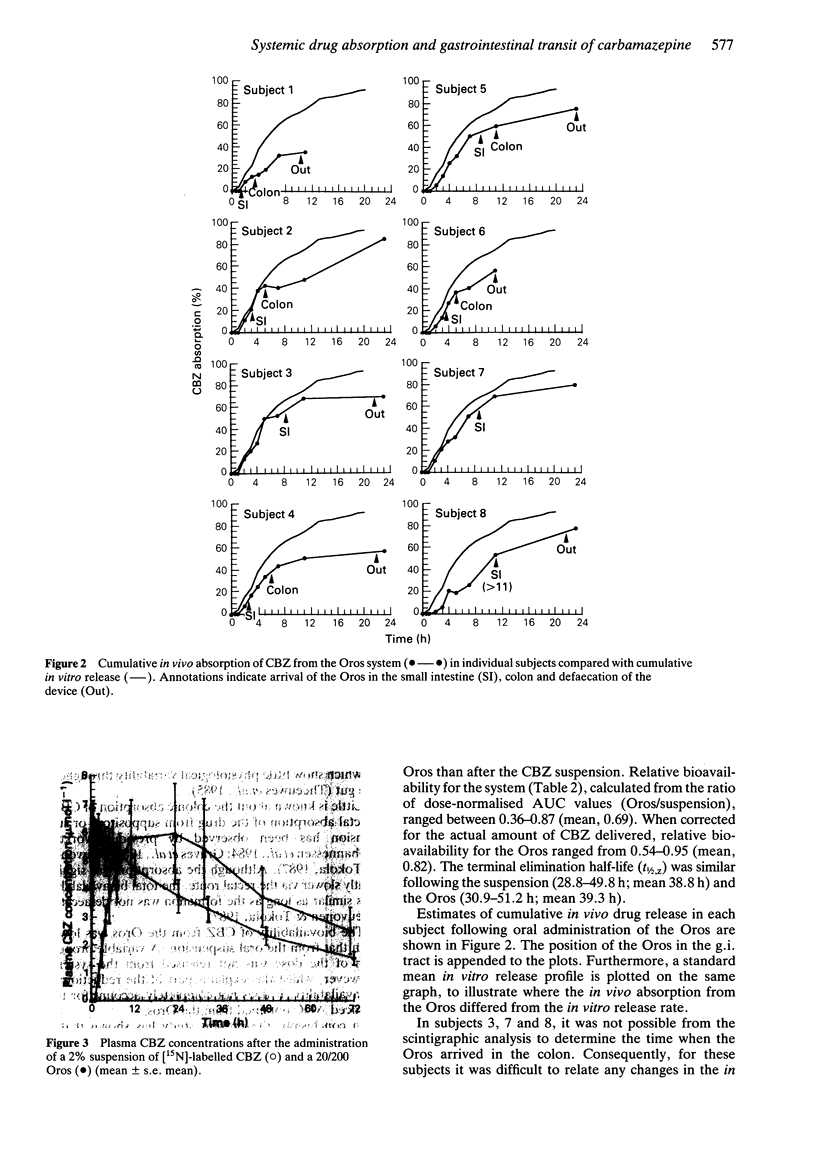
1. Plasma drug concentrations after a single oral administration of a suspension of carbamazepine (CBZ) and a 20/200 CBZ Oros osmotic pump system were measured in eight healthy male volunteers. The oral suspension contained 100 mg CBZ labelled with the stable isotope nitrogen-15, whilst the Oros contained 200 mg unlabelled CBZ. Plasma concentrations of [15N]-CBZ and CBZ were measured simultaneously by gas chromatography-mass spectrometry. 2. The position of the CBZ Oros (labelled with indium-111) in the gastrointestinal tract was followed by gamma scintigraphy. Plasma drug concentrations after the two treatments were used to relate pharmacokinetic with transit data. 3. The Oros was taken after breakfast and gastric emptying occurred between 1.1- greater than h post-dosing (median, 5.3 h). Small intestinal transit times ranged from 1.5- greater than 3.6 h, with a median of 2.2 h. There were wide individual variations in colonic transit, and the total transit time ranged from 10-60 h (median, 22 h). 4. Relative systemic bioavailability of CBZ from the Oros was reduced compared with that from the suspension (mean dose normalised AUC ratio = 0.69 +/- 0.17; mean dose-normalised AUC ratio = 0.85 +/- 0.13, allowing for actual release from the Oros system). 5. The in vivo absorption of drug into the systemic circulation from the Oros was estimated using the Wagner-Nelson method. This showed that absorption of CBZ was rapid when the Oros was present in the stomach and small intestine, the rate being determined by the release of drug from the system.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan K. K., Sawchuk R. J., Thompson T. A., Redalieu E., Wagner W. E., Jr, LeSher A. R., Weeks B. J., Hall N. R., Gerardin A. Bioequivalence of carbamazepine chewable and conventional tablets: single-dose and steady-state studies. J Pharm Sci. 1985 Aug;74(8):866–870. doi: 10.1002/jps.2600740813. [DOI] [PubMed] [Google Scholar]
