Abstract
The K1 protein of Kaposi's sarcoma-associated herpesvirus (KSHV) contains an immunoreceptor tyrosine-based activation motif (ITAM) in its cytoplasmic region and elicits cellular signal transduction through this motif. To investigate the role of K1 signal transduction in KSHV replication, we expressed full-length K1 and CD8-K1 chimeras in BCBL1 cells. Unlike its strong signaling activity in uninfected B lymphocytes, K1 did not induce intracellular calcium mobilization or NF-AT activation at detectable levels in KSHV-infected BCBL1 cells. Instead, K1 signaling dramatically suppressed KSHV lytic reactivation induced by tetradecanoyl phorbol acetate (TPA) stimulation, but not by ORF50 ectopic expression. Mutational analysis showed that the cytoplasmic ITAM sequence of K1 was required for this suppression. Viral microarray and immunoblot analyses demonstrated that K1 signaling suppressed the TPA-mediated increase in the expression of a large subset of viral lytic genes in KSHV-infected BCBL1 cells. Furthermore, electrophoretic mobility shift assays demonstrated that TPA-induced activation of AP-1, NF-κB, and Oct-1 activities was severely diminished in BCBL1 cells expressing the K1 cytoplasmic domain. The reduced activities of these transcription factors may confer the observed reduction in viral lytic gene expression. These results demonstrate that K1-mediated signal transduction in KSHV-infected cells is profoundly different from that in KSHV-negative cells. Furthermore, K1 signal transduction efficiently suppresses TPA-mediated viral reactivation in an ITAM-dependent manner, and this suppression may contribute to the establishment and/or maintenance of KSHV latency in vivo.
A novel member of the herpesvirus group, called Kaposi's sarcoma-associated herpesvirus (KSHV) or human herpesvirus 8 (HHV8), has been identified in Kaposi's sarcoma (KS) tumors from both human immunodeficiency virus (HIV)-positive and HIV-negative patients (14, 56, 62). KSHV has also been identified in primary effusion lymphoma (PEL) and some forms of Castleman's disease (69). The genomic sequence indicates that KSHV is a gamma-2 herpesvirus that is closely related to herpesvirus saimiri (HVS) (63) and rhesus monkey rhadinovirus (2, 70). DNA sequence analysis of the entire 140.5-kb KSHV genome has revealed a number of genes homologous to those associated with the pathogenesis of other viruses (63). These include a virus-encoded interleukin-6 (IL-6) (51, 53, 55), the chemokine macrophage inflammatory protein-1 (MIP-1) (36, 56), a bcl-2 homolog (67), a virus-encoded interferon regulatory factor (vIRF) (11, 44), v-cyclin (45, 68), vIL-8 receptor (5), vFLIP (7, 72), and vOX2 (17).
A hallmark of herpesvirus is the establishment of a lifelong persistent infection in the host (59). KSHV is primarily found in endothelial cells, thought to be the principal tumor cells in KS lesions (52). As with other gammaherpesviruses, KSHV DNA is also found in vivo in CD19-positive B lymphocytes, which are a potential reservoir for viral dissemination (10, 21). In KS lesions and PEL cells, the virus is primarily present in a latent state, with transcription restricted to a small set of viral genes and no detectable production of viral progeny (54). Currently, there is no efficient cell culture system for KSHV replication. The only viable experimental system is cell lines generated by culture of PEL specimens (62). KSHV primarily displays latent infection, both in vivo in KS lesions and PEL and in vitro in most PEL lines (26, 30). However, treatment of these cells with phorbol esters or other stimulants induces lytic replication (50, 56, 60, 62). In addition, KSHV ORF50, a homolog of Epstein-Barr virus (EBV) Rta, is essential and sufficient to drive the entire viral lytic cycle and is the only known lytic-switch gene for viral reactivation from latency (28, 48, 50, 71).
At a position equivalent to that of the STP (saimiri transformation protein) of HVS (32) and latent membrane protein 1 (LMP1) of EBV (23), KSHV contains a distinct open reading frame (ORF) called K1 (37, 43, 75). The K1 gene is expressed at low levels in PEL, and its expression is significantly induced during the lytic phase of the viral life cycle (37). The K1 protein is predicted to have a signal peptide sequence at the amino terminus, an extracellular domain, a transmembrane domain, and a short cytoplasmic tail at the carboxyl terminus (42). The predicted extracellular domain of the K1 protein demonstrates regional homology with the variable region of the lambda chain of the immunoglobulin (Ig) light chain (42). In addition, as with Igα and Igβ, the cytoplasmic region of K1 contains a functional immunoreceptor tyrosine-based activation motif (ITAM), which transduces extracellular signals to elicit cellular activation events (39, 42). In addition, the amino-terminal region of K1 specifically interacts with the μ chains of B-cell antigen receptor (BCR) complexes, and this interaction inhibits the intracellular transport of BCR, resulting in downregulation of BCR surface expression (41). A recent report has also shown that ITAM-dependent signaling by K1 functions to modestly augment lytic replication in KSHV-infected PEL cells, indicating that K1 has multiple roles in cellular signal transduction and viral lytic replication (38).
If latently infected B cells in the body were to undergo fortuitous reactivation, the released virus would be neutralized by antibodies, and other infected cells would be killed by cytotoxic T-cell attack. In addition, in vitro herpesvirus replication and production of infectious viral progeny are ultimately associated with cell death. Since viral persistence and replication are mutually exclusive, it is reasonable that viral proteins should be involved in the suppression of lytic replication in order to establish and/or maintain viral latency. EBV LMP2A and HVS tyrosine kinase-interacting protein (Tip) are two viral proteins known to inhibit viral reactivation (34, 47). Both proteins recruit and prevent lymphocyte tyrosine kinases from participating in cellular signal transduction, resulting in the suppression of viral lytic reactivation (33, 34, 49). In addition, we and others have shown that the KSHV K15 membrane protein interacts with a major B-lymphocyte tyrosine kinase, Lyn, and that this interaction inhibits BCR signal transduction; thus, K15 may have an inhibitory role in KSHV reactivation (16, 27). Finally, EBV LMP1 has recently been shown to suppress viral reactivation induced by anti-IgM or tetradecanoyl phorbol acetate (TPA) treatment by mimicking CD40 signal transduction, suggesting that LMP1 may have a signaling role in the maintenance of EBV latency (1). Thus, the signal transduction from various oncoproteins and signal modulators of gammaherpesviruses is involved in downregulating viral lytic reactivation, which ultimately plays an important role in establishing and/or maintaining viral latency.
In this report, we show that KSHV K1 mediates signal transduction pathways in virus-infected PEL cells distinct from those in normal B cells and that this signal transduction efficiently suppresses TPA-mediated KSHV lytic reactivation in an ITAM-dependent manner. In addition, an electrophoretic mobility shift assay (EMSA) suggests that a dramatic reduction in TPA-induced activation of AP-1, NF-κB, and Oct-1 activities by K1 signaling likely confers the suppression of viral lytic gene expression. These results suggest that, like EBV LMP1 and LMP2A and HVS Tip, KSHV K1 may assist in maintaining viral latency.
MATERIALS AND METHODS
Reagents and antibodies.
TPA and sodium vanadate (Na2VO3) were purchased from Calbiochem (San Diego, Calif.) and Sigma Chemicals (St. Louis, Mo.). The anti-phosphotyrosine antibody 4G10 was purchased from Upstate Biotechnology Inc. (Lake Placid, N.Y.). Polyclonal anti-K8.1, anti-K8, anti-vIRF, anti-LANA (46), anti-K3, and anti-K5 antibodies were prepared as previously described (31). Anti-gpK8.1 was a generous gift from B. Chandran at the University of Kansas Medical Center. Anti-vIL-6 was kindly provided by P. Moore and Y. Chang at Columbia University. An anti-actin antibody was purchased from Santa Cruz (Santa Cruz, Calif.).
Cell cultures.
BCBL1 and BJAB cells were maintained in complete medium (RPMI 1640 supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 1 mM β-mercaptoethanol). To establish stable BCBL1 and BJAB cell lines, cells were electroporated with expression plasmids in serum-free medium at 200 V and 975 μF. After 20 h, live cells were separated from dead cells by Ficoll-Hypaque centrifugation, and live cells were selected with 2 mg of G418/ml for 5 to 6 weeks. Cells were then stained with an appropriate antibody and sorted for positive cells by use of Dynal (Lake Success, N.Y.) beads. Sorted cells were maintained in complete medium supplemented with 200 μg of G418/ml until they were used for further experiments.
Plasmid constructions.
CD8 chimeras with the cytoplasmic domain of K1 have been described previously (42). Flag-tagged full-length K1 and mutant K1 Y1,2F were kindly provided by D. Ganem at the University of California, San Francisco. The ORF50 cDNA was subcloned into the pTracer-GFP vector (Invitrogen, Carlsbad, Calif.).
Flow cytometry.
Cells (5 × 105) were washed with complete medium and stained either with a phycoerythrin (PE)-, fluorescein isothiocyanate (FITC)-, or allophycocyanin (APC)-conjugated primary antibody or with an unconjugated primary antibody, followed by a PE- or FITC-conjugated secondary antibody at 4°C. After a final wash, the cells were fixed with 2% paraformaldehyde, and flow cytometry was performed by fluorescence activated cell scan (or sorter) (FACS) (Becton Dickinson, Mountain View, Calif.). Appropriate antibodies were used for isotype controls.
Calcium mobilization analysis.
