Abstract
The objectives of this study were to compare the molecular and biological characteristics of recent porcine reproductive and respiratory syndrome virus (PRRSV) field isolates to those of a modified live virus (MLV) PRRS vaccine and its parent strain. One hundred seventeen, 4-week-old pigs were randomly assigned to six groups. Group 1 (n = 20) served as sham-inoculated negative controls, group 2 (n = 19) was inoculated with Ingelvac PRRS MLV vaccine, group 3 (n = 20) was inoculated with the parent strain of the vaccine (ATCC VR2332), group 4 (n = 19) was inoculated with vaccine-like PRRSV field isolate 98-38803, group 5 (n = 19) was inoculated with PRRSV field isolate 98-37120, and group 6 (n = 20) was inoculated with known high-virulence PRRSV isolate ATCC VR2385. The levels of severity of gross lung lesions (0 to 100%) among the groups were significantly different at both 10 (P < 0.0001) and 28 days postinoculation (p.i.) (P = 0.002). At 10 days p.i., VR2332 (26.5% ± 4.64%) and VR2385 (36.4% ± 6.51%) induced gross lesions of significantly greater severity than 98-38803 (0.0% ± 0.0%), 98-37120 (0.8% ± 0.42%), Ingelvac PRRS MLV (0.9% ± 0.46%), and negative controls (2.3% ± 1.26%). At 28 days p.i., 98-37120 (17.2% ± 6.51%) induced gross lesions of significantly greater severity than any of the other viruses. Analyses of the microscopic-interstitial-pneumonia-lesion scores (0 to 6) revealed that VR2332 (2.9 ± 0.23) and VR2385 (3.1 ± 0.35) induced significantly more severe lesions at 10 days p.i. At 28 days p.i., VR2385 (2.5 ± 0.27), VR2332 (2.3 ± 0.21), 98-38803 (2.6 ± 0.29), and 98-37120 (3.0 ± 0.41) induced significantly more severe lesions than Ingelvac PRRS MLV (0.7 ± 0.17) and controls (0.7 ± 0.15). The molecular analyses and biological characterizations suggest that the vaccine-like isolate 98-38803 (99.5% amino acid homology based on the ORF5 gene) induces microscopic pneumonia lesions similar in type to, but different in severity and time of onset from, those observed with virulent strains VR2385 and the parent strain of the vaccine. Our data strongly suggest that isolate 98-38803 is a derivative of Ingelvac PRRS MLV and that the isolate is pneumovirulent.
Porcine reproductive and respiratory syndrome (PRRS) was first reported in the United States in 1987 (19). The causal agent of PRRS was first isolated in The Netherlands in 1991 (37) and was determined to be a small, enveloped, positive-sense, single-strand RNA virus referred to as PRRS virus (PRRSV). PRRSV is classified in the order Nidovirales, family Arteriviridae, genus Arterivirus (6).
PRRSV is endemic in most swine-producing countries, and today it is associated with major economic losses. Clinical signs of PRRS in growing pigs include fever, anorexia, and respiratory disease characterized by dyspnea and tachypnea. Reproductive failure associated with PRRSV is characterized by mid- to late-term abortions, increased numbers of mummified fetuses, decreased numbers of pigs born alive, increased numbers of weak-born pigs, and generally poor reproductive performance (10, 11).
Modified live commercial PRRSV (MLV) vaccines such as Ingelvac PRRS MLV (Boehringer Ingelheim Vetmedica, Inc.) for prevention and control have been available since June 1994. Shedding and spread of vaccine virus between vaccinated pigs and nonvaccinated contact controls have been reported to occur (36). Spread of vaccine strains of PRRSV in semen from boars in vaccinated boar studs to naive breeding herds has also been demonstrated to occur and to induce disease (34). A U.S. study demonstrated that boars vaccinated with PRRS MLV shed vaccine virus in semen up to 2 weeks after vaccination. But, after challenge with a wild-type virus, the shedding of the wild-type virus was shortened or eliminated. The semen quality in vaccinated boars after challenge was significantly reduced (9).
In 1996, veterinary practitioners and diagnosticians began to report disease outbreaks described as swine abortion and mortality syndrome, atypical PRRS, or acute PRRS (5, 17). Many of the affected herds had been vaccinated multiple times with modified live PRRSV vaccine, yet they experienced clinical outbreaks characterized by mid- or late-term abortions, with an incidence of 10 to 50% of the herd affected in a 1- to 5-week period. In the majority of the acute PRRS cases, diagnosticians observed microscopic lesions typical of PRRSV (interstitial pneumonia, encephalitis, and myocarditis). In some field cases of severe reproductive failure in breeding herds and respiratory disease in growing pigs, the only infectious agent detected was a PRRSV isolate that, based on genomic sequencing and restriction fragment length polymorphism patterns, was highly homologous to the vaccine (Ingelvac PRRS MLV) used in the herd (38). This raised concern from practitioners and producers over vaccine efficacy and safety.
Concerns over PRRS MLV vaccine safety arose in Denmark following the use of PRRS MLV vaccine in 1996 as part of a national PRRSV control program. The program included the vaccination of PRRSV-seropositive herds and of boars in artificial-insemination centers with a PRRS MLV vaccine. In 1997 there were multiple outbreaks of “severe PRRS” in Danish swine herds, both vaccinated and unvaccinated. The only PRRSV isolated from these affected herds was a virus with 99.2 to 99.5% sequence identity to the Ingelvac PRRS MLV vaccine (4, 22), which was the vaccine used in the control program. PRRSV isolates from these cases showed 99.0 to 99.3% nucleotide sequence identities with VR2332 (22). The North American type of PRRSV had not previously been recognized in Denmark or elsewhere in Europe. A comparison of the sequences of the open reading frame 1 (ORF1) regions of Ingelvac PRRS MLV vaccine, the parental strain of the vaccine (VR2332), vaccine-derived field isolates collected from vaccinated herds 2 to 5 months after vaccination, and two isolates from nonvaccinated herds isolated 6 months after the beginning of the federal vaccination program was carried out. Five mutations that occurred in all three examined vaccine-derived isolates indicated a strong parallel pressure on these positions in the vaccine virus (33). Taken together, the data suggested that the MLV vaccine had spread from the vaccinated herds to other nonvaccinated PRRSV-naive herds.