- Davis S. S., Hardy J. G., Fara J. W. Transit of pharmaceutical dosage forms through the small intestine. Gut. 1986 Aug;27(8):886–892. doi: 10.1136/gut.27.8.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S. S., Norring-Christensen F., Khosla R., Feely L. C. Gastric emptying of large single unit dosage forms. J Pharm Pharmacol. 1988 Mar;40(3):205–207. doi: 10.1111/j.2042-7158.1988.tb05220.x. [DOI] [PubMed] [Google Scholar]
- Davis S. S., Washington N., Parr G. D., Short A. H., John V. A., Lloyd P., Walker S. M. Relationship between the rate of appearance of oxprenolol in the systemic circulation and the location of an oxprenolol Oros 16/260 drug delivery system within the gastrointestinal tract as determined by scintigraphy. Br J Clin Pharmacol. 1988 Oct;26(4):435–443. doi: 10.1111/j.1365-2125.1988.tb03403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves N. M., Kriel R. L., Jones-Saete C., Cloyd J. C. Relative bioavailability of rectally administered carbamazepine suspension in humans. Epilepsia. 1985 Sep-Oct;26(5):429–433. doi: 10.1111/j.1528-1157.1985.tb05675.x. [DOI] [PubMed] [Google Scholar]
- Hardy J. G., Wilson C. G., Wood E. Drug delivery to the proximal colon. J Pharm Pharmacol. 1985 Dec;37(12):874–877. doi: 10.1111/j.2042-7158.1985.tb04992.x. [DOI] [PubMed] [Google Scholar]
- John V. A., Shotton P. A., Moppert J., Theobald W. Gastrointestinal transit of Oros drug delivery systems in healthy volunteers: a short report. Br J Clin Pharmacol. 1985;19 (Suppl 2):203S–206S. doi: 10.1111/j.1365-2125.1985.tb02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuvonen P. J., Tokola O. Bioavailability of rectally administered carbamazepine mixture. Br J Clin Pharmacol. 1987 Dec;24(6):839–841. doi: 10.1111/j.1365-2125.1987.tb03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes F., Swanson D. R., Guittard G., Ayer A., Khanna S. Osmotic delivery systems for the beta-adrenoceptor antagonists metoprolol and oxprenolol: design and evaluation of systems for once-daily administration. Br J Clin Pharmacol. 1985;19 (Suppl 2):69S–76S. doi: 10.1111/j.1365-2125.1985.tb02745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGNER J. G., NELSON E. KINETIC ANALYSIS OF BLOOD LEVELS AND URINARY EXCRETION IN THE ABSORPTIVE PHASE AFTER SINGLE DOSES OF DRUG. J Pharm Sci. 1964 Nov;53:1392–1403. doi: 10.1002/jps.2600531126. [DOI] [PubMed] [Google Scholar]



