Abstract
Analysis of abnormal prion protein glycoform patterns from chronic wasting disease (CWD)-affected deer and elk, scrapie-affected sheep and cattle, and cattle with bovine spongiform encephalopathy failed to identify patterns capable of reliably distinguishing these transmissible spongiform encephalopathy diseases. However, PrP-res patterns sometimes differed among individual animals, suggesting infection by different or multiple CWD strains in some species.
Chronic wasting disease (CWD) of deer and elk is of particular concern to scientists and the general public because the potential for transmission to humans or livestock is unknown. Rapid methods by which to identify the source of transmissible spongiform encephalopathy (TSE) following cross-species transmission of the causal agent would be valuable in identifying expanding host ranges of known TSEs. Various TSE diseases have been distinguished on the basis of brain-derived PrP-res glycoform patterns (1, 2, 5, 8-10, 14). We therefore analyzed brain tissue from CWD-affected deer and elk, scrapie-affected sheep and cattle, and cattle with bovine spongiform encephalopathy (BSE) to determine if distinct abnormal prion protein (PrP-res) profiles capable of differentiating these diseases could be identified.
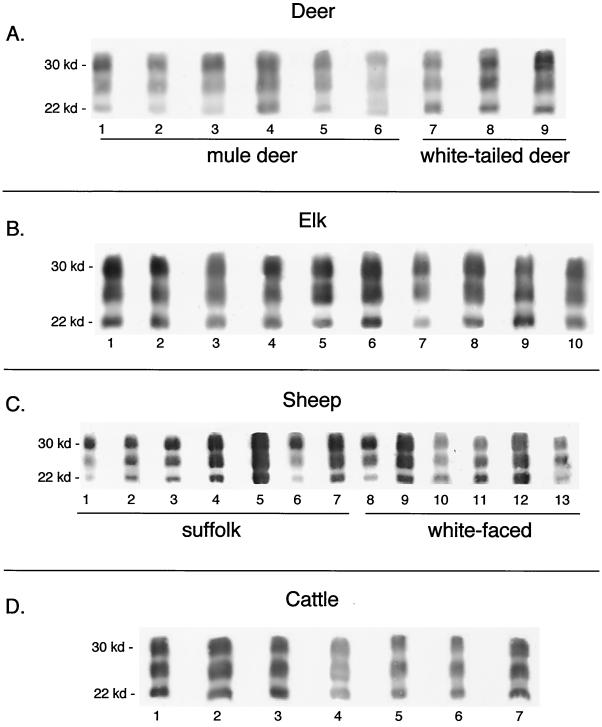
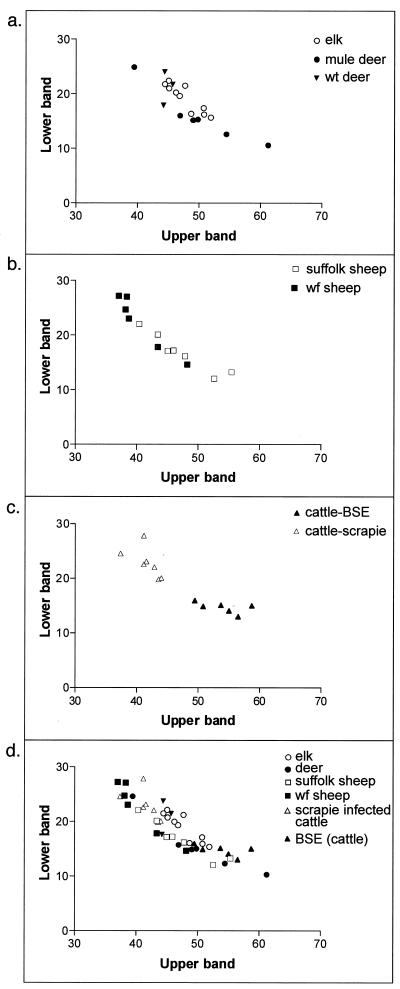
The PrP-res immunoblot profiles of brain tissues from six mule deer, three white-tailed deer, and 10 elk, all with CWD, as well as 13 sheep and seven cattle with scrapie and six cattle with BSE, were analyzed. For all of the deer and elk, the strongest PrP-res signal was observed in the uppermost diglycosylated (30-kDa) band and the weakest signal was observed in the lowest nonglycosylated (22-kDa) PrP-res band. Some animal-to-animal variation was observed, especially among the deer (Fig. 1 and 2). We also analyzed the proportions of the uppermost and lowest PrP-res bands for each deer and elk and expressed the data on a scattergraph (Fig. 3 a). The patterns for elk were more tightly grouped than those for deer, suggesting that the elk and deer were possibly infected by different CWD strains.
FIG. 1.