In 1999, the ORF5 and ORF7 genes of 20 Danish field isolates were sequenced to determine relatedness to the Ingelvac PRRS MLV vaccine. It was found that MLV vaccines such as the Ingelvac PRRS MLV have a tendency to spread in a swine population and to revert to a state of greater virulence (35). In all 20 isolates, a total of 52 nucleotide mutations in the ORF5 gene sequence compared to the ORF5 sequence of the vaccine virus were found. Only seven of these mutations were synonymous. At amino acid positions 13 and 151, the sequences of many of the Danish isolates had reverted to that of the wild-type parental strain (VR2332).
It has been documented that PRRSV strains differ in virulence (14-16, 30) and vary genetically (23-27). Concerns that vaccine strains or derivatives of the vaccine strains may induce disease continue to be discussed (20, 28, 36). The objective of this research was to compare the pathogenicity of two U.S. PRRSV isolates with a high sequence homology with Ingelvac vaccine virus from cases of severe PRRS outbreaks to that of a known high-virulence PRRSV isolate (ATCC VR2385), the Ingelvac PRRS MLV vaccine, and the parent strain of the vaccine (ATCC VR2332) in a well-established PRRSV model (14, 15).
MATERIALS AND METHODS
Animal source.
One hundred seventeen, specific-pathogen-free pigs from a PRRSV-free herd were purchased, weaned, and transported to the Iowa State University research facility at 12 to 14 days of age. To confirm the negative PRRSV status of the pigs, a commercial enzyme-linked immunosorbent assay (ELISA; PRRSV antibody test kit; IDEXX Laboratories, Inc., Westbrook, Mass.) was performed on serum samples collected on arrival and again prior to inoculation at 4 weeks of age.
Virus inocula.
Five different PRRSV isolates were used: ATCC VR2385 (28), ATCC VR2332 (31), which is the parent strain of Ingelvac PRRS MLV vaccine, Ingelvac PRRS MLV vaccine (lot JA-621C-416) in a 2-ml dose in an extralabel fashion (intranasal inoculation), isolate 98-37120 (5, 20), and isolate 98-38803. Isolate 98-37120 was isolated from a farm in Iowa containing nonvaccinated swine that experienced characteristic symptoms of acute PRRS (5). Isolate 98-38803 was from a 300-sow herd in Iowa that experienced 80 abortions over a 5-week period and high preweaning (50%) and growing-pig mortality (15%) associated with PRRSV-induced pneumonia and secondary infections. Gilts in this herd were vaccinated with Ingelvac PRRS MLV approximately 60 days prior to introduction to the herd.
The virus inocula were prepared as previously described (40). For each virus inoculum, a plaque-purified virus stock was propagated in confluent monolayers of MARC-145 cells (21). All virus inocula were adjusted to a final titer of 105.5 50% tissue culture infectious doses/2 ml. A 2-ml inoculation was given intranasally by slowly dripping the inoculum into the nostrils. Controls were inoculated with uninfected cell culture lysates.
Experiment design.
The pigs were randomly assigned to one of six groups of 19 or 20 animals each. Each group was housed in a separate power-ventilated room. At 4 weeks of age all groups were inoculated intranasally with 2 ml of one of the different PRRSV isolates (field isolates 98-38803 and 98-37120, VR2332, and VR2385) or the vaccine (Ingelvac PRRS MLV vaccine) or were sham inoculated with cell culture media from uninfected cells. Clinical observations including respiratory disease scores ranging from 0 to 6 (0, normal; 6, severe) and rectal temperature were recorded every other day from 0 to 28 days postinoculation (p.i.) (14, 15). Blood was collected prior to inoculation and at 6, 14, 21, and 28 days p.i. for virus assays and serology. Necropsy was performed on 10 randomly selected pigs from each group at 10 days p.i. and on the remainder at 28 days p.i. Bronchoalveolar lavage (BAL) fluid was collected as described previously (29) at necropsy at 10 and 28 days p.i.
Serology.
A commercial PRRSV ELISA (IDEXX Laboratories, Inc.) kit was used to detect and compare the anti-PRRSV antibody responses of the pigs prior to and after inoculation. According to the manufacturer, samples with sample-to-positive (S/P) ratios ≥0.4 were considered positive for antibodies against PRRSV.
Gross pathology and histopathology.
Complete necropsies were performed on all pigs, and all organ systems were examined. All lungs were examined in blind fashion and given a subjective score for severity of gross lung lesions using an established scoring system that estimates the percentage of lung affected by pneumonia (14, 15). Samples of lung (two sections from the cranial lobe and one from each of middle, accessory, and caudal lung lobes), heart, kidney, ileum, spleen, tonsil, and tracheobronchial and mediastinal lymph nodes were collected and fixed in 10% neutral buffered formalin and routinely processed for histopathological examination. The microscopic sections were examined in blind fashion and assigned a score for severity of interstitial pneumonia (0 to 6) as previously described (14, 15).
Immunohistochemistry.
Immunohistochemistry of paraffin-embedded blocks of lung and lymph nodes for detection of PRRSV antigen was performed as previously described (13) by using a cocktail of two different mouse monoclonal antibodies, SDOW-17 in a dilution of 1/5,000 and SR-30 in a dilution of 1/1,500.
RT-PCR.
