Abstract
Recombinant bovine respiratory syncytial virus (rBRSV) and an rBRSV deletion mutant lacking the G gene (rBRSVΔG) were characterized in calves with respect to replication competence, attenuation, and protective efficacy as live-attenuated BRSV vaccines. Both recombinant viruses were safe and induced protection against a BRSV challenge infection. rBRSV replicated efficiently in the upper respiratory tract. Intranasal immunization with rBRSVΔG led to infection but not to mucosal virus replication. Neutralizing antibodies were induced by rBRSV and rBRSVΔG. Thus, the BRSV attachment glycoprotein G seems to be dispensable in vaccinating calves against BRSV.
Bovine respiratory syncytial virus (BRSV) is an enveloped, nonsegmented negative-strand RNA virus, genus Pneumovirus, of the Paramyxoviridae family (6). The BRSV genome is 15,140 nt in length and comprises 10 genes in a modular organization, and it encodes 11 proteins (2). Of these, the envelope-associated fusion glycoprotein F and attachment glycoprotein G (18, 20) represent the main viral antigenic determinants (28). As a major viral cause of respiratory disease in cattle, BRSV has great economic impact (33), and a reliable vaccine is needed. Experimental studies showed that priming calves with or without maternal antibodies for a mucosal and serum antibody response was achieved only by applying live virus via the respiratory tract (7, 17, 19, 35).
Of the three envelope-associated glycoproteins, the BRSV SH and G glycoproteins are dispensable in cell culture (14). The in vitro growth phenotype of rBRSV lacking the G gene (rBRSVΔG) is similar to that of rBRSV. In the absence of G protein, attachment is mediated by the BRSV F protein (14). As for HRSV, the BRSV attachment protein not only represents one of the major immunogenic viral proteins but also is suspected to participation in undesirable immune responses, which manifest in an enhanced clinical disease upon infection of previously immunized animals by wild-type virus (11, 22). The purpose of this study was to determine the in vivo phenotype of recombinant BRSV and of rBRSVΔG in the natural host with intranasal administration and to investigate whether previous immunization with rBRSV can protect calves from disease after subsequent infection with a virulent BRSV isolate.
In vivo replication competence and pathogenicity of rBRSV and rBRSVΔG.
Eleven conventionally reared mixed-breed calves which were free from BRSV, bovine parainfluenzavirus type 3, bovine viral diarrhea virus, and bovine herpesvirus 1 were taken to isolation facilities (BL3 equivalent) when they were between 2 and 8 days of age. At the age of 2 months, two groups of four animals each were inoculated intranasally with 8 × 106 PFU each of either rBRSV (2) (derived from BRSV strain ATue51908; GenBank accession no. AF092942) or rBRSVΔG, which lacks the complete BRSV G gene (ATue51908; nucleotides [nt] 4674 to 5539) (14). The virus stocks were propagated on MDBK cells as described previously (14). Three animals were inoculated with MDBK cell culture supernatant and kept as a mock-immunized control group.
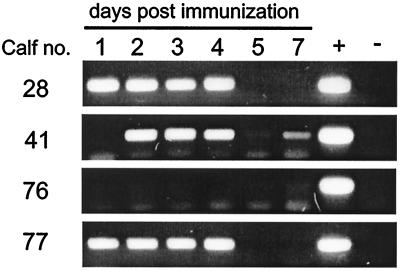
Virus replication in the upper respiratory tract was monitored by daily nasal swabs which were collected in 2 ml of minimal essential medium. For isolation of virus, monolayers of Kop-R cells (Kop-R, CCLVRIE244; a permanent cell line generated from oesopharyngeal tissue of a newborn calf, obtained from Roland Riebe, Insel Riems, Germany) were incubated with serial 10-fold nasal swab sample dilutions. After immunization with rBRSV, virus could be reisolated from all calves from days 2 to 4 until day 7 or 8 (Table 1). Peak titers of up to 4.1 log10 PFU per ml were determined, giving evidence of extensive replication of rBRSV in the upper respiratory tract. After immunization with rBRSVΔG, recovery of virus from nasal swabs was unsuccessful, and reverse transcription-PCR (RT-PCR) which was carried out to detect BRSV genomic RNA from nasal swabs was negative (not shown). Additionally, RT-PCR was performed to detect viral mRNA transcription from the nasal swab material of the rBRSVΔG immunized group, with positive results for three out of four animals (Fig. 1). The BRSV nucleoprotein (N) mRNA was chosen as a template because it is among the most abundantly expressed mRNAs of BRSV. From these results, it can be concluded that inoculation with rBRSVΔG leads to infection and mRNA expression over several days. As expected, after mock immunization, virus isolation and RT-PCRs from nasal swabs were negative.