Immunoblot analysis of brain-derived PrP-res from CWD-affected deer and elk and scrapie-affected sheep and cattle. PrP-res was purified as previously described (11). Immunoblot assays were done as previously described (12), except that a primary antibody designated R35, made against PrP peptide 5′-CGQGGTHGQWNKPSK-3′, was used. This antibody has broad reactivity against PrP from deer, elk, sheep, cattle, mice, hamsters, and possibly other species. Gels were developed with an ECF kit (Amersham-Pharmacia) that allows quantitation of individual protein bands with a phosphorimager (Molecular Dynamics). Panel A shows PrP-res in brain tissue from nine CWD-affected mule or white-tailed deer. Lanes contain 5- to 25-mg equivalents of brain tissue. No bands were seen when 100 mg of brain tissue from normal deer was analyzed (data not shown). Panel B shows 5- to 25-mg equivalents of brain tissue from each of 10 CWD-affected elk. One hundred milligrams of brain tissue from normal elk gave no PrP bands (data not shown). Panel C shows PrP-res derived from brain tissue of scrapie-affected sheep. Lanes 1 to 7 contain 5- to 25-mg equivalents of brain tissue from naturally infected Suffolk sheep of U.S. origin, and lanes 8 to 13 contain brain tissue from white-faced breeds of sheep, including Leicester (lane 8), Shropshire (lanes 9 and 10), Rambouillet (lane 11), and Cheviot (lanes 12 and 13). Panel D shows PrP-res from 5 to 25 mg of brain tissue from scrapie-affected cattle. Details relating to these animals have been published before (6). kd, kilodaltons.
FIG. 2.
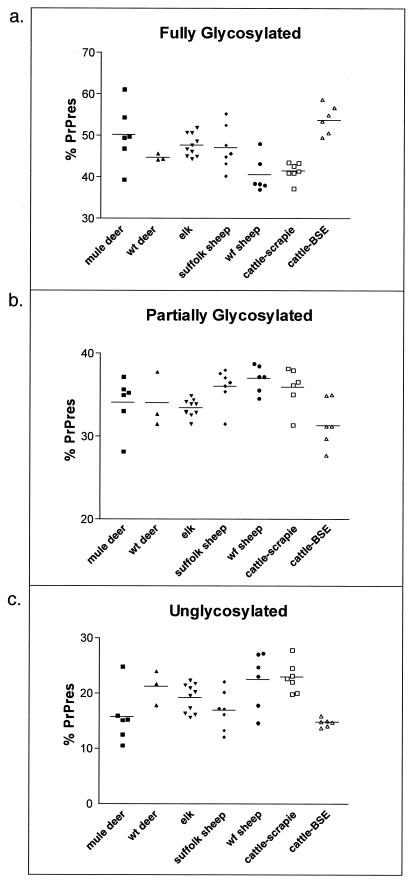
Brain samples from 10 elk, six mule deer, and three white-tailed (wt) deer, all affected with CWD, and seven scrapie-affected cattle were each immunoblotted four times. Brain samples from 13 scrapie-affected sheep were each immunoblotted twice, and brain samples from six BSE-affected cattle were immunoblotted five times. The values shown are the average amounts of PrP-res per protein band (bars) for all individuals (points) within the indicated species. Panel a shows a species comparison of the uppermost fully glycosylated PrP-res band. Mule deer and elk were statistically significantly different from white-face (wf) sheep (P < 0.05) and from scrapie-affected cattle (P < 0.05). BSE-affected cattle were different from white-face sheep and scrapie-affected cattle (P < 0.05). Panel b shows species comparisons of the middle diglycosylated band. Elk differed statistically sig-nificantly from white-face sheep (P < 0.05), and BSE-affected cattle differed from white-face sheep and scrapie-affected cattle (P < 0.05). Panel c shows species comparisons of the lowest unglycosylated PrP-res band. Mule deer differed statistically significantly from white-face sheep and scrapie-affected cattle (P < 0.05). Suffolk sheep differed from white-face sheep and scrapie-affected cattle (P < 0.05), while BSE-affected cattle were different from white-face sheep and scrapie-affected cattle (P < 0.05).
FIG. 3.
Glycoform ratios of PrP-res from TSE-affected ruminants. Glycoform ratios are presented as plots of the percentage of the total PrP-res found in the upper diglycosylated PrP-res band versus the percentage found in the lowest nonglycosylated band. Each point represents the average value for multiple immunoblots of each brain. Panel a compares mule deer, white-tailed (wt) deer, and elk. Panel b compares Suffolk and white-face (wf) sheep. Panel c compares scrapie-affected and BSE-affected cattle, and panel d compares all of the above.