Reverse transcription-PCR (RT-PCR) and PRRSV isolation from BAL fluid were attempted (39). In the pigs where the BAL fluid tested negative for PRRSV, serum was tested by PCR. If serum also tested negative for PRRSV, tonsils from those pigs were tested by PCR. BAL fluid was collected aseptically with 50 ml of lavage fluid consisting of Earle's balanced salt solution (Sigma Chemical Co., St. Louis, Mo.). The BAL fluid was kept at −70°C until used for RT-PCR. Prior to RNA extraction, the BAL fluid was frozen and thawed three times. After centrifugation at 2,000 × g for 10 min at 4°C, 140 μl of the supernatant was used for RNA extraction with the QIAamp viral RNA minikit (Qiagen Inc., Valencia, Calif.) according to the instructions of the manufacturer. RT-PCR was carried out by using the One Step RT-PCR kit (Qiagen Inc.). Eight microliters of the RNA template was added to 42 μl of the RT-PCR master mixture. PCR was performed with a set of primers corresponding to conserved regions in the ORF7 as previously described (7, 8).
Viral sequence analysis.
Two PCR products amplified from each group, one at 10 days p.i. and one at 28 days p.i., were sequenced in the ORF5 gene (GenBank accession no. PRU87392; primer locations: P5F, positions 13696 to 13714; P5R, positions 14459 to 14437; PCR product length: 764 bp) to confirm that the isolate used in the respective inoculum was the same as the one replicating in the respective pigs. The PCR amplification of the target gene and preparation for sequencing were performed as previously described (7).
Sequencing was performed at the Iowa State University Nucleic Acid Facility (Ames, Iowa). Sequences were aligned and analyzed with the DNAstar (DNASTAR Inc., Madison, Wis.) computer program.
Statistical analysis.
The data from repeated measurements of rectal temperature were analyzed by using multivariate analysis of variance, which accounts for the within-subject correlation. The asymmetries of the histograms in terms of percentage of gross lung lesions and the sample size of 10 per group suggested that it was appropriate and conservative to use the nonparametric Kruskal-Wallis analysis of variance (ANOVA) to test for a difference in group distributions. If the ANOVA was significant (P < 0.05), post hoc tests (pair-wise Wilcoxon tests) were then used to determine the relationships among the groups. Gross lesions and microscopic-interstitial-pneumonia scores were ordinal variables, so Kruskal-Wallis ANOVA was used to test for a difference in the group distributions.
RESULTS
Clinical signs.
The negative-control group and the Ingelvac PRRS MLV-inoculated group showed no clinical signs of illness at any time during the experiment. Rectal temperatures and respiratory disease scores from these two groups were within normal range. Pigs in the VR2332 and VR2385 groups exhibited dyspnea, tachypnea, and lethargy beginning at 5 to 7 days p.i.; symptoms became moderate to severe by 10 days p.i. and resolved by 14 to 21 days p.i. In contrast, pigs inoculated with field isolates 98-38803 and 98-37120 had a different temporal pattern of disease manifestation. Pigs in these groups did not display clinical signs until 21 days p.i. Respiratory disease (dyspnea and tachypnea), fever, and lethargy in these groups were mild to moderate from 21 days through the termination of the study at 28 days p.i.
Statistical evaluation of the clinical-sign scores suggested no significant differences between the groups. The scores for the control group and the Ingelvac PRRS MLV group were generally lower than the scores for the other groups. The variation among groups decreased as days p.i. increased. The statistical analysis of the data from repeated measurements of rectal temperature resulted in a statistically significant (P < 0.05) group by time interaction. The relationship among the group averages changed with days p.i., and no group average was uniformly different from the others over time. Inspection of the plot of temperature means versus days p.i. showed no clear patterns, such as trends, except a reduction in variation at 14, 16, and 18 days p.i.
Gross lesions.
Gross-lung-lesion scores are summarized in Table 1. When observed, the PRRSV-induced lesions were similar in type but different in severity among the groups. PRRSV-induced gross lesions were characterized by failure of the lung to collapse and mottled tan, moderately well demarcated areas of pneumonia. The lesions were observed most often in the cranioventral portions but were also found multifocally throughout the lung. At 10 days p.i., gross lung lesions in the VR2385 and VR2332 groups were significantly (P < 0.0001) more severe than those in the other groups. At 28 days p.i., there were significantly (P = 0.002) more severe gross lesions in the 98-37120 group than in all other groups.
TABLE 1.
Comparison of PRRSV-induced gross and microscopic lung lesions at 10 and 28 days p.i.c
| Group | Gross-lung-lesion score (%)a at:
|
Microscopic-lesion scoreb at:
|
||
|---|---|---|---|---|
| 10 days p.i. | 28 days | 10 days p.i. | 28 days p.i. | |
| Negative control | 2.3 (1.26)A | 4.3 (1.03)A | 0.4 (0.22)A | 0.7 (0.15)A |
| Ingelvac PRRSV | 0.9 (0.46)A | 4.2 (1.06)A | 0.0 (0.0)A | 0.7 (0.17)A |
| VR2332 | 26.5 (4.64)B | 9.6 (1.9)A | 2.9 (0.23)B | 2.3 (0.21)B |
| 98-38803 | 0.0 (0.0)A | 7.1 (1.9)A | 0.3 (0.15)A | 2.6 (0.29)B |
| 98-37120 | 0.8 (0.42)A | 17.2 (6.51)B | 0.6 (0.27)A | 3.0 (0.41)B |
| VR2385 | 36.4 (6.51)B | 3.8 (0.7)A | 3.1 (0.35)B | 2.5 (0.27)B |
Mean (standard error of the mean) gross-lung-lesion score estimates the percentage of the lung affected by pneumonia.
Interstitial-pneumonia score. Range, 0 to 6. 0, no microscopic lesions; 1, mild multifocal lesions; 2, mild diffuse lesions; 3, moderate multifocal lesions; 4, moderate diffuse lesions; 5, severe multifocal lesions; 6, severe diffuse interstitial-pneumonia lesions.
Different superscripts (A and B) within each column indicate significantly different values as determined by Kruskal-Wallis ANOVA.
Microscopic lesions.