Cells (2 × 106) were loaded with 1 μM indo-1 in 2 ml of RPMI complete medium for 20 min at 37°C as described previously (42). Baseline calcium levels were established for 1 min prior to the addition of the antibody. Cells were stimulated with 10 μg of a mouse anti-CD8 antibody (OKT8) or 5 μg of a mouse anti-Flag antibody/ml, followed by reaction with10 μg of goat anti-mouse Ig (GAM)/ml. Baseline absolute intracellular calcium levels were determined by using an ionophore and EGTA. Data were collected and analyzed on a FACS Vantage (Becton-Dickinson).
Immunoblot analysis.
Cells were lysed in lysis buffer (50 mM HEPES [pH 8.0], 150 mM NaCl, 1% NP-40) containing protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 mM Na2VO3, 1 mM NaF, 1 μg of aprotinin/ml, 5 μg of leupeptin/ml, and 1 μg of pepstatin/ml). Cell lysates were resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred to a nitrocellulose membrane. Primary antibodies were used at 1:500 to 1:2,000 dilutions, and secondary antibodies were used at a 1:2,000 dilution in 7% nonfat dry milk. After a final wash, nitrocellulose membranes were used for an enhanced chemiluminescence assay with a Fuji phosphorimager.
Nuclear extracts and EMSA.
Nuclear extracts were prepared by modified protocols as described previously (6). EMSA was performed according to the manufacturer's recommendations (Promega, Madison, Wis.). Briefly, binding reactions were performed by mixing 10 μg of nuclear extract with 2 μg of poly(dI-dC)-poly(dI-dC) and 2 μl of 5× binding buffer in a total volume of 9 μl. Reaction mixtures were preincubated for 15 min at room temperature, and 1 μl of radiolabeled oligonucleotide (∼5 × 104 cpm) was added. The reaction mixtures were further incubated for 20 min, followed by the addition of sample buffer. Reaction products were separated by electrophoresis on a 5% nondenaturing polyacrylamide gel in 0.5× Tris-borate-EDTA buffer. The gels were fixed, dried, and analyzed with a Fuji phosphorimager.
Reporter assay.
BCBL1 and BJAB cells (107) were transfected with 10 μg of mixed plasmid DNAs (the NF-AT-luc reporter plasmid and the K1 expression plasmid). All transfections included 2 μg of pGKβgal, which expresses β-galactosidase from a phosphoglucokinase promoter. At 48 h after transfection, cells were washed once in phosphate-buffered saline (PBS) and lysed in 200 μl of reporter lysis buffer. A luciferase activity assay was carried out according to the manufacturer's instructions (Promega), and activity was measured with a luminometer. Values were normalized to β-galactosidase activity.
RNA preparation.
Cells were treated with 20 nM TPA and collected on days 0, 1, 2, and 3 after induction. For RNA isolation, 3.0 × 107 cells were used. Poly(A)+ RNA was isolated by using the Fast Track 2.0 kit (Invitrogen) according to the manufacturer's instructions.
DNA microarray analysis.
Poly(A)+ RNA was reverse transcribed by using an oligo(dT) primer (Invitrogen). BCBL1 CD8Δ samples were labeled with Cy3-dUTP, and BCBL1 CD8-K1 samples were labeled with Cy5-dUTP (Amersham Pharmacia Biotech, Piscataway, N.J.) as previously described (58). Hybridizations were repeated at least three independent times. The data obtained in the separate trials were similar.
Slides were scanned with a GenePix 4000 scanner (Axon Instruments, Inc., Foster City, Calif.) and analyzed by using a collection of extensions developed by Chen and coworkers (8, 15) based on IP Lab Spectrum software (Scanalytics, Inc., Fairfax, Va.). The final reported probe intensity for each spot is the average fluorescence intensity for the spot minus the local background intensity. The ratios of emission fluorescence for all targets were determined by taking the final reported probe intensity at each spot in the Cy5-labeled image and dividing by the final probe intensity of the same spot in the Cy3-labeled CD8Δ sample. To ensure that the ratios obtained were not affected by differential labeling or hybridization efficiency, a normalization factor, based on the distribution of ratios for 88 cellular genes, was applied to ratios from the viral targets, and calibrated ratios were reported (8, 15). Calibrated expression ratios for each gene were cataloged by using a hierarchical clustering program (22) with Pearson's correlation coefficient, an average linkage algorithm, and a noncentered metric. A quality score associated with each ratio measurement was incorporated into the calculation to ensure that the measurements were not sensitive to unreliable data points, which may result from low target intensity, high local background, and small target sizes. In the tabulated and displayed data, calibrated ratios receiving a quality score of 0.3 or lower were flagged.
RESULTS
The K1 signal is not capable of eliciting intracellular calcium mobilization or NF-AT activity in KSHV-infected BCBL1 cells.
Previously, it has been shown that expression of the CD8/K1 chimera in KSHV-negative BJAB cells induces cellular tyrosine phosphorylation and intracellular calcium mobilization upon stimulation with an anti-CD8 antibody (42). In addition, K1 expression has also been shown to induce NF-AT transcriptional activity in BJAB cells (39). Mutational analysis shows that the cytoplasmic ITAM sequence of K1 is required for its signal-transducing activity. To assess the role of K1 signal transduction in the KSHV life cycle, KSHV-infected BCBL1 cells were used to establish stable lines expressing the CD8/K1 chimera in which 27 amino acids of the cytoplasmic tail of human CD8Δ protein were replaced with 38 amino acids of the cytoplasmic tail of K1 (CD8/K1-C [Fig. 1A]). As a control, we used CD8Δ, which expresses CD8 lacking a cytoplasmic region (Fig. 1A). Since mutations of the tyrosine residues of the K1 ITAM sequence abolish its signal-transducing capacity, we also included previously described K1 mutants containing deletions or point mutations in the cytoplasmic ITAM (42). These mutants were CD8/K1-TYF (containing tyrosine-to-phenylalanine mutations at positions 271, 272, and 282), CD8/K1-D1 (containing the negatively charged conserved region with a proximal YXXL motif), CD8/K1-D2 (containing only the negatively charged conserved region), and CD8/K1-D4 (containing the distal YXXP motif) (Fig. 1A). After electroporation of the expression vectors into BCBL1 cells, cell lines were selected by growth in a medium containing 2 mg of neomycin/ml for 5 weeks. Since CD8 is not expressed on the surfaces of BCBL1 cells, neomycin-resistant cells were sorted by their adhesion to CD8 antibody-coated magnetic beads. The expression levels of CD8 were similar on most of the sorted cells expressing CD8/K1 chimeras (Fig. 1B).
FIG. 1.
CD8/K1 chimeras and their signaling activities in BCBL1 cells. (A) Schematic diagram of CD8/K1-C chimeras. The cytoplasmic region of human CD8α was replaced with the cytoplasmic region of K1. Shaded boxes and circles indicate the extracellular and transmembrane domains (amino acids 1 to 96) of CD8, respectively. Open boxes indicate the cytoplasmic region (amino acids 251 to 289) of K1. All constructs have been described previously (42). (B) Flow cytometric analysis of surface CD8 expression on BCBL1 cells. Live cells were stained and sorted for the surface expression of CD8. Two hundred thousand events were collected. As a control, each experimental histogram (solid) is overlaid with a histogram of untransfected BCBL1 cells (open). (C) Induction of intracellular free calcium. Calcium mobilization was monitored over time by changes in the ratio of violet to blue (405 to 485 nm) fluorescence of cells loaded with the calcium-sensitive dye indo-1 and analyzed by flow cytometry. Data are presented as a histogram of the number of cells with a particular fluorescence ratio (y axis) versus time (x axis). Arrowheads indicate the addition of anti-CD8 (αCD8) and the secondary antibody GAM. The breaks in the graphs indicate the time intervals during addition of antibody. Data were similar in three independent experiments. (D) Induction of tyrosine phosphorylation upon antibody stimulation. BJAB CD8Δ, BJAB CD8/K1-C, BCBL1 CD8Δ, and BCBL1 CD8/K1-C cells were stimulated with 10 μg of anti-CD8 antibody/ml for 0, 1, or 3 min. Cell lysates were used for immunoblotting with an anti-phosphotyrosine antibody (top) or an anti-actin antibody (bottom).
To determine the ability of the K1 ITAM to elicit an increase in cytoplasmic free calcium levels, BCBL1 cells expressing CD8Δ or CD8/K1-C were stimulated with an anti-CD8 antibody (OKT8), followed by treatment with GAM. Intracellular free calcium levels were monitored by flow cytometry. As shown previously (42), BJAB CD8/K1-C cells exhibited a prolonged increase in intracellular calcium concentrations immediately after anti-CD8 stimulation (Fig. 1C). In striking contrast, BCBL1 CD8/K1-C cells did not induce intracellular calcium mobilization under the same conditions (Fig. 1C). The four BCBCL cell lines expressing mutant CD8/K1 chimeras were analyzed in the same assay. None of these cell lines was able to elicit an increase in intracellular free calcium concentrations (data not shown). Furthermore, tyrosine phosphorylation did not significantly increase in BCBL1 CD8/K1-C cells upon CD8 stimulation, unlike the results in BJAB CD8/K1-C cells (Fig. 1D).