Because sheep scrapie is believed to be the cause of BSE in Europe (15) and has been implicated as a potential source of CWD, we also determined the PrP-res patterns of scrapie-affected sheep. Both Suffolk and white-face sheep were analyzed. For all but one of the sheep (Fig. 1C, lane 12), the uppermost PrP-res band gave the strongest PrP-res signal although the relative amounts of PrP-res in the upper band varied among individuals. The relative intensity of the lowest PrP-res band also varied among the sheep, being weak for some but stronger for others (Fig. 1C and 2). One Suffolk sheep gave an overall pattern similar to that of the white-face sheep, and one white-face sheep gave a pattern similar to that of the Suffolk sheep (Suffolk sheep, lane 5; white-face sheep, lane 13), making it unlikely that scrapie-affected Suffolk sheep could be differentiated from affected white-face sheep on the basis of the PrP-res glycoform pattern alone. The proportions of the uppermost and lowest PrP-res bands of the Suffolk and white-face sheep were also compared. Suffolk sheep segregated somewhat from the white-face sheep, but there were several animals that were not clearly in a specific group (Fig. 3b). Thus, on the basis of the range of the PrP-res glycoform pattern possibilities, we concluded that the scrapie-affected Suffolk sheep could not be distinguished from the white-face sheep and that, in general, the scrapie-affected sheep could not be distinguished from CWD-affected cervids (Fig. 1 to 3).
We reasoned that if sheep scrapie is the source of BSE, one might expect sheep with scrapie, cattle with BSE, and scrapie-affected cattle to give similar PrP-res glycoform patterns, further supporting this hypothesis. We therefore determined the PrP-res glycoform pattern of cattle with sheep scrapie or BSE and compared the patterns to those previously determined for scrapie-affected sheep. The PrP-res profiles for the scrapie-affected cattle were uniform (Fig. 1D and 2), possibly reflecting the fact that the cattle were infected at the same time with the same inoculum, exposed to the same environment, and sacrificed at similar stages of clinical disease. The PrP-res patterns of these animals were not different enough to allow differentiation of individual animals from CWD-affected cervids or scrapie-affected sheep. However, the PrP-res pattern was clearly different from that of BSE-affected cattle (Fig. 2 and 3c). Furthermore, the PrP-res patterns of BSE-affected cattle were different from those of scrapie-affected cattle and white-face sheep, especially when the ratios of the uppermost and lowest PrP-res bands were compared (Fig. 2 and 3). BSE-affected cattle also tended to cluster away from the other species, but there was overlap with individual deer, elk, and Suffolk sheep, which precluded differentiation of the cervid TSE diseases and scrapie from BSE (Fig. 2 and 3).
There is no direct evidence that CWD has been transmitted to humans (3). However, because CWD is a relatively new TSE, it is unlikely that enough people have consumed enough CWD-affected cervids to result in a clinically or pathologically recognizable disease attributable to CWD, especially considering the very long incubation periods characteristic of TSE diseases. Also unknown is whether sheep or cattle can be infected with CWD under natural circumstances or whether people might be susceptible to CWD passaged through sheep or cattle. In the past, differences in PrP-res glycoform profiles have reliably differentiated certain TSE agent strains from others (1, 2, 4, 5, 8, 9, 14). However, inconsistent results were reported when sheep scrapie and BSE were analyzed. One study stated that scrapie-affected and BSE-affected sheep could be differentiated on the basis of differences in brain-associated PrP-res glycoforms (7), whereas a second study concluded that they could not be distinguished (2). In the study reported here, brain-derived PrP-res glycoform patterns sometimes varied among individual deer, elk, or sheep. Variation primarily involved differences in the ratio of the three PrP-res protein bands relative to each other in individuals rather than differences in the molecular weights of the various bands. The basis for the PrP-res glycoform variation that we observed among individual animals is not clear. Variation could simply reflect the range expected from randomly selected, heterogeneous populations of TSE-affected ruminants. Alternatively, it is possible that the different PrP-res patterns represent infection by one or more specific TSE strains not yet characterized in these species. For example, the relatively tight grouping of CWD-affected elk, compared to that of mule deer, might indicate that elk are infected with a single strain of CWD agent whereas mule deer are infected with multiple or different strains. The brain region could influence the glycoform pattern. However, we observed no differences in PrP-res glycoform patterns when six distinct brain regions of clinically affected deer or elk were analyzed (data not shown).
The general similarity of PrP-res glycoform patterns in scrapie-affected sheep and CWD-affected cervids seems to support the hypothesis that CWD arose from sheep scrapie, as do in vitro analyses (13). Sheep scrapie has been present in the United States since at least 1947, and in many geographical areas, sheep, deer, and elk share pastures and rangeland. If scrapie-affected sheep were present in these situations, then cross-species transmission might have occurred. Sheep scrapie is not thought to cause disease in humans, although passage through cattle appears to have changed this characteristic. It remains to be determined if the same will be true of CWD.