TABLE 1.
Replication of rBRSV and rBRSVΔG in the upper respiratory tracts of calves
| Groupa | Calf no. | Nasal swab titer (log10 PFU/ml) or passageb at day postimmunization
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| rBRSV | 29 | 1.3 | 1.4 | 2.2 | 1.7 | 1st | |||||
| 35 | 1.1 | 1.0 | 1.0 | 3.2 | 0.8 | ||||||
| 74 | 1.1 | 1.2 | 1.8 | 3.8 | 3.2 | 4.1 | 2nd | ||||
| 79 | 0.4 | 1.2 | 3.1 | 3.5 | 2.3 | ||||||
| rBRSVΔG | 28, 41, 76, 77 | ||||||||||
| Mock | 26, 36, 37 | ||||||||||
Calves were immunized intranasally with 8 × 106 PFU of the indicated virus on day 0. Nasal swab samples were collected on indicated days. Virus reisolation was done on Kop-R cells and confirmed by an indirect immunofluorescence assay. Titers of virus are shown as log10 PFU per ml of sample.
Italics indicate passages of Kop-R cells needed for virus isolation after inoculation with nasal swab material.
FIG. 1.
Detection of rBRSVΔG mRNA transcription. To detect BRSV mRNA transcription in the rBRSVΔG immunized group, RNA was prepared from nasal swab material and RT-PCR was performed. First-strand cDNA was synthezised using a primer complementary to the N mRNA (ATue51908; nt 1676 to 1657), and RT-PCR was done using a primer downstream of the first-strand primer (ATue51908; nt 1613 to 1594) and a primer with the sequence of the N open reading frame 3′ end (ATue51908; nt 1144 to 1163), resulting in an RT-PCR product of 470 bp. +, positive control (RT-PCR from total RNA from rBRSVΔG-infected cells); -, negative control.
After immunization, no clinical signs were observed in any of the groups, showing that the virulence of recombinant BRSV, strain ATue51908, and of rBRSVΔG for calves is very low. This is noteworthy, taking into account that rBRSV was found to replicate very efficiently in the upper respiratory tract of all inoculated animals after a singular intranasal immunization.
Humoral immune response is induced by intranasal immunization with rBRSV and rBRSVΔG.
The 50% neutralization titer (ND50) of calf sera was determined as described previously (25). The sera obtained on days 8, 15, 29, and 35 after immunization from the calves of the rBRSV and rBRSVΔG groups showed neutralizing activity, with ND50 titers ranging from 2.5 log2 to 6 log2, as early as 8 days after intranasal immunization. In the group immunized with rBRSV, the ND50 subsequently increased to values between 5 and 8 log2, which resembles the immune response following natural infection (5, 21). The ND50 of the rBRSVΔG group increased to moderate levels of 3 to 4 log2 until day 35 after vaccination. Prior to challenge, none of the calf sera of the mock-immunized group contained BRSV-specific neutralizing antibodies.