To further investigate the lack of K1 signaling activity, the mammalian expression vector pcDEF3 containing the amino-terminal Flag-tagged, full-length K1 or K1 Y1,2F mutant (39) was electroporated into BJAB cells and KSHV-infected BCBL1 cells. At 48 h after electroporation, cells were selected by growth in a medium containing 2 mg of neomycin/ml for 5 weeks. Neomycin-resistant cells were sorted by staining with an anti-Flag antibody, followed by GAM-coated magnetic beads. Comparable levels of Flag surface expression were detected on BJAB DEF/K1, BJAB DEF/K1 Y1,2F, BCBL1 DEF/K1, and BCBL1 DEF/K1 Y1,2F cells (Fig. 2A). To test the ability of K1 to elicit an increase in cytoplasmic free calcium levels, these cells were stimulated with an anti-Flag antibody, followed by GAM. K1 strongly increased intracellular calcium mobilization in BJAB cells upon antibody stimulation, whereas the K1 Y1,2F mutant did not (Fig. 2B). By contrast, neither wild-type (wt) K1 nor the K1 Y1,2F mutant showed any intracellular calcium mobilization in BCBL1 cells under the same conditions (Fig. 2B). Since a low concentration of ionomycin drastically induced intracellular calcium expression in BCBL1 DEF/K1 cells, these cells were apparently capable of responding to an extracellular signal to elicit signal transduction (Fig. 2B). Furthermore, we examined the activation of NF-AT activity by K1 expression. BJAB and BCBL1 cells were electroporated with the pEF3/K1 expression vector and the NF-AT-luc reporter plasmid. In addition, all electroporations included 2 μg of pGKβgal, which expresses β-galactosidase from a phosphoglucokinase promoter. At 48 h posttransfection, cells were washed once in PBS and assayed for luciferase. Luciferase values were normalized to β-galactosidase activity in order to control for transfection efficiency. As previously shown (39), K1 expression strongly induced NF-AT promoter activity in BJAB cells, whereas it did not do so in BCBL1 cells (Fig. 2C). Although tyrosine phosphorylation of the K1 protein was induced in both BJAB DEF/K1 and BCBL1 DEF/K1 cells upon anti-Flag antibody stimulation, the extent of the increase in overall tyrosine phosphorylation was much lower in BCBL1/K1 cells than in BJAB DEF/K1 cells (Fig. 2D). The K1 Y1,2F mutant neither induced tyrosine phosphorylation nor was phosphorylated in BCBL1 cells under the same conditions (Fig. 2D). This indicated that KSHV-infected BCBL1 cells might have significant differences from BJAB cells in the expression or activity of downstream signaling molecules. To test this, we surveyed several cellular signaling molecules for their expression. No expression of SH-PTP1 and weak expression of PLC-γ2 and Cbl were detected in BCBL1 DEF/K1 cells compared to BJAB DEF/K1 cells, whereas Syk, Lyn, and SH-PTP2 were expressed at equivalent levels in both types of cells (Fig. 2E). In addition, tyrosine phosphorylation of Syk was strongly induced in both BJAB DEF/K1 and BCBL1 DEF/K1 cells upon anti-Flag stimulation (Fig. 2F). In contrast, strong phosphorylation of the 90-kDa cellular protein associated with Lyn kinase was detected in BJAB DEF/K1 cells during anti-Flag stimulation, whereas it was not detected in BCBL1 DEF/K1 cells (Fig. 2E). These results indicate that, in contrast to its strong signaling activity in KSHV-negative BJAB cells, K1 is not capable of eliciting intracellular calcium mobilization and NF-AT activity in KSHV-infected BCBL1 cells and that BCBL1 cells likely have significant differences from BJAB cells in the expression and activity of cellular signaling molecules.
FIG. 2.
Comparison of full-length K1 signaling activity in BCBL1 versus BJAB cells. (A) Flow cytometric analysis of K1 surface expression. Live cells were stained for surface expression of Flag-tagged K1. Two hundred thousand events were collected. As a control, each experimental histogram (solid) is overlaid with a histogram of untransfected BCBL1 and BJAB cells (open). (B) Intracellular free calcium concentrations upon anti-Flag antibody stimulation. Calcium mobilization was monitored over time by changes in the ratio of violet to blue (405 to 485 nm) fluorescence of cells loaded with indo-1, and results were analyzed by flow cytometry. Data are presented as a histogram of the number of cells with a particular fluorescence ratio (y axis) versus time (x axis). Arrowheads indicate addition of anti-Flag, GAM, and ionomycin (5 nM). Ionomycin was added as a control for intracellular calcium mobilization. Breaks in the graphs indicate the time intervals during addition of antibodies or ionomycin. Data were similar in three independent experiments. (C) NF-AT activation by K1 expression. BJAB and BCBL1 cells were electroporated with a control vector (hatched bars) or the pEF3/K1 expression vector (solid bars), along with the NF-AT-luc reporter plasmid and the pGKβgal transfection control plasmid. At 48 h after transfection, cells were washed once in PBS and assayed for luciferase. Luciferase values were normalized to β-galactosidase activity in order to control for transfection efficiency. The y axis indicates fold induction of NF-AT by K1 expression. The results are averaged from three independent assays. (D) Induction of tyrosine phosphorylation upon anti-Flag antibody stimulation. (Top panel) BJAB DEF, BJAB DEF/K1, BCBL1 DEF, and BCBL1 DEF/K1 cells were stimulated with 10 μg of anti-Flag antibody/ml for 0, 1, or 5 min. Cell lysates were used for immunoblotting with an anti-phosphotyrosine antibody. Arrowheads indicate tyrosine-phosphorylated proteins. (Bottom two panels) Lysates of these cells were also used for immunoprecipitation with an anti-Flag antibody, followed by immunoblotting with an anti-phosphotyrosine antibody to detect K1 tyrosine phosphorylation (K1 Y-P) or with an anti-Flag antibody to detect K1 protein (K1). (E) Expression of cellular signaling molecules. The same amount of polypeptides of BJAB, BJAB DEF/K1, BCBL1 DEF, BCBL1 DEF/K1, and BCBL1 DEF/K1 Y1,2F cells were used for immunoblot assays with the indicated antibodies. (F) Tyrosine phosphorylation of Syk, Lyn, and SH-PTP2. Upon anti-Flag stimulation, lysates of BJAB DEF/K1 and BCBL1 DEF/K1 cells were also used for immunoprecipitation with antibodies of Syk, Lyn, and SH-PTP2, followed by immunoblotting with an anti-phosphotyrosine antibody.
K1 inhibits TPA-mediated K8.1 glycoprotein surface expression.
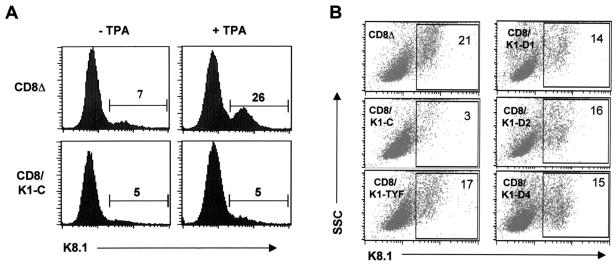
The inability of K1 to induce calcium mobilization, tyrosine phosphorylation, and NF-AT activity in BCBL1 cells suggested that K1 might target a different cellular signal transduction pathway in KSHV-infected cells from that in uninfected BJAB cells. To address this point, we examined the potential role of K1 signal transduction in KSHV lytic reactivation. BCBL1 CD8Δ and BCBL1 CD8/K1-C cells were treated with TPA (20 ng/ml), which has been shown to efficiently induce KSHV lytic reactivation (13, 62). The assay used a polyclonal antibody that recognizes the K8.1 envelope glycoprotein expressed with late kinetics (12, 46). This protein is specific to lytically infected cells, since it is expressed only in cells that are committed to viral replication. In parental BCBL1 cells, approximately 5 to 10% of BCBL1 CD8Δ cells showed spontaneous expression of K8.1 surface expression before TPA stimulation, whereas approximately 30% showed K8.1 surface expression 48 h after TPA stimulation (Fig. 3A). In striking contrast, BCBL1 CD8/K1-C cells showed little or almost no increase in K8.1 surface expression after TPA stimulation (Fig. 3A). To test the specificity of K1 for viral reactivation, BCBL1 cells containing CD8/K1 mutant chimeras were examined under the same conditions. This experiment showed that all five BCBL1 cells expressing CD8/K1 mutant chimeras were capable of inducing K8.1 surface expression as efficiently as BCBL1 CD8Δ cells (Fig. 3B). Thus, the effect of K1 signaling on TPA-mediated KSHV reactivation is specific, and this activity is dependent on its cytoplasmic ITAM sequence (Fig. 3B).
FIG. 3.
Suppression of TPA-induced K8.1 surface expression by CD8/K1 chimera expression. (A) Flow cytometric analysis of K8.1 surface expression. At 48 h after stimulation with or without 20 ng of TPA/ml, cells (BCBL1 CD8Δ and BCBL1 CD8/K1-C) were fixed with paraformaldehyde and reacted with K8.1 rabbit sera, followed by an FITC-conjugated anti-rabbit secondary antibody. Numbers are percentages of K8.1-positive cells. The data were reproduced in three independent experiments. (B) Flow cytometric analysis of K1 mutants. At 48 h after stimulation with 20 ng of TPA/ml, transfected cells (BCBL1 CD8Δ, BCBL1 CD8/K1-C, BCBL1 CD8/K1-D1, BCBL1 CD8/K1-D2, BCBL1 CD8/K1-D4, and BCBL1 CD8/K1-TYF) were fixed and reacted with K8.1 rabbit sera, followed by an FITC-conjugated anti-rabbit secondary antibody. The x axis indicates K8.1-FITC, and the y axis indicates side scattering (SSC). Numbers are percentages of K8.1-positive cells. The data were similar in three independent experiments.
To further test the effect of K1 signaling on viral reactivation, BCBL1 cells expressing the amino-terminal Flag-tagged, full-length K1 or K1 Y1,2F mutant were used for the same assay in the presence or absence of anti-Flag antibody stimulation. Full-length K1 expression also slightly suppressed TPA-induced K8.1 surface expression, and this suppression was significantly enhanced by anti-Flag antibody stimulation (Fig. 4). In contrast, expression of the vector alone or of the K1 Y1,2F mutant showed no effect on TPA-mediated K8.1 surface expression with or without anti-Flag antibody stimulation (Fig. 4). It is of note that the level of suppression of TPA-induced K8.1 expression by wt K1 was lower than the level of suppression by the CD8/K1-C chimera. These results indicate that K1 signaling specifically suppresses TPA-mediated K8.1 surface expression and that a functional ITAM sequence in the K1 cytoplasmic region is required for this activity.