Microscopic lung lesions in the PRRSV-inoculated groups were similar in type but varied in severity and timing of onset and resolution. The lesions were characterized by type 2 pneumocytic hypertrophy and hyperplasia, septal infiltration with mononuclear cells, and increased amounts of alveolar exudate and necrotic debris. Lesions in the VR2332 and VR2385 groups were moderate to severe at 10 days p.i. and mild to moderate by 28 days p.i. Lung lesions in groups 98-37120 and 98-38803 were minimal or absent at 10 days p.i. but were mild to moderate at 28 days p.i. (Fig. 1). Lesions in controls and Ingelvac PRRS MLV groups were essentially unremarkable at both 10 and 28 days p.i. Microscopic lung lesions in the VR2385 and VR2332 groups were significantly (P < 0.0001) more severe than those in all other groups at 10 days p.i. At 28 days p.i., the microscopic lesions in the VR2385, VR2332, 98-37120 and 98-38803 groups were significantly (P < 0.0001) more severe than those in controls and the Ingelvac PRRS MLV group (Table 1).
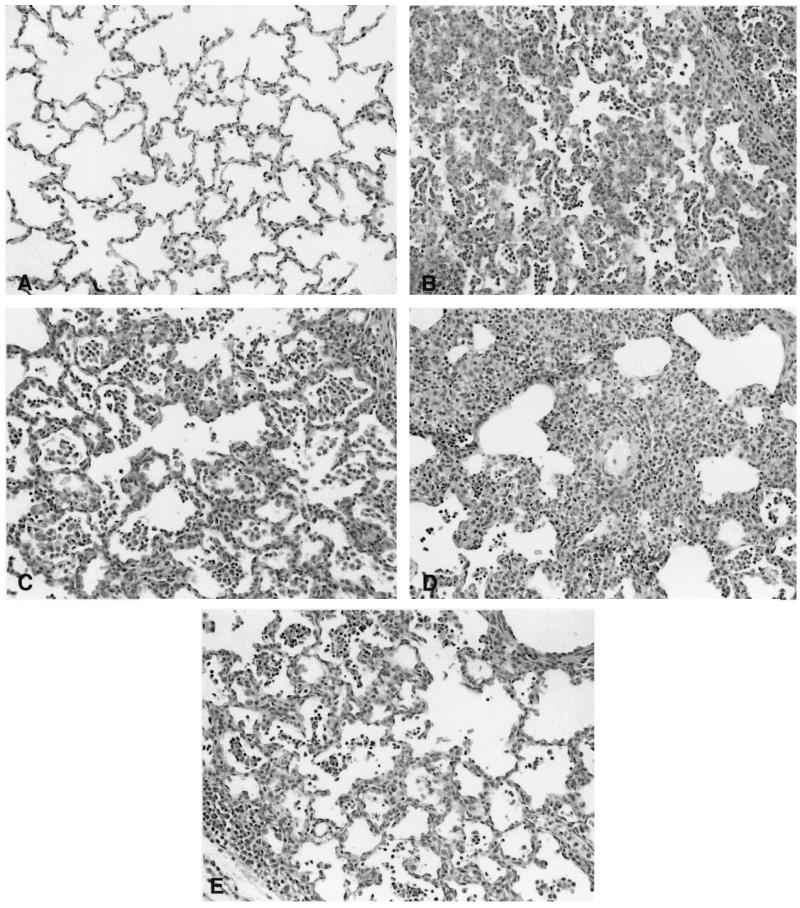
FIG. 1.
Comparison of PRRSV-induced microscopic lung lesions at 28 days p.i. Shown are sections of lungs from pigs in Ingelvac PRRS MLV (A), VR2332 (B), 98-38803 (C), 98-37120 (D), and VR2385 (E) groups. Lesion severity ranges from no lesions (A) to moderate (B, C, and E) to severe (D) interstitial pneumonia.
None of the pigs in the control or Ingelvac PRRS MLV group had microscopic evidence of myocarditis, whereas 7 of 19, 8 of 19, 8 of 20, and 8 of 20 pigs in the 98-38803, 98-37120, VR2385, and VR2332 groups, respectively, had mild lymphoplasmacytic myocarditis.
Antibody response.
All pigs were negative (S/P < 0.4) for PRRSV serum antibodies at the time of arrival (10 to 12 days of age) and at the time of inoculation (4 weeks of age). Negative-control pigs remained negative for PRRSV antibodies throughout the study.
Pigs in the VR2332 and VR2385 groups all seroconverted to anti-PRRSV between 6 and 14 days p.i. and showed similar antibody responses (Fig. 2). The serological profile for the 98-38803 group was similar to those for the VR2332 and VR2385 groups. None of the pigs seroconverted before 14 days p.i., and the highest peak in S/P ratio was seen at 21 days p.i. In the 98-37120 group, the antibody response to PRRSV was first detected at 14 days p.i., with four of nine pigs positive; by ELISA, seven of nine pigs were positive at 21 days p.i. and eight of nine pigs were positive at 28 days p.i. The serological profile of group 2 (Ingelvac PRRS MLV vaccine) was somewhat different from those of the other groups: 0 of 20 pigs at 6 days p.i., 2 of 9 pigs at 14 days p.i., 4 of 9 pigs at 21 days p.i., and 5 of 9 pigs at 28 days p.i. were positive.
FIG. 2.
Comparison of the PRRSV ELISA means (S/P ratios) among the PRRSV-inoculated groups. DPI, days p.i.
RT-PCR.
The sham-inoculated negative-control group was free of PRRSV throughout the study. All pigs in the VR2332 and VR2385 groups were positive for PRRSV nucleic acids by RT-PCR of BAL fluid at 10 and 28 days p.i. In the 98-38803 group, 9 of 10 pigs were positive by PCR at 10 days p.i. and BAL fluid for 9 of 9 pigs was positive at 28 days p.i. In group 98-37120 BAL fluid for 7 of 10 pigs was positive by PCR at 10 days p.i. Among the three pigs in the 98-37120 group whose BAL fluid was PCR negative, one of three had positive serum and the remaining two had negative serum and tonsil. At 28 days p.i., BAL fluid from eight of nine of the 98-37120 pigs was positive by PCR. The negative pig also had negative serum and tonsil. In contrast, in the Ingelvac PRRS MLV group, 1 of 10 pigs was positive by RT-PCR at 10 days p.i. and 2 of 9 pigs had BAL fluid positive by RT-PCR at 28 days p.i. RT-PCR of serum samples of Ingelvac PRRS MLV-inoculated pigs demonstrated that four of nine pigs were positive at 10 days p.i. and three of seven pigs were positive at 28 days p.i. In the Ingelvac PRRS MLV-inoculated pigs whose BAL fluid and serum were negative by RT-PCR, tonsil was also tested and one additional positive animal was identified. Overall 6 of 10 Ingelvac PRRS MLV-inoculated pigs were positive at 10 days p.i. and 5 of 9 were positive at 28 days p.i.