Acknowledgments
We thank Anita Mora for graphic-art assistance, Irene Cook Rodriguez and Cam Ruark for helping with the preparation of the manuscript, and Bruce Chesebro, Byron Caughey, and Sue Priola for discussions relating to the manuscript. Personnel of the Wyoming Game and Fish Department and the Colorado Division of Wildlife are also appreciated and were critical to collection of samples.
REFERENCES
- 1.Baron, T. G., and A. G. Biacabe. 2001. Molecular analysis of the abnormal prion protein during coinfection of mice by bovine spongiform encephalopathy and a scrapie agent. J. Virol. 75:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron, T. G., J. Y. Madec, and D. Calavas. 1999. Similar signature of the prion protein in natural sheep scrapie and bovine spongiform encephalopathy-linked diseases. J. Clin. Microbiol. 37:3701-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belay, E. D., P. Gambetti, L. B. Schonberger, P. Parchi, D. R. Lyon, S. Capellari, J. H. McQuiston, K. Bradley, G. Dowdle, J. M. Crutcher, and C. R. Nichols. 2001. Creutzfeldt-Jakob disease in unusually young patients who consumed venison. Arch. Neurol. 58:1673-1678. [DOI] [PubMed] [Google Scholar]
- 4.Bessen, R. A., and R. F. Marsh. 1992. Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J. Virol. 66:2096-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collinge, J., K. C. L. Sidle, J. Meads, J. Ironside, and A. F. Hill. 1996. Molecular analysis of prion strain variation and the etiology of ′new variant' CJD. Nature 383:685-690. [DOI] [PubMed] [Google Scholar]
- 6.Cutlip, R. C., J. M. Miller, R. E. Race, A. L. Jenny, J. B. Katz, H. D. Lehmkuhl, B. M. DeBey, and M. M. Robinson. 1994. Intracerebral transmission of scrapie to cattle. J. Infect. Dis. 169:814-820. [DOI] [PubMed] [Google Scholar]
- 7.Hill, A. F., K. C. Sidle, S. Joiner, P. Keyes, T. C. Martin, M. Dawson, and J. Collinge. 1998. Molecular screening of sheep for bovine spongiform encephalopathy. Neurosci. Lett. 255:159-162. [DOI] [PubMed] [Google Scholar]
- 8.Hope, J., S. C. Wood, C. R. Birkett, A. Chong, M. E. Bruce, D. Cairns, W. Goldmann, N. Hunter, and C. J. Bostock. 2000. Molecular analysis of ovine prion protein identifies similarities between BSE and an experimental isolate of natural scrapie, CH1641. J. Gen. Virol. 81:851. [DOI] [PubMed] [Google Scholar]
- 9.Kascsak, R. J., R. Rubenstein, P. A. Merz, R. I. Carp, N. K. Robakis, H. M. Wisniewski, and H. Diringer. 1986. Immunological comparison of scrapie-associated fibrils isolated from animals infected with four different scrapie strains. J. Virol. 59:676-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuczius, T., I. Haist, and M. H. Groschup. 1998. Molecular analysis of bovine spongiform encephalopathy and scrapie strain variation. J. Infect. Dis. 178:693-699. [DOI] [PubMed] [Google Scholar]
- 11.Oldstone, M. B. A., R. Race, D. Thomas, H. Lewicki, D. Homann, S. Smelt, A. Holz, P. Koni, D. Lo, B. Chesebro, and R. Flavell. 2002. Lymphotoxin-α- and lymphotoxin-β-deficient mice differ in susceptibility to scrapie: evidence against dendritic cell involvement in neuroinvasion. J. Virol. 76:4357-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Race, R., A. Raines, G. J. Raymond, B. Caughey, and B. Chesebro. 2001. Long-term subclinical carrier state precedes scrapie replication and adaptation in a resistant species: analogies to bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease in humans. J. Virol. 75:10106-10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raymond, G. J., A. Bossers, L. D. Raymond, K. I. O'Rourke, L. E. McHolland, P. K. Bryant III, M. W. Miller, E. S. Williams, M. Smits, and B. Caughey. 2000. Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO J. 19:4425-4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somerville, R. A. 1999. Host and transmissible spongiform encephalopathy agent strain control glycosylation of PrP. J. Gen. Virol. 80:1865-1872. [DOI] [PubMed] [Google Scholar]
- 15.Wilesmith, J. W., G. A. H. Wells, M. P. Cranwell, and J. B. M. Ryan. 1988. Bovine spongiform encephalopathy: epidemiological studies. Vet. Rec. 123:638-644. [PubMed] [Google Scholar]