FIG. 4.
Suppression of TPA-induced K8.1 surface expression in BCBL1 cells by K1 expression. At 72 h after stimulation with or without 20 ng of TPA/ml in the presence or absence of 10 μg of anti-Flag antibody, cells (BCBL1 DEF, BCBCL1 DEF/K1, and BCBL1 DEF/K1 Y1,2F) were fixed and reacted with K8.1 rabbit sera, followed by an FITC-conjugated anti-rabbit secondary antibody. Numbers are percentages of K8.1-positive cells. The data were similar in three independent experiments.
K1 cytoplasmic signaling inhibits TPA-induced viral protein expression in BCBL1 cells.
To further elucidate the effect of K1 signaling on KSHV lytic reactivation, we compared the levels of viral proteins in the absence or presence of constitutive K1 signal transduction. Since the CD8/K1 chimera provided constitutive signaling, BCBL1 CD8/K1-C cells were chosen for further analysis. We stimulated 5 × 105 BCBL1 CD8Δ, BCBL1 CD8/K1-C, or BCBL1 CD8/K1-D2 cells with TPA for 0, 24, 48, or 72 h, and whole-cell lysates were subjected to immunoblot assays with antibodies against various KSHV proteins. These proteins were latency-associated nuclear antigen (LANA) (20, 35, 61) for detection of latent infection; K8 (73), MIR1 (K3) (18, 31), and MIR2 (K5) (19, 29, 31) for immediate-early lytic replication; vIRF (11, 25, 44) and vIL-6 (4, 55) for early lytic replication; and the K8.1 glycoprotein (12) for late replication. Finally, cellular actin was included as a control for protein loading. LANA, MIR1 (K3), MIR2 (K5), K8, vIRF, vIL-6, and K8.1 were detected at the expected time points in the KSHV life cycle in BCBL1 CD8Δ and BCBL1 CD8/K1-D2 cells upon TPA stimulation (Fig. 5). In contrast, TPA-mediated induction of MIR1 (K3), MIR2 (K5), K8, K8.1, vIRF, and vIL-6 protein expression was reduced at least three- to fivefold in BCBL1 CD8/K1-C cells from that in BCBL1 CD8Δ and BCBL1 CD8/K1-D2 cells (Fig. 5). The level of latently expressing LANA protein was not altered by TPA stimulation in any of the three cell lines (Fig. 5). These results demonstrate that K1 signaling significantly suppresses TPA-induced viral lytic protein expression and that this activity is dependent on the cytoplasmic ITAM sequence.
FIG. 5.
Suppression of TPA-induced KSHV lytic protein expression by the CD8/K1 chimera. Cells (BCBL1 CD8Δ, BCBL1 CD8/K1-C, and BCBL1 CD8/K1-D2) were stimulated with 20 ng of TPA/ml for the indicated times. Cell lysates were used for immunoblotting with antibodies to K8, MIR1 (K3), MIR2 (K5), vIL-6, vIRF, K8.1, and LANA. A donkey anti-actin antibody was used as an internal control.
Effect of K1 signal transduction on viral gene expression.
To further delineate the inhibition of TPA-induced viral gene expression by K1, we utilized a recently described KSHV DNA microarray (58). The viral array, which contains nearly every known KSHV ORF, allowed us to monitor the global changes in KSHV gene expression due to K1 signal transduction. RNAs from BCBL1 CD8Δ and BCBL1 CD8/K1-C cells were reverse transcribed, and the labeled cDNAs (Cy3-CD8Δ and Cy5-CD8/K1) were hybridized to the viral array. Calibrated fluorescence intensity ratios were determined for the KSHV ORFs (Table 1) and used to generate gene expression profiles (Fig. 6). A hierarchical clustering algorithm (9, 22) was utilized to group the KSHV genes on the basis of similarity by Pearson's correlation coefficient. The dendrogram in Fig. 6 assembles all the genes into a single tree in which ORFs with the most closely related expression patterns are joined by a branch.
TABLE 1.
KSHV gene expression
| ORF | Putative function | Expression levela on:
|
|||
|---|---|---|---|---|---|
| Day 0 | Day 1 | Day 2 | Day 3 | ||
| ORF 60 | Ribonucleotide reductase, small subunit | 0.42 | 0.34 | 0.34 | 0.33 |
| ORF 50 | Rta transactivator | 0.63 | 0.61 | 0.56 | 0.54 |
| ORF 38 | EBV BBLF 1 homolog | 0.40 | 0.36 | 0.43 | 0.28 |
| ORF 37 | Alkaline exonuclease | 0.52 | 0.45 | 0.44 | 0.33 |
| ORF 64 | Tegument protein | 0.51 | 0.54 | 0.67 | 0.43 |
| ORF 54 | dUTPase | 0.70 | 0.53 | 0.66 | 0.50 |
| ORF 52 | EBV BLRF 2 homolog | 0.54 | 0.42 | 0.57 | 0.28 |
| ORF 39 | Glycoprotein M | 0.60 | 0.37 | 0.59 | 0.27 |
| ORF 18 | 0.60 | 0.39 | 0.36 | 0.24 | |
| ORF 21 | Thymidine kinase | 0.75 | 0.58 | 0.57 | 0.44 |
| ORF 65 | Capsid protein (SCIP) | 0.61 | 0.32 | 0.40 | 0.24 |
| ORF K6 | vMIP-1α | 0.41 | 0.12 | 0.36 | 0.14 |
| ORF K4.1 | vMIP-3 | 0.78 | 0.50 | 0.66 | 0.53 |
| ORF 69 | 0.69 | 0.27 | 0.45 | 0.32 | |
| ORF K7 | Poly(A) nuclear transcript | 0.78 | 0.31 | 0.45 | 0.29 |
| ORF 19 | Tegument protein | 0.47 | 0.44 | 0.30 | 0.40 |
| ORF 34 | EBV BGLF 3 homolog | 0.60 | 0.49 | 0.35 | 0.51 |
| ORF 30 | EBV BDLF 3.5 homolog | 0.55 | 0.41 | 0.27 | 0.38 |
| ORF 55 | EBV BSRF 1 homolog | 0.65 | 0.46 | 0.35 | 0.42 |
| ORF K2 | IL-6 homolog | 0.73 | 0.48 | 0.39 | 0.53 |
| ORF 49 | EBV BRRF 1 homolog | 0.63 | 0.62 | 0.40 | 0.39 |
| ORF 7 | Transport protein | 0.42 | 0.35 | 0.14 | 0.24 |
| ORF 42 | EBV BBRF 2 homolog | 0.55 | 0.45 | 0.21 | 0.29 |
| ORF 27 | HVS ORF 27 homolog | 0.49 | 0.34 | 0.14 | 0.19 |
| ORF 23 | EBV BTRF1 homolog | 0.49 | 0.44 | 0.15 | 0.22 |
| ORF 17 | Minor capsid protein | 0.73 | 0.37 | 0.21 | 0.25 |
| ORF 10 | 0.74 | 0.41 | 0.23 | 0.37 | |
| T1.1 | Latency-associated transcript | 0.81 | 0.30 | 0.20 | 0.27 |
| ORF K5 | BHV4-E1 homolog (ZMP-B) | 0.79 | 0.35 | 0.23 | 0.36 |
| ORF 11 | EBV Raji LF2 homolog | 0.84 | 0.37 | 0.27 | 0.44 |
| ORF 68 | Glycoprotein | 0.71 | 0.47 | 0.22 | 0.45 |
| ORF K9 | IRF-1 homolog | 1.05 | 0.64 | 0.44 | 0.60 |
| ORF 26 | Minor capsid protein | 0.53 | 0.35 | 0.21 | 0.17 |
| ORF 46 | Uracil DNA glucosidase | 0.74 | 0.60 | 0.46 | 0.36 |
| ORF 33 | HVS ORF 33 homolog | 0.75 | 0.40 | 0.22 | 0.20 |
| ORF 41 | Helicase/primase subunit | 0.75 | 0.51 | 0.27 | 0.24 |
| ORF 29B | Packaging protein | 0.70 | 0.46 | 0.33 | 0.18 |
| ORF 45 | Acidic domain transactivation | 0.96 | 0.65 | 0.35 | 0.33 |
| ORF 57 | Immediate-early protein | 0.82 | 0.33 | 0.37 | 0.45 |
| ORF 22 | Glycoprotein H | 0.58 | 0.35 | 0.37 | 0.09 |
| ORF 63 | Tegument protein | 0.67 | 0.51 | 0.38 | 0.05 |
| ORF 47 | Glycoprotein L | 1.04 | 0.65 | 0.80 | 0.44 |
| ORF 74 | GCR homolog | 1.31 | 0.55 | 0.64 | 0.32 |
| ORF 75 | Tegument protein, FGARAT | 1.33 | 0.69 | 0.50 | 0.27 |
| ORF K11 | IRF-2 homolog | 0.98 | 0.84 | 0.93 | 0.40 |
| ORF 66 | EBV BFRF 2 homolog | 0.89 | 0.94 | 0.54 | 0.29 |
| ORF 70 | Thymidylate synthase | 1.04 | 1.03 | 0.76 | 0.61 |
| ORF 73 | LANA | 1.07 | 1.07 | 0.66 | 0.67 |
| ORF K12 | Kaposin | 1.82 | 0.81 | 0.32 | 0.30 |
| ORF 53 | EBV BLRF 1 homolog | 0.60 | 0.54 | 0.88 | 0.55 |
| ORF K3 | BHV4-IEIB homolog | 0.63 | 0.35 | 0.78 | 0.53 |
| ORF 9 | DNA polymerase | 0.44 | 0.32 | 0.87 | 0.61 |
| ORF 2 | DHFR | 0.75 | 0.39 | 0.78 | 0.37 |
| ORF 32 | EBV BGLF 1 homolog | 0.70 | 0.66 | 0.90 | 0.38 |
| ORF 29A | Packaging protein | 0.75 | 0.66 | 0.93 | 0.43 |
| ORF 36 | Protein kinase | 0.62 | 0.63 | 1.04 | 0.46 |
| ORF 31 | EBV BDLF 4 homolog | 0.43 | 0.46 | 1.16 | 0.24 |
| ORF 25 | Major capsid protein | 0.58 | 0.54 | 1.13 | 0.42 |