Nucleotide sequencing and sequence analysis.
All isolates described in this paper can be found in the GenBank database under accession numbers AF066183 (Ingelvac PRRS MLV vaccine), PRU87392 (VR2332), AF535152 (98-38803), AF339499 (98-37120), and U03040 (VR2385). Sequence analysis of the ORF5 gene identified three phylogenetically distinct clusters: Ingelvac PRRS MLV, VR2332, and 98-38803 are closely related, while groups 98-37120 and VR2385 are located in other branches (Fig. 3). Based on the sequence of ORF5 gene, 98-38803 is most closely related (99.5% amino acid sequence identity) to the Ingelvac PRRS MLV vaccine strain (Table 2). 98-37120 and Ingelvac PRRS MLV have 96% amino acid sequence identity in the products of their ORF5 genes. Of the PRRSV isolates used in this study, VR2385 appears to be the most distinct.
FIG. 3.
Dendrogram of the ORF5 nucleotide sequences (positions 13696 to 14459 of the PRRSV gene; GenBank accession no. PRU87392) of PRRSV isolates recovered from pigs at 10 (pigs 9, 41, 61, and 81) and 28 days p.i. (pigs 11, 51, 71, and 99). 98-38803, pigs 9 and 11; 98-37120, pigs 22 and 31; VR2385, pigs 41 and 51; VR2332, pigs 61 and 71; Ingelvac PRRS MLV, pigs 81 and 99. The phylogenetic relationships were estimated by using the ClustalV method.
TABLE 2.
Pair-wise comparison of the amino acid sequences of the ORF5 gene products among PRRSV inocula used in the study
Amino acid sequence identity between virus isolates recovered from pigs and the respective original inocula in groups VR2332, VR2385, 98-37120, and 98-38803 was 100%. The virus isolate recovered from pigs in the Ingelvac PRRS MLV group had 99.5 to 100% sequence identity with the original virus inoculum.
The sequence of a virus isolate from one pig in the Ingelvac PRRS MLV group (pig 81, necropsied at 10 days p.i.) was found to be 99.5% identical to the sequence of the virus inoculum (Ingelvac PRRS MLV) and 99.5% identical to the sequence of VR2332 (the parent strain of the vaccine). There are only two amino acid differences in the products of the ORF5 genes of Ingelvac PRRS MLV and VR2332, one at position 13 and the other at position 151. VR2332 has an arginine at both positions, whereas Ingelvac PRRS MLV has a glutamine at position 13 and a glycine at position 151. The isolate from pig 81 had the change from glycine to arginine at position 151, and therefore its ORF5 gene was as closely related to that of Ingelvac PRRS MLV as to that of VR2332.
Nucleotide sequence accession number.
The nucleotide sequence of the ORF5 gene of PRRSV isolate 98-38803 has been submitted to GenBank (accession no. AF535152).
DISCUSSION
PRRSV 98-38803 was isolated from a sow herd with PRRSV-induced abortions and PRRSV-related respiratory disease in growing pigs. The gilts prior to introduction into the herd were vaccinated with Ingelvac PRRSV MLV vaccine. PRRSV was isolated from the tissues of affected sows via passage in cell culture. The product of the ORF5 gene of the 98-38803 isolate from this herd has 99.5% amino acid sequence identity to that of the Ingelvac PRRSV MLV vaccine. Although the two viruses are genetically very similar, the results of this study, using a respiratory model, indicate that there are significant biological differences between 98-38803 and the Ingelvac MLV vaccine. The 98-38803 isolate replicated better in pigs and induced higher ELISA S/P ratios than Ingelvac PRRS MLV. Isolate 98-38803 also induced significantly more severe microscopic lung lesions (P < 0.0001) at 28 days p.i. than Ingelvac PRRS MLV or controls. In addition, none of the pigs in the Ingelvac PRRS MLV group had myocarditis, whereas 7 of 19 pigs in the 98-38803 group had myocarditis. Isolate 98-38803 is considerably less virulent than highly virulent PRRSV strain VR2385 and produces a later onset of respiratory symptoms. Pigs infected with 98-38803 showed evidence of mild clinical disease nearly 2 weeks after the group inoculated with the highly virulent strain did. When VR2385 pigs were recovering from the disease, pigs in the 98-38803 group began to show signs of respiratory disease and fevers. This was further supported by observation of mild-to-moderate interstitial pneumonia lesions in the 98-38803 group at 28 days p.i.
The second field isolate used in this study, 98-37120, biologically behaved most similar to 98-38803 in onset, duration, and severity of disease and appears to be moderate in virulence. The virus was one of eight acute PRRSV isolates genetically analyzed by Key et al. (20). Among the ORF5 genes of these acute PRRSV isolates, that of 98-37120 had the highest sequence identity to those of Ingelvac PRRS MLV and VR2332. Our sequence analysis showed that the ORF5 gene product of the 98-37120 isolate has 96% amino acid sequence identity to those of Ingelvac PRRS MLV and VR2332 and 95.5% amino acid sequence identity to that of 98-38803. Between the 200-amino-acid ORF5 gene products of Ingelvac PRRS MLV and 98-37120 there are six amino acid differences. The positions are 29 (valine), 30 (aspartic acid), 34 (asparagine), 35 (asparagine), 58 (glutamic acid), and 151 (arginine). 98-37120 and VR2385 have three of these changes in common, positions 29, 34, and 151. Isolates 98-37120, VR2332, and Ingelvac PRRS MLV all have an isoleucine at position 189. Consequently, sequence analysis did not suggest that isolate 98-37120 is a direct derivative of the Ingelvac PRRS MLV.
There is still the question of whether field isolate 98-38803 is a direct derivative of the Ingelvac PRRS MLV vaccine, a possible recombinant of the vaccine virus and a wild-type virus, or a truly wild-type virus that is highly homologous to the original parent vaccine strain, VR2332, which may be still circulating in the field. Although answering such a question may require whole-genome sequencing, sequence analysis of ORF5, a highly variable gene of PRRSV, provides some clues. ORF5 is a region containing 603 bases and encoding the major envelope glycoprotein (25 kDa). Although ORF5 is only a small part of the entire PRRSV genome, which contains >15,000 bases, it is known to have the most variable sequence among isolates (18). Amino acid homology in the product of ORF5 revealed a very close relationship between 98-38803 and the Ingelvac PRRS MLV vaccine, as isolate 98-38803 showed 99.5% identity, while the isolate has 98.5% homology with the vaccine parent strain, VR2332. Our conclusion may be supported by a recent study by Chang et al. (7) suggesting that sequence divergence of ORF5 between the parental strain and its derivatives should be less than 1% after 1 year of in vivo replication in pigs. The only difference in the product of ORF5 between Ingelvac PRRS MLV vaccine and 98-38803 was found at position 189: the 98-38803 isolate had a valine, whereas the Ingelvac PRRS MLV had an isoleucine. Furthermore, like the Ingelvac PRRS MLV, isolate 98-38803 had a glycine at position 151. Wesley et al. (38) described the glycine at residue 151 as an amino acid marker that identifies the vaccine. Unlike the Ingelvac PRRSV MLV vaccine, its parental strain, VR2332, has arginine at position 151, which appears to be more stable and common among wild-type PRRSVs (1, 2, 20). However, reversion from glycine to arginine during in vivo replication of the vaccine virus and cell-culture-adapted virus has been demonstrated (7, 38). In the context of the previous vaccination history in the index herd, partial sequence analysis suggests that PRRSV 98-38803 is likely a direct derivative of the vaccine.
Provided that isolate 98-38803 is a descendant of Ingelvac PRRS MLV, the question that still remains to be answered is why 98-38803 is more virulent than the vaccine. Reversion of the MLV vaccine strain to a virulent strain has also been demonstrated by Storgaard et al. (35) using European field isolates and by Allende et al. (2) using U.S. field isolates. Although no genetic markers for the virulence of PRRSV have been identified yet, it is interesting that isolate 98-38803 has valine at position 189 like the known highly virulent PRRSV VR2385 whereas Ingelvac PRRS MLV, VR2332, and isolate 98-37120 have isoleucine. Furthermore, this was the only amino acid substituted among amino acids constituting the C terminal region of the product of ORF5 (amino acids 164 to 200). The exact biological function of this region is unknown. However, in many cases the C terminus of the envelope protein might play a role in signal transduction related to virus replication. Whether such a substitution is of biological significance in the process of reversion to virulence remains to be further studied.
While genetic markers for virulence remains to be found, reversion of the vaccine virus to a virulent strain could be attributed to recombination event between the vaccine strain and field isolate. Experiments with distinct PRRSV isolates growing in the same cell culture have shown that the ability of the virus to build recombination is relatively high (32, 41, 42). One group estimated that the frequency of such recombination is from <2 to 10% in a 1,182-base fragment analyzed (41). Although no recombinant PRRSV was obtained from pigs inoculated with two different PRRSV field isolates or two different PRRSV MLV vaccines (32, 41), the possibility of recombination between vaccine and field strains due to vaccination of PRRSV-infected herds must always be kept in mind.
In summary, our data strongly suggest that isolate 98-38803 is a derivative of Ingelvac PRRS MLV and that the isolate is pneumovirulent. The obtained sequencing data indicate that, based on the ORF5 gene, 98-38803 isolate is more closely related to Ingelvac PRRS MLV than to VR2332 and that isolate 98-38803 is a descendant of the MLV. Whole-genome sequencing for a better assessment of this remains to be conducted. Inoculation of pregnant sows with the 98-38803 isolate would provide further insight into the reproductive virulence of the isolate. Consequently, when producers and veterinarians choose to use MLV vaccines, they should consider the potential risk of reversion to virulence. However, MLV vaccines are also an effective way of inducing immunity and protecting herds from losses associated with infections by highly virulent strains of PRRSV (3, 12). Producers and practitioners must continually weigh the risks of vaccination versus no vaccination. Utilization of a protocol that focuses on population immune management with vaccination or planned exposure may minimize this risk.
Acknowledgments
We thank Prem Paul (University of Nebraska, Lincoln) for providing PRRSV isolate VR2385 and John Landgraf (NVSL, Ames, Iowa) for providing acute PRRSV isolate 98-37120. We also thank Ryan Royer for his assistance in animal work during the entire project.
This study was supported by a grant from Boehringer Ingelheim Vetmedica Inc., Ames, Iowa.