| ORF 16 | BCL-2 homolog | 0.55 | 0.36 | 1.09 | 0.27 |
| ORF 4 | Complement binding protein | 0.60 | 0.35 | 1.11 | 0.30 |
| ORF 6 | ssDNA binding protein | 0.48 | 0.29 | 1.28 | 0.16 |
| ORF 40 | Helicase/primase subunit | 0.72 | 0.57 | 1.22 | 0.45 |
| ORF 20 | EBV BXRF1 homolog (fusion protein) | 0.65 | 0.41 | 1.36 | 0.27 |
| ORF 24 | EBV BcRF 1 homolog | 0.46 | 0.34 | 1.40 | 0.18 |
| ORF 44 | Helicase/primase subunit | 0.65 | 0.57 | 1.29 | 0.37 |
| ORF 56 | DNA replication protein | 0.53 | 0.41 | 1.55 | 0.32 |
| ORF 67 | Tegument protein | 0.63 | 0.50 | 1.29 | 0.25 |
| ORF 61 | Ribonucleotide reductase, large subunit | 0.44 | 0.36 | 1.55 | 0.06 |
| 89600-90541 | IRF homolog | 0.64 | 0.63 | 1.44 | 0.31 |
| ORF 59 | Processivity factor (PF-8) | 0.51 | 0.50 | 1.42 | 0.53 |
| ORF 28 | 0.37 | 0.31 | 2.31 | 0.33 | |
| ORF 35 | EBV BGLF 3.5 homolog | 0.81 | 0.52 | 1.29 | 0.50 |
| ORF 62 | Assembly DNA maturation protein | 0.70 | 0.69 | 1.50 | 0.48 |
| ORF 43 | Minor capsid protein | 0.57 | 0.42 | 1.89 | 0.39 |
| ORF K14 | Ox-2 homolog | 0.66 | 0.45 | 1.81 | 0.35 |
| ORF 8 | Glycoprotein B | 0.73 | 0.41 | 1.83 | 0.35 |
| ORF K10 | 0.65 | 0.46 | 2.41 | 0.31 | |
| 90173-90643 | Herpes glycoprotein X | 0.60 | 0.58 | 2.04 | 0.47 |
| ORF K13 | vFLIP | 0.94 | 1.31 | 0.91 | 0.71 |
| ORF K15 | Membrane protein (LAMP) | 0.50 | 0.76 | 2.59 | 1.84 |
| ORF K1 | Membrane protein | 1.00 | 1.54 | 2.65 | 1.11 |
| ORF 48 | EBV BRRF 2 homolog | 0.82 | 1.52 | 2.99 | 2.75 |
| ORF 72 | Cyclin D homolog | 1.28 | 1.86 | 3.00 | 2.30 |
Calibrated fluorescence intensity ratios (CD8/K1-C versus CDBΔ) were determined for the expression of each KSHV gene. Calibrated expression ratios with low-quality scores are italicized.
FIG.6.
Hierarchical clustering of KSHV gene expression data. The calibrated expression ratios from Table 1 are listed based on hierarchical clustering (average-linkage algorithm). The expression ratios of the genes were compared pairwise and grouped according to their similarity (Pearson's correlation coefficient). On the left, the dendrogram clusters the viral genes based on similarity between gene expression patterns. ORFs with the most closely related expression patterns are joined by a branch. Branch lengths reflect degrees of similarity between gene expression patterns. On the right, the data are presented in a colored mosaic matrix where each column represents a time point following TPA induction and each row displays the expression profile of an individual ORF. The calibrated expression ratios are color coded as follows: green, lower gene expression in BCBL1 CD8/K1 cells; red, higher levels of gene expression in BCBL1 CD8/K1 cells; black, relatively equal expression in BCBL1 CD8Δ and BCBL1 CD8/K1 cells; gray, spots with low-quality scores. Those viral genes upregulated in BCBL1 CD8/K1-C cells, such as ORF48, K1, and K15, appear in the cluster at the bottom.
BCBL1 CD8/K1-C cells clearly showed an overall downregulation of TPA-mediated viral lytic gene expression compared to BCBL1 CD8Δ cells (Table 1 and Fig. 6). A number of KSHV genes had twofold lower expression in BCBL1 CD8/K1-C cells than in BCBL1 CD8Δ cells by day 1 after TPA treatment (Fig. 6 and Table 1). Several KSHV genes showed lower levels of expression in BCBL1 CD8/K1 cells than in BCBL1 CD8Δ cells prior to TPA induction (Fig. 6 and Table 1). While K1 downregulated the expression of the majority of KSHV genes, it did not affect the expression of all the KSHV genes; furthermore, it differentially affected TPA-mediated KSHV gene expression. For example, ORFs 50 and 60 showed a steady downregulation of gene expression throughout the 3 days of TPA treatment, while expression of ORFs 23, 27, 42, 68, and K12 showed a twofold reduction in expression at day 2. In contrast, levels of gene expression for a subset of genes, including ORF48, K1, and K15, were upregulated in BCBL1 CD8/K1-C cells compared to those in BCBL1 CD8Δ cells (Fig. 6). Finally, expression levels of two latency genes, LANA and vFLIP, in the two cell types were not considerably different (Fig. 6 and Table 1). These results suggest that K1 signaling selectively downregulates the expression of KSHV genes.
TPA-mediated activation of AP1, NF-κB, and Oct1 transcription factor activity is suppressed by K1 signaling in BCBL1 cells.
TPA stimulation has pleiotropic effects on cellular signal transduction, which induces the activation of various transcriptional factors, resulting in viral lytic reactivation. To further assess an inhibitory effect of K1 signal transduction on viral lytic gene expression, we examined whether K1 signaling affected TPA-mediated activation of basic cellular transcription factors NF-κB, NF-AT, AP-1, AP-2, Oct-1, and SP-1. BCBL1 CD8Δ and BCBL1 CD8/K1-C cells were treated with 20 nM TPA for the indicated times, and their nuclear extracts were used for EMSA. The labeled DNA probes used were the specific consensus motifs of the six transcription factors. Activation of AP-1 complex was readily detected in BCBL1 CD8Δ cells 1 h after TPA stimulation and reached a maximum at 6 h, whereas it was not detected in BCBL1 CD8/K1-C cells until 12 h after TPA stimulation and remained at a low level (Fig. 7A). In addition, a basal level of Oct-1 activity was weakly detected in BCBL1 CD8Δ cells before TPA stimulation, and its activation reached a maximum at 12 h and declined thereafter (Fig. 7A). By contrast, no Oct-1 activation was detected in BCBL1 CD8/K1-C cells upon TPA stimulation (Fig. 7A). A basal level of low-molecular-weight NF-κB complex was already present in both BCBL1 CD8Δ cells and BCBL1 CD8/K1-C cells before TPA stimulation (Fig. 7A). Both high- and low-molecular-weight NF-κB complexes in BCBL1 CD8Δ cells were strongly detected an hour after TPA stimulation, reached a maximum at 3 h, and declined afterward (Fig. 7A). In contrast, BCBL1 CD8/K1-C cells showed an increase in only the low-molecular-weight NF-κB complexes, which reached a maximum at 6 h after stimulation and declined rapidly thereafter (Fig. 7A). No significant level of activation of NF-AT, AP-2, or SP-1 activity by TPA stimulation was observed in either BCBL1 CD8Δ cells or BCBL1 CD8/K1-C cells (Fig. 7B). These results suggest that K1 signaling significantly alleviated TPA-mediated activation of AP-1, NF-κB, and Oct-1 transcriptional activity, which may confer suppression of KSHV lytic gene expression.
FIG. 7.
Suppression of the TPA-mediated activation of transcription factor DNA-binding activity by K1 signal transduction. BCBL1 CD8Δ and BCBL1 CD8/K1-C cells were stimulated with 20 ng of TPA/ml for the times indicated. EMSAs were performed on nuclear extracts by using a radioactively labeled oligonucleotide probe containing a specific transcription factor binding site. In lanes without nuclear extracts (lanes C), no band shift was detected. Arrowheads indicate low- and high-molecular-weight forms of NF-κB. F, free probe. (A) Results for AP1, Oct-1, and NF-κB. (B) Results for NF-AT, AP-2, and SP-1.
K1 signal transduction slightly augments K8.1 expression induced by ectopic expression of ORF50.