REFERENCES
- 1.Allende, R., T. L. Lewis, Z. Lu, D. L. Rock, G. F. Kutish, A. Ali, A. R. Doster, and F. A. Osorio. 1999. North American and European porcine reproductive and respiratory syndrome viruses differ in non-structural protein coding regions. J. Gen. Virol. 80:307-315. [DOI] [PubMed] [Google Scholar]
- 2.Allende, R., G. F. Kutish, W. Laegreid, Z. Lu, T. L. Lewis, D. L. Rock, J. Friesen, J. A. Galeota, A. R. Doster, and F. A. Osorio. 2000. Mutations in the genome of porcine reproductive and respiratory syndrome virus responsible for the attenuation phenotype. Arch. Virol. 145:1149-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson, J. E., M. J. Yaeger, and K. M. Lager. 2000. Effect of porcine reproductive and respiratory syndrome virus (PRRSV) exposure dose on fetal infection in vaccinated and nonvaccinated swine. Swine Health Product. 8:155-160. [Google Scholar]
- 4.Bøtner, A., B. Strandbygaard, K. J. Sørensen, P. Have, K. G. Madsen, E. S. Madsen, and S. Alexandersen. 1997. Appearance of acute PRRS-like symptoms in sows after vaccination with a modified live PRRS vaccine. Vet. Rec. 141:497-499. [DOI] [PubMed] [Google Scholar]
- 5.Bush, E. J., B. Corso, J. Zimmerman, S. Swenson, D. Pyburn, and T. Burkgren. 1999. Update on the acute PRRS investigative study. Swine Health Product. 7:179-180. [Google Scholar]
- 6.Cavanaugh, D. 1997. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 142:629-633. [PubMed] [Google Scholar]
- 7.Chang, C. C., K.-J. Yoon, J. J. Zimmerman, K. M. Harmon, P. M. Dixon, C. M. T. Dvorak, and M. P. Murtaugh. 2002. Evolution of porcine reproductive and respiratory syndrome virus during sequential passages in pigs. J. Virol. 76:4750-4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christopher-Hennings, J., E. A. Nelson, J. K. Nelson, R. J. Hines, S. L. Swenson, H. T. Hill, J. J. Zimmerman, J. B. Katz, M. J. Yaeger, C. C. L. Chase, and D. A. Benfield. 1995. Detection of porcine reproductive and respiratory syndrome virus in boar semen by PCR. J. Clin. Microbiol. 33:1730-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christopher-Hennings, J., E. A. Nelson, J. K. Nelson, and D. A. Benfield. 1997. Effects of a modified-live virus vaccine against porcine reproductive and respiratory syndrome in boars. Am. J. Vet. Res. 58:40-45. [PubMed] [Google Scholar]
- 10.Chung, W. B., W. F. Chang, M. Hsu, and P. C. Yang. 1997. Persistence of porcine reproductive and respiratory syndrome virus in intensive farrow-to-finish pig herds. Can. J. Vet. Res. 61:292-298. [PMC free article] [PubMed] [Google Scholar]
- 11.Done, S. H., D. J. Paton, and M. E. C. White. 1996. Porcine reproductive and respiratory syndrome (PRRS): a review with emphasis on pathological, virological and diagnostic aspects. Br. Vet. J. 152:153-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorcyca, D., G. Spronk, R. Morrison, and D. Polson. 1995. Field evaluation of a new MLV PRRS virus vaccine: applications for PRRS prevention and control in swine herds, p. 401-411. In Proceedings of the 26th Annual Meeting of the American Association of Swine Practitioners, Omaha, Nebr. American Society of Swine Veterinarians, Perry, Iowa.
- 13.Halbur, P. G., J. J. Andrews, E. L. Hoffman, P. S. Paul, X. J. Meng, and Y. Niyo. 1994. Development of a streptavidin-biotin immunoperoxidase procedure for the detection of porcine reproductive and respiratory syndrome virus antigen in porcine lung. J. Vet. Diagn. Investig. 6:254-257. [DOI] [PubMed] [Google Scholar]
- 14.Halbur, P. G., P. S. Paul, M. L. Frey, J. Landgraf, K. Eernisse, X. J. Meng, M. A. Lum, J. J. Andrews, and J. A. Rathje. 1995. Comparison of the pathogenicity of two U.S. porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 34:648-660. [DOI] [PubMed] [Google Scholar]
- 15.Halbur, P. G., P. S. Paul, X. J. Meng, M. A. Lum, J. J. Andrews, and J. A. Rathje. 1996. Comparative pathogenicity of nine U.S. porcine reproductive and respiratory syndrome virus (PRRSV) isolates in a 5-week-old cesarean-derived-colostrum-deprived pig model. J. Vet. Diagn. Investig. 8:11-20. [DOI] [PubMed] [Google Scholar]
- 16.Halbur, P. G., P. S. Paul, M. L. Frey, J. Landgraf, K. Eernisse, X. J. Meng, J. J. Andrews, M. A. Lum, and J. A. Rathje. 1996. Comparison of the antigen distribution of two U.S. porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 33:159-170. [DOI] [PubMed] [Google Scholar]
- 17.Halbur, P. G., and E. Bush. 1997. Update on abortion storms and sow mortality. Swine Health Product. 5:73. [Google Scholar]
- 18.Kapur, V., M. R. Elam, T. M. Pawlovich, and M. P. Murtaugh. 1996. Genetic variation in porcine reproductive and respiratory syndrome virus isolates in the midwestern United States. J. Gen. Virol. 77:1271-1276. [DOI] [PubMed] [Google Scholar]
- 19.Keffaber, K. 1989. Reproductive failure of unknown aetiology. Am. Assoc. Swine Pract. Newsl. 1:1-10. [Google Scholar]
- 20.Key, K. F., G. Haqshenas, D. K. Guenette, S. L. Swenson, T. E. Toth, and X. J. Meng. 2001. Genetic variation and phylogenetic analyses of the ORF5 gene of acute porcine reproductive and respiratory syndrome virus isolates. Vet. Microbiol. 83:249-263. [DOI] [PubMed] [Google Scholar]
- 21.Kim, H. S., J. Kwang, K.-J. Yoon, H.-S. Joo, and M. L. Frey. 1993. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch. Virol. 133:477-483. [DOI] [PubMed] [Google Scholar]
- 22.Madsen, K. G., C. M. Hansen, E. S. Madsen, B. Strandbygaard, A. Bøtner, and K. J. Sørensen. 1998. Sequence analysis of porcine reproductive and respiratory syndrome virus of the American type collected from Danish swine herd. Arch. Virol. 143:1683-1700. [DOI] [PubMed] [Google Scholar]