KSHV ORF50, a homolog of EBV Rta, is essential and sufficient to drive the entire viral lytic cycle and is the only known lytic-switch gene for viral reactivation from latency (50, 71, 74). ITAM-dependent K1 signal transduction has been shown to modestly augment ORF50-mediated KSHV lytic reactivation (38). To investigate whether K1 signaling affects ORF50-mediated lytic reactivation, we used the pTracer-GFP/ORF50 vector where green fluorescent protein (GFP) is expressed from a cytomegalovirus early promoter and ORF50 is expressed from an elongation factor-1 promoter. We electroporated BCBL1 CD8Δ, BCBL1 CD8/K1-C, BCBL1 DEF, BCBL1 DEF/K1, and BCBL1 DEF/K1 Y1,2F cells with the pTracer-GFP/ORF50 vector. At 3 days after electroporation, the GFP-positive cell population was separated by flow cytometry, and the surface level of K8.1 glycoprotein was assessed. As shown previously (38), ectopic expression of the ORF50 gene robustly induced K8.1 expression on BCBL1 CD8Δ, BCBL1 CD8/K1-C, BCBL1 DEF, and BCBL1 DEF/K1 cells (Fig. 8). With slight variations, approximately 20 to 40% of BCBL1 CD8Δ, BCBL1 CD8/K1-C, BCBL1 DEF, and BCBL1 DEF/K1 cells exhibited K8.1 surface expression 3 days after ectopic expression of the ORF50 gene (Fig. 8). As described previously (38), we also observed a slight augmentation of K8.1 surface expression induced by ORF50 in the presence of K1 signaling (Fig. 8). This indicates that while K1 signaling efficiently suppresses TPA-mediated KSHV lytic reactivation, it weakly augments K8.1 expression induced by ectopic expression of the ORF50 transcription factor.
FIG. 8.
K1 signaling slightly augments K8.1 surface expression induced by ectopic expression of ORF50. Cells were electroporated with the pTracer-GFP/ORF50 vector, and the level of surface K8.1 glycoprotein in the GFP-positive cell population was assessed by flow cytometry 3 days after electroporation. Numbers are percentages of K8.1-positive cells. (A) Two independent experimental results each for BCBL1 CD8Δ and BCBL1 CD8/K1-C cells. (B) Two independent experimental results each for BCBL1 DEF, BCBL1 DEF/K1, and BCBL1 DEF/K1 Y1,2F cells.
DISCUSSION
In this study, we demonstrated that K1 signal transduction in KSHV-infected BCBL1 cells is significantly different from that in uninfected B lymphocytes and that the K1 signal efficiently suppresses TPA-mediated viral lytic reactivation. This suppression requires the cytoplasmic ITAM sequence. Extensive analyses including viral microarray, immunoblots, and EMSAs demonstrated that K1 signaling suppresses KSHV lytic gene expression through the inhibition of TPA-induced activation of AP-1, NF-κB, and Oct-1. This suggests that K1-mediated signal transduction may help to establish and/or maintain KSHV viral latency.
It has previously been demonstrated that the cytoplasmic ITAM sequence of K1 is able to elicit intracellular calcium mobilization and tyrosine phosphorylation and to activate NF-AT and NF-κB activity (37, 42, 65). However, most or all previous studies of K1 signal transduction were performed in KSHV-negative cell lines, including human BJAB B lymphocytes and 293 epithelial cells (37, 42). To our surprise, we found that K1 signaling was not able to induce intracellular calcium mobilization and minimally induced tyrosine phosphorylation in KSHV-infected BCBL1 cells (Fig. 1 and 2). Nor did it induce NF-AT activity in BCBL1 cells at any detectable level, while it greatly induced NF-AT activity in BJAB cells (Fig. 2). In fact, it has also been shown that K1 expression does not induce NF-AT activity in a cultured KS spindle cell line (SLK) (38). This suggests that K1 likely acts through different signal transduction pathways in different cell types. K1 signal transduction induces NF-AT/NF-κB activity, calcium mobilization, and tyrosine phosphorylation in CD19-positive circulating B lymphocytes in infected individuals. However, since PEL cells have significant differences in expression and activity of cellular signaling molecules from mature B lymphocytes, K1 may target different cellular molecules and induce distinct signal transduction in virus-harboring PELs. Furthermore, this K1-mediated signal transduction pathway may overlap with the TPA-induced cellular signaling pathway. Upon activation, K1-mediated signal transduction may compete for the recruitment of cellular signaling molecules with TPA-induced signal transduction, which ultimately leads to the suppression of TPA-induced KSHV reactivation. This hypothesis is under active investigation.
Viral microarrays demonstrated an overall downregulation of TPA-induced KSHV gene expression by K1. Although K1 may decrease the expression of the majority of KSHV genes, it does not inhibit the expression of all of them. A small subset of KSHV genes, including ORF48 and K15, showed increased levels of expression. Our array results also showed that K1 itself was upregulated in BCBL1 CD8/K1-C cells upon TPA stimulation, suggesting that there is a potential autoregulation during the latter part of the infection cycle. This suggests that transfected K1 or CD8-K1 chimera genes may increase K1 gene expression, which ultimately amplifies the K1 effect on TPA-mediated lytic reactivation. Since K1 decreased the expression of a broadly active and potent transactivator, ORF50, both before and during lytic infection, a majority of the effects of K1 could be mediated through downregulation of ORF50 expression by K1. Thus, K1 signal transduction may primarily target ORF50 expression to control KSHV lytic reactivation. The detailed mechanisms by which K1 leads to the decrease (or, in a few cases, to an increase) in KSHV expression remain to be determined.
TPA stimulation has pleiotropic effects on cellular signal transduction, and these signal transduction pathways induce various transcriptional factors, resulting in viral lytic reactivation. EMSAs showed that K1 signaling significantly alleviated TPA-mediated activation of AP-1, NF-κB, and Oct-1 transcriptional activity. It is intriguing that while both high- and low-molecular-weight NF-κB complexes were strongly detected in BCBL1 CD8Δ cells an hour after TPA stimulation, only low-molecular-weight NF-κB complexes increased in BCBL1 CD8/K1-C cells. The high-molecular-weight NF-κB complex consists of a p65/p50 heterodimer, which is known to be transcriptionally active (24). By contrast, the low-molecular-weight complex contains p50, presumably present as a homodimer or as a heterodimer with another NF-κB family member, such as p52. The p50/p52 complex has usually been described as transcriptionally silent (11, 57). This indicates that K1 signaling specifically targets the transcriptionally active form of NF-κB complexes upon TPA stimulation, while it leaves the transcriptionally inactive form of NF-κB complexes unaffected under the same conditions. Both B-cell-specific transcription factors, Oct-1, which plays an important role in Ig gene expression in B cells (64), and AP-1, which is a major regulatory protein that mediates TPA stimulation (3, 40), display reduced levels of activation in the presence of K1 signal transduction. In addition, we observed no significant NF-AT activation by TPA stimulation in either BCBL1 CD8Δ cells or BCBL1 CD8/K1-C cells. Thus, K1 signaling significantly suppresses the TPA-mediated activation of AP-1, NF-κB, and Oct-1 transcriptional activity, which may confer suppression of KSHV lytic gene expression.
Full-length K1 required antibody cross-linking to show an inhibitory effect on TPA-induced lytic reactivation, whereas the CD8/K1-C chimera did not require anti-CD8 antibody cross-linking to show an inhibitory effect on TPA-induced lytic reactivation. Furthermore, the inhibitory activity of CD8/K1-C in the absence of anti-CD8 antibody cross-linking was much stronger than that of full-length K1 in the presence of anti-Flag antibody cross-linking. In fact, we have found that the presence of continuous anti-CD8 antibody stimulation elicits markedly strong signal transduction, which eventually induces the differentiation of BCBL1 CD8/K1-C cells (unpublished data). These cells undergo morphological alteration to an adherent cell type, induce cytoskeleton reorganization, and produce polarized lamellipodia, the results of active cellular signal transduction. This suggests that the CD8/K1 chimera may elicit constitutively active signaling in the absence of antibody cross-linking and that antibody stimulation further enhances its signaling activity to induce B-lymphocyte differentiation. In contrast, antibody cross-linking is necessary to generate the signal transduction activity of the full-length K1 protein. This suggests that K1 protein may require the potential ligands that exist on the surfaces of virus-infected cells or neighboring cells and that this interaction may induce K1-mediated signal transduction. If identified, this ligand may be a key factor in the modulation of K1 signal-transducing activity and thereby in the regulation of viral reactivation and development of virus-associated pathogenesis. Thus, additional study is needed to provide evidence for or against this hypothesis.
Recently, EBV LMP1 transforming protein has been shown to suppress viral reactivation triggered by either anti-IgM or TPA (1). In addition, signal transduction of cellular CD40 that mimics LMP1 is also able to inhibit EBV lytic reactivation in culture (1) and to block reactivation of mouse gammaherpesvirus 68 in vivo (66). At a position equivalent to that of the LMP1 of EBV, KSHV contains the K1 gene as a functional homolog: both deregulate cell growth control (39, 42). Furthermore, our finding that K1 signal transduction inhibits TPA-mediated KSHV reactivation is another example of the functional similarity between these gammaherpesvirus transforming proteins. After primary infection of B cells, K1, together with or independent of K15, may suppress the lytic cycle in KSHV-infected cells. This suppression may establish KSHV latency in infected individuals. However, a major drawback of this hypothesis is the lack of evidence for K1 expression during viral latency. While a low level of K1 transcripts is detected in PEL cells, it may reflect spontaneous lytic reactivation of KSHV in culture and probably does not reflect latent expression of K1. However, no attempt has been made to detect latent K1 expression in KS lesions or PELs because of the lack of a specific anti-K1 antibody. Thus, future studies should be directed toward developing tools, such as a K1-specific antibody, to examine K1 expression in KSHV-infected tissues and cells and toward dissecting K1-mediated signal transduction in virus-infected cells. In summary, we have shown that K1 mediates a set of signal transduction pathways in virus-infected cells distinct from that in normal B lymphocytes and that this signal transduction significantly suppresses TPA-mediated lytic reactivation in culture.