- 23.Meng, X. J., P. S. Paul, and P. G. Halbur. 1994. Molecular cloning and nucleotide sequencing of the 3′-terminal genomic RNA of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 75:1795-1801.8021610 [Google Scholar]
- 24.Meng, X. J., P. S. Paul, P. G. Halbur, and M. A. Lum. 1995. Phylogenic analyses of the putative M (ORF 6) and N (ORF 7) genes of porcine reproductive and respiratory syndrome virus (PRRSV): implication for the existence of two genotypes of PRRSV in the USA and Europe. Arch. Virol. 140:745-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng, X. J., P. S. Paul, P. G. Halbur, and I. Morozov. 1995. Sequence comparison of open reading frames 2 to 5 of low and high virulence United States isolates of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 76:3181-3188. [DOI] [PubMed] [Google Scholar]
- 26.Meng, X. J., P. S. Paul, P. G. Halbur, and M. A. Lum. 1996. Characterization of a high-virulence US isolate of porcine reproductive and respiratory syndrome virus in a continuous cell line, ATCC CRL11171. J. Vet. Diagn. Investig. 8:374-381. [DOI] [PubMed] [Google Scholar]
- 27.Meng, X. J., P. S. Paul, I. Morozov, and P. G. Halbur. 1996. A nested set of six or seven subgenomic mRNAs is formed in cells infected with different isolates of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 77:1265-1270. [DOI] [PubMed] [Google Scholar]
- 28.Meng, X. J. 2000. Heterogeneity of porcine reproductive and respiratory syndrome virus: implications for current vaccine efficacy and future vaccine development. Vet. Microbiol. 74:309-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mengeling, W. L., A. C. Vorwald, K. M. Lager, and S. L. Brockmeier. 1996. Diagnosis of porcine reproductive and respiratory syndrome using infected alveolar macrophages collected from live pigs. Vet. Microbiol. 49:105-115. [DOI] [PubMed] [Google Scholar]
- 30.Mengeling, W. L., K. M. Lager, and A. C. Vorwald. 1998. Clinical consequences of exposing pregnant gilts to strains of porcine reproductive and respiratory syndrome (PRRS) virus isolated from field cases of “atypical” PRRS. Am. J. Vet. Res. 59:1540-1544. [PubMed] [Google Scholar]
- 31.Murtaugh, M. P., M. R. Elam, and L. T. Kakach. 1995. Comparison of the structural protein coding sequences of the VR-2332 and Lelystad virus strains of the PRRS virus. Arch. Virol. 140:1451-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murtaugh, M. P., E. A. Nelson, and K. S. Faaberg. 2002. Genetic interaction between porcine reproductive and respiratory syndrome virus (PRRSV) strains in cell culture and in animals. Swine Health Product. 10:15-21. [Google Scholar]
- 33.Nielsen, H. S., M. B. Oleksiewicz, R. Forsberg, T. Stadejek, A. Bøtner, and T. Storgaard. 2001. Reversion of a live porcine reproductive and respiratory syndrome virus vaccine investigated by parallel mutations. J. Gen. Virol. 82:1263-1272. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen, T. L., J. Nielsen, P. Have, P. Bækbo, R. Hoff-Jørgensen, and A. Bøtner. 1997. Examination of virus shedding in semen from vaccinated and from previously infected boars after experimental challenge with porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 54:101-112. [DOI] [PubMed] [Google Scholar]
- 35.Storgaard, T., M. Oleksiewicz, and A. Bøtner. 1999. Examination of the selective pressures on a live PRRS vaccine virus. Arch. Virol. 144:2389-2401. [DOI] [PubMed] [Google Scholar]
- 36.Torrison, J. L., M. Knoll, and B. Wiseman. 1996. Evidence of pig-to-pig transmission of a modified live vaccine, p. 89-91. In Proceedings of the 27th Annual Meeting of the American Association of Swine Practitioners, Nashville, Tenn. American Society of Swine Veterinarians, Perry, Iowa.
- 37.Wensvoort, G., C. Terpstra, J. M. A. Pol, E. A. ter Laak, M. Bloemraad, E. P. de Kluyver, C. Kragten, L. van Buiten, A. den Besten, F. Wagenaar, J. M. Broekhuijsen, P. L. J. M. Moonen, T. Zetstra, E. A. de Boer, H. J. Tibben, M. F. de Jong, P. van't Veld, G. J. R. Groenland, J. A. van Gennep, M. T. H. Voets, J. H. M. Verheijden, and J. Braamskamp. 1991. Mystery swine disease in the Netherlands: the isolation of Lelystad virus. Vet. Q. 13:121-130. [DOI] [PubMed] [Google Scholar]
- 38.Wesley, R. D., W. L. Mengeling, K. M. Lager, A. C. Vorwald, and M. B. Roof. 1999. Evidence for divergence of restriction fragment length polymorphism patterns following in vivo replication of porcine reproductive and respiratory syndrome virus. Am. J. Vet. Res. 60:463-467. [PubMed] [Google Scholar]
- 39.Yoon, K.-J., J. J. Zimmerman, C. C. Chang, S. Cancel-Tirado, K. M. Harmon, and M. J. McGinley. 1999. Effect of challenge dose and route on porcine reproductive and respiratory syndrome virus infection in young swine. Vet. Res. 6:629-638. [PubMed] [Google Scholar]
- 40.Yoon, K.-J., J. J. Zimmerman, S. L. Swenson, M. J. McGinley, K. A. Eernisse, A. Brevik, L. L. Rhinehart, M. L. Frey, H. T. Hill, and K. B. Platt. 1995. Characterization of the humoral immune response to porcine reproduction and respiratory syndrome (PRRS) virus infection. J. Vet. Diagn. Investig. 7:305-312. [DOI] [PubMed] [Google Scholar]
- 41.Yuan, S., C. J. Nelsen, M. P. Murtaugh, B. J. Schmitt, and K. S. Faaberg. 1999. Recombination between North American strains of porcine reproductive and respiratory syndrome virus. Virus Res. 61:87-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan, S., M. P. Murtaugh, and K. S. Faaberg. 2000. Heteroclite subgenomic RNAs are produced in porcine reproductive and respiratory syndrome virus infection. Virology 275:158-169. [DOI] [PubMed] [Google Scholar]