Acknowledgments
We thank D. Ganem, B. Chandran, P. Moore, and Y. Chang for providing reagents and Jennifer Macke for editing the text.
This work was partly supported by U.S. Public Health Service grants CA82057, CA91819, and RR00168; ACS grant RPG001102; the NIH Intramural AIDS Targeted Antiviral Program; and an intramural research award from the Center for Cancer Research, NCI. J. U. Jung is a Leukemia and Lymphoma Society Scholar.
REFERENCES
- 1.Adler, B., E. Schaadt, B. Kempkes, U. Zimber-Strobl, B. Baier, and G. W. Bornkamm. 2002. Control of Epstein-Barr virus reactivation by activated CD40 and viral latent membrane protein 1. Proc. Natl. Acad. Sci. USA 99:437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, L., L. Denekamp, A. Knapp, M. R. Auerbach, B. Damania, and R. C. Desrosiers. 2000. The primary sequence of rhesus monkey rhadinovirus isolate 26-95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J. Virol. 74:3388-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angel, P., M. Imagawa, R. Chiu, B. Stein, R. J. Imbra, H. J. Rahmsdorf, C. Jonat, P. Herrlich, and M. Karin. 1987. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell 49:729-739. [DOI] [PubMed] [Google Scholar]
- 4.Aoki, Y., E. S. Jaffe, Y. Chang, K. Jones, J. Teruya-Feldstein, P. S. Moore, and G. Tosato. 1999. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood 93:4034-4043. [PubMed] [Google Scholar]
- 5.Arvanitakis, L., E. Geras-Raaka, A. Varma, M. C. Gershengorn, and E. Cesarman. 1997. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385:347-350. [DOI] [PubMed] [Google Scholar]
- 6.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1991. Current protocols in molecular biology, p. 12.1-12.2. John Wiley & Sons, Inc., New York, N.Y.
- 7.Belanger, C., A. Gravel, A. Tomoiu, M. E. Janelle, J. Gosselin, M. J. Tremblay, and L. Flamand. 2001. Human herpesvirus 8 viral FLICE-inhibitory protein inhibits Fas-mediated apoptosis through binding and prevention of procaspase-8 maturation. J. Hum. Virol. 4:62-73. [PubMed] [Google Scholar]
- 8.Bittner, M., P. Meltzer, Y. Chen, Y. Jiang, E. Seftor, M. Hendrix, M. Radmacher, R. Simon, Z. Yakhini, A. Ben-Dor, N. Sampas, E. Dougherty, E. Wang, F. Marincola, C. Gooden, J. Lueders, A. Glatfelter, P. Pollock, J. Carpten, E. Gillanders, D. Leja, K. Dietrich, C. Beaudry, M. Berens, D. Alberts, and V. Sondak. 2000. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature 406:536-540. [DOI] [PubMed] [Google Scholar]
- 9.Bittner, M., P. Meltzer, and J. Trent. 1999. Data analysis and integration: of steps and arrows. Nat. Genet. 22:213-215. [DOI] [PubMed] [Google Scholar]
- 10.Bosing, T., F. Bellos, F. W. Cremer, C. Gemmel, G. Moldenhauer, A. D. Ho, H. Goldschmidt, and M. Moos. 2000. CD19+ and CD20+ B cells from the peripheral blood of patients with multiple myeloma are not infected with human herpesvirus 8. Leukemia 14:1330-1331. [DOI] [PubMed] [Google Scholar]
- 11.Burysek, L., W. S. Yeow, and P. M. Pitha. 1999. Unique properties of a second human herpesvirus 8-encoded interferon regulatory factor (vIRF-2). J. Hum. Virol. 2:19-32. [PubMed] [Google Scholar]
- 12.Chandran, B., C. Bloomer, S. R. Chan, L. Zhu, E. Goldstein, and R. Horvat. 1998. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology 249:140-149. [DOI] [PubMed] [Google Scholar]
- 13.Chandran, B., M. S. Smith, D. M. Koelle, L. Corey, R. Horvat, and E. Goldstein. 1998. Reactivities of human sera with human herpesvirus-8-infected BCBL-1 cells and identification of HHV-8-specific proteins and glycoproteins and the encoding cDNAs. Virology 243:208-217. [DOI] [PubMed] [Google Scholar]
- 14.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 15.Chen, Y., E. Dougherty, and M. Bittner. 1997. Ratio-based decision and quantitative analysis of cDNA microarray images. J. Biomed. Optics 2:364-374. [DOI] [PubMed] [Google Scholar]
- 16.Choi, J. K., B. S. Lee, S. N. Shim, M. Li, and J. U. Jung. 2000. Identification of the novel K15 gene at the rightmost end of the Kaposi's sarcoma-associated herpesvirus genome. J. Virol. 74:436-446. [PMC free article] [PubMed] [Google Scholar]
- 17.Chung, Y. H., R. E. Means, J. K. Choi, B. S. Lee, and J. U. Jung. 2002. Kaposi's sarcoma-associated herpesvirus OX2 glycoprotein activates myeloid lineage cells to induce inflammatory cytokine production. J. Virol. 76:4688-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coscoy, L., and D. Ganem. 2000. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl. Acad. Sci. USA 97:8051-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coscoy, L., and D. Ganem. 2001. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J. Clin. Investig. 107:1599-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cotter, M. A., II, and E. S. Robertson. 1999. The latency-associated nuclear antigen tethers the Kaposi's sarcoma-associated herpesvirus genome to host chromosomes in body cavity-based lymphoma cells. Virology 264:254-264. [DOI] [PubMed] [Google Scholar]
- 21.Dittmer, D., C. Stoddart, R. Renne, V. Linquist-Stepps, M. E. Moreno, C. Bare, J. M. McCune, and D. Ganem. 1999. Experimental transmission of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) to SCID-hu Thy/Liv mice. J. Exp. Med. 190:1857-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisen, M. B., P. T. Spellman, P. O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95:14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eliopoulos, A. G., and L. S. Young. 2001. LMP1 structure and signal transduction. Semin. Cancer Biol. 11:435-444. [DOI] [PubMed] [Google Scholar]
- 24.Fujita, T., G. P. Nolan, S. Ghosh, and D. Baltimore. 1992. Independent modes of transcriptional activation by the p50 and p65 subunits of NF-κB. Genes Dev. 6:775-787. [DOI] [PubMed] [Google Scholar]
- 25.Gao, S. J., C. Boshoff, S. Jayachandra, R. A. Weiss, Y. Chang, and P. S. Moore. 1997. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 15:1979-1985. [DOI] [PubMed] [Google Scholar]
- 26.Gao, S. J., Y. J. Zhang, J. H. Deng, C. S. Rabkin, O. Flore, and H. B. Jenson. 1999. Molecular polymorphism of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) latent nuclear antigen: evidence for a large repertoire of viral genotypes and dual infection with different viral genotypes. J. Infect. Dis. 180:1466-1476. [DOI] [PubMed] [Google Scholar]
- 27.Glenn, M., L. Rainbow, F. Aurad, A. Davison, and T. F. Schulz. 1999. Identification of a spliced gene from Kaposi's sarcoma-associated herpesvirus encoding a protein with similarities to latent membrane proteins 1 and 2A of Epstein-Barr virus. J. Virol. 73:6953-6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gradoville, L., J. Gerlach, E. Grogan, D. Shedd, S. Nikiforow, C. Metroka, and G. Miller. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 74:6207-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haque, M., K. Ueda, K. Nakano, Y. Hirata, C. Parravicini, M. Corbellino, and K. Yamanishi. 2001. Major histocompatibility complex class I molecules are down-regulated at the cell surface by the K5 protein encoded by Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8. J. Gen. Virol. 82:1175-1180. [DOI] [PubMed] [Google Scholar]
- 30.Horenstein, M. G., R. G. Nador, A. Chadburn, E. M. Hyjek, G. Inghirami, D. M. Knowles, and E. Cesarman. 1997. Epstein-Barr virus latent gene expression in primary effusion lymphomas containing Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8. Blood 90:1186-1191. [PubMed] [Google Scholar]
- 31.Ishido, S., C. Wang, B. S. Lee, G. B. Cohen, and J. U. Jung. 2000. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 74:5300-5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung, J. U., J. K. Choi, A. Ensser, and B. Biesinger. 1999. Herpesvirus saimiri as a model for gammaherpesvirus oncogenesis. Semin. Cancer Biol. 9:231-239. [DOI] [PubMed] [Google Scholar]
- 33.Jung, J. U., S. M. Lang, U. Friedrich, T. Jun, T. M. Roberts, R. C. Desrosiers, and B. Biesinger. 1995. Identification of Lck-binding elements in tip of herpesvirus saimiri. J. Biol. Chem. 270:20660-20667. [DOI] [PubMed] [Google Scholar]
- 34.Jung, J. U., S. M. Lang, T. Jun, T. M. Roberts, A. Veillette, and R. C. Desrosiers. 1995. Downregulation of Lck-mediated signal transduction by tip of herpesvirus saimiri. J. Virol. 69:7814-7822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kedes, D. H., M. Lagunoff, R. Renne, and D. Ganem. 1997. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi's sarcoma-associated herpesvirus. J. Clin. Investig. 100:2606-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kledal, T. N., M. M. Rosenkilde, F. Coulin, G. Simmons, A. H. Johnsen, S. Alouani, C. A. Power, H. R. Luttichau, J. Gerstoft, P. R. Clapham, I. Clark-Lewis, T. N. Wells, and T. W. Schwartz. 1997. A broad-spectrum chemokine antagonist encoded by Kaposi's sarcoma-associated herpesvirus. Science 277:1656-1659. [DOI] [PubMed] [Google Scholar]
- 37.Lagunoff, M., and D. Ganem. 1997. The structure and coding organization of the genomic termini of Kaposi's sarcoma-associated herpesvirus. Virology 236:147-154. [DOI] [PubMed] [Google Scholar]
- 38.Lagunoff, M., D. M. Lukac, and D. Ganem. 2001. Immunoreceptor tyrosine-based activation motif-dependent signaling by Kaposi's sarcoma-associated herpesvirus K1 protein: effects on lytic viral replication. J. Virol. 75:5891-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lagunoff, M., R. Majeti, A. Weiss, and D. Ganem. 1999. Deregulated signal transduction by the K1 gene product of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 96:5704-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamph, W. W., P. Wamsley, P. Sassone-Corsi, and I. M. Verma. 1988. Induction of proto-oncogene JUN/AP-1 by serum and TPA. Nature 334:629-631. [DOI] [PubMed] [Google Scholar]
- 41.Lee, B. S., X. Alvarez, S. Ishido, A. A. Lackner, and J. U. Jung. 2000. Inhibition of intracellular transport of B cell antigen receptor complexes by Kaposi's sarcoma-associated herpesvirus K1. J. Exp. Med. 192:11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee, H., J. Guo, M. Li, J. K. Choi, M. DeMaria, M. Rosenzweig, and J. U. Jung. 1998. Identification of an immunoreceptor tyrosine-based activation motif of K1 transforming protein of Kaposi's sarcoma-associated herpesvirus. Mol. Cell. Biol. 18:5219-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee, H., R. Veazey, K. Williams, M. Li, J. Guo, F. Neipel, B. Fleckenstein, A. Lackner, R. C. Desrosiers, and J. U. Jung. 1998. Deregulation of cell growth by the K1 gene of Kaposi's sarcoma-associated herpesvirus. Nat. Med. 4:435-440. [DOI] [PubMed] [Google Scholar]
- 44.Li, M., H. Lee, J. Guo, F. Neipel, B. Fleckenstein, K. Ozato, and J. U. Jung. 1998. Kaposi's sarcoma-associated herpesvirus viral interferon regulatory factor. J. Virol. 72:5433-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li, M., H. Lee, D. W. Yoon, J. C. Albrecht, B. Fleckenstein, F. Neipel, and J. U. Jung. 1997. Kaposi's sarcoma-associated herpesvirus encodes a functional cyclin. J. Virol. 71:1984-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li, M., J. MacKey, S. C. Czajak, R. C. Desrosiers, A. A. Lackner, and J. U. Jung. 1999. Identification and characterization of Kaposi's sarcoma-associated herpesvirus K8.1 virion glycoprotein. J. Virol. 73:1341-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Longnecker, R., B. Druker, T. M. Roberts, and E. Kieff. 1991. An Epstein-Barr virus protein associated with cell growth transformation interacts with a tyrosine kinase. J. Virol. 65:3681-3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lukac, D. M., J. R. Kirshner, and D. Ganem. 1999. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 73:9348-9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller, C. L., A. L. Burkhardt, J. H. Lee, B. Stealey, R. Longnecker, J. B. Bolen, and E. Kieff. 1995. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity 2:155-166. [DOI] [PubMed] [Google Scholar]
- 50.Miller, G., L. Heston, E. Grogan, L. Gradoville, M. Rigsby, R. Sun, D. Shedd, V. M. Kushnaryov, S. Grossberg, and Y. Chang. 1997. Selective switch between latency and lytic replication of Kaposi's sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J. Virol. 71:314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore, P. S., C. Boshoff, R. A. Weiss, and Y. Chang. 1996. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 274:1739-1744. [DOI] [PubMed] [Google Scholar]
- 52.Moses, A. V., K. N. Fish, R. Ruhl, P. P. Smith, J. G. Strussenberg, L. Zhu, B. Chandran, and J. A. Nelson. 1999. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J. Virol. 73:6892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neipel, F., J. C. Albrecht, A. Ensser, Y. Q. Huang, J. J. Li, A. E. Friedman-Kien, and B. Fleckenstein. 1997. Human herpesvirus 8 encodes a homolog of interleukin-6. J. Virol. 71:839-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neipel, F., J. C. Albrecht, and B. Fleckenstein. 1999. Human herpesvirus 8: is it a tumor virus? Proc. Assoc. Am. Physicians 111:594-601. [DOI] [PubMed] [Google Scholar]
- 55.Nicholas, J., V. R. Ruvolo, W. H. Burns, G. Sandford, X. Wan, D. Ciufo, S. B. Hendrickson, H. G. Guo, G. S. Hayward, and M. S. Reitz. 1997. Kaposi's sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat. Med. 3:287-292. [DOI] [PubMed] [Google Scholar]
- 56.Nicholas, J., J. C. Zong, D. J. Alcendor, D. M. Ciufo, L. J. Poole, R. T. Sarisky, C. J. Chiou, X. Zhang, X. Wan, H. G. Guo, M. S. Reitz, and G. S. Hayward. 1998. Novel organizational features, captured cellular genes, and strain variability within the genome of KSHV/HHV8. J. Natl. Cancer Inst. Monogr. 23:79-88. [DOI] [PubMed] [Google Scholar]
- 57.Nolan, G. P., T. Fujita, K. Bhatia, C. Huppi, H. C. Liou, M. L. Scott, and D. Baltimore. 1993. The bcl-3 proto-oncogene encodes a nuclear IκB-like molecule that preferentially interacts with NF-κB p50 and p52 in a phosphorylation-dependent manner. Mol. Cell. Biol. 13:3557-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paulose-Murphy, M., N. K. Ha, C. Xiang, Y. Chen, L. Gillim, R. Yarchoan, P. Meltzer, M. Bittner, J. Trent, and S. Zeichner. 2001. Transcription program of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus). J. Virol. 75:4843-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ploegh, H. L. 1998. Viral strategies of immune evasion. Science 280:248-253. [DOI] [PubMed] [Google Scholar]
- 60.Poole, L. J., Y. Yu, P. S. Kim, Q. Z. Zheng, J. Pevsner, and G. S. Hayward. 2002. Altered patterns of cellular gene expression in dermal microvascular endothelial cells infected with Kaposi's sarcoma-associated herpesvirus. J. Virol. 76:3395-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rainbow, L., G. M. Platt, G. R. Simpson, R. Sarid, S. J. Gao, H. Stoiber, C. S. Herrington, P. S. Moore, and T. F. Schulz. 1997. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J. Virol. 71:5915-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 63.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sakakibara, S., K. Ueda, J. Chen, T. Okuno, and K. Yamanishi. 2001. Octamer-binding sequence is a key element for the autoregulation of Kaposi's sarcoma-associated herpesvirus ORF50/Lyta gene expression. J. Virol. 75:6894-6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samaniego, F., S. Pati, J. Karp, O. Prakash, and D. Bose. 2001. Human herpesvirus 8 K1-associated nuclear factor-κb-dependent promoter activity: role in Kaposi's sarcoma inflammation? J. Natl. Cancer Inst. Monogr. 28:15-23. [DOI] [PubMed] [Google Scholar]
- 66.Sarawar, S. R., B. J. Lee, S. K. Reiter, and S. P. Schoenberger. 2001. Stimulation via CD40 can substitute for CD4 T cell function in preventing reactivation of a latent herpesvirus. Proc. Natl. Acad. Sci. USA 98:6325-6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sarid, R., T. Sato, R. A. Bohenzky, J. J. Russo, and Y. Chang. 1997. Kaposi's sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nat. Med. 3:293-298. [DOI] [PubMed] [Google Scholar]
- 68.Sarid, R., J. S. Wiezorek, P. S. Moore, and Y. Chang. 1999. Characterization and cell cycle regulation of the major Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) latent genes and their promoter. J. Virol. 73:1438-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schultz, T. F., Y. Chang, and P. S. Moore. 1998. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8), p. 87-134. In D. J. McCance (ed.), Human herpesviruses. ASM Press, Washington, D.C.
- 70.Searles, R. P., E. P. Bergquam, M. K. Axthelm, and S. W. Wong. 1999. Sequence and genomic analysis of a rhesus macaque rhadinovirus with similarity to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J. Virol. 73:3040-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thome, M., P. Schneider, K. Hofmann, H. Fickenscher, E. Meinl, F. Neipel, C. Mattmann, K. Burns, J. L. Bodmer, M. Schroter, C. Scaffidi, P. H. Krammer, M. E. Peter, and J. Tschopp. 1997. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386:517-521. [DOI] [PubMed] [Google Scholar]
- 73.Wu, F. Y., J. H. Ahn, D. J. Alcendor, W. J. Jang, J. Xiao, S. D. Hayward, and G. S. Hayward. 2001. Origin-independent assembly of Kaposi's sarcoma-associated herpesvirus DNA replication compartments in transient cotransfection assays and association with the ORF-K8 protein and cellular PML. J. Virol. 75:1487-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu, F. X., T. Cusano, and Y. Yuan. 1999. Identification of the immediate-early transcripts of Kaposi's sarcoma-associated herpesvirus. J. Virol. 73:5556-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zong, J. C., D. M. Ciufo, D. J. Alcendor, X. Wan, J. Nicholas, P. J. Browning, P. L. Rady, S. K. Tyring, J. M. Orenstein, C. S. Rabkin, I. J. Su, K. F. Powell, M. Croxson, K. E. Foreman, B. J. Nickoloff, S. Alkan, and G. S. Hayward. 1999. High-level variability in the ORF-K1 membrane protein gene at the left end of the Kaposi's sarcoma-associated herpesvirus genome defines four major virus subtypes and multiple variants or clades in different human populations. J. Virol. 73:4156-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]