Six weeks after immunization, the calves were challenged by aerosolization with virulent wild-type BRSV, strain CA-1 (5 × 104 PFU in a 10-ml volume per calf). The challenge virus was propagated on bovine turbinate cells, frozen in −70°C, and thawed prior to challenge infection. Eight days after challenge, the ND50 in the rBRSVΔG group increased four- to sixfold, reaching titers of 6.5 to 10 log2, indicating that they were efficiently primed for BRSV by the previous immunization with rBRSVΔG. Only in one out of four rBRSV immunized calves was a slight increase in neutralizing activity found after challenge, which is consistent with the fact that challenge virus replication was highly restricted in this group. In the mock-infected group, on day 7 or 8 after challenge, an initial rise in neutralizing activity (2 to 3.5 log2) was detected.
Intranasal immunization with rBRSV and rBRSVΔG protects against infection.
To monitor shedding of challenge virus, nasal swabs were taken daily for 8 days after challenge. All calves of the mock-immunized group shed challenge virus, with titers between 1.6 log10 and 3.4 log10 PFU per ml (Table 2). In the vaccinated groups, at least one passage of cells inoculated with nasal swab material was necessary to reisolate challenge virus, with the exception of one animal of the rBRSVΔG group, which showed low nasal swab titers of up to 1.6 log10 on three consecutive days (Table 2).
TABLE 2.
Replication of challenge virus CA-1 in the upper respiratory tract of calves and clinical signs postchallenge
| Groupa | Calf no. | Nasal swab titer (log10 PFU/ml) or passageb at day postchallenge
|
Day postchallengec at which clinical sign observed
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Feverd | Lung sounds | Coughe | Respiration ratef | ||
| rBRSV | 29 | 1st | |||||||||||
| 35 | 1st | 1st | |||||||||||
| 74 | |||||||||||||
| 79 | |||||||||||||
| rBRSVΔG | 28 | 2nd | |||||||||||
| 41 | 1.0 | 1.6 | 1.6 | 1st | 5, 7 | ||||||||
| 76 | 1st | ||||||||||||
| 77 | 1st | 1st | 1st | ||||||||||
| Mock | 26 | 3.4 | 3.0 | 3.2 | 2.7 | 2.6 | 5, 6, 7 | 6, 7 | +5, ++6, ++7 | 5, 6, 7 | |||
| 36 | 2.0 | 2.5 | 2.6 | 2.4 | |||||||||
| 37 | 2.0 | 1.6 | 2.3 | 2.0 | 2.4 | 1st | 3, 4, 5, 6, 7, 8 | ||||||
Six weeks after intranasal immunization with the indicated virus, calves were challenged with 5 × 104 PFU of virulent BRSV, strain CA-1 (42). After challenge, nasal swab samples were collected on indicated days. Titers of virus are shown as log10 PFU/ml of sample.
Italics indicate passage needed for virus isolation.
After challenge, calves were monitored daily for clinical signs.
Rectal temperature exceeding the mean value for at least 1°C.
+, spontaneous coughing; ++, frequent spontaneous coughing.
Rate at least twofold increased compared to the mean value.
Challenge virus replication in the lower respiratory tract was assessed by postmortem virus isolation on day 7 or 8 after challenge. Challenge virus was reisolated from lung tissue homogenate and lung lavage fluid from a calf in the mock-immunized group which, due to heavy respiratory distress, had been euthanatized on day 7 after challenge. In both samples, 5 log10 PFU per ml was present. On day 8 postchallenge, challenge virus was isolated from lung tissue from a second mock-immunized calf after the first cell culture passage. From neither the animals previously immunized with rBRSV nor those previously immunized with rBRSVΔG was challenge virus isolated from lung tissue samples, lavage fluids, or lymph nodes. This shows that mucosal immunization with rBRSV, as well as with rBRSVΔG, protects against challenge virus replication in the upper and lower airways, even though after immunization, rBRSVΔG could not be reisolated from nasal swabs.
Intranasal immunization with rBRSV or rBRSVΔG protects against disease upon challenge with wild-type virus and prevents BRSV-induced lung pathology.
After the challenge, calves were monitored daily for 10 days for clinical signs (Table 2). In the rBRSV group, clinical signs were absent after challenge. In one out of four calves immunized with rBRSVΔG, a rise in rectal temperature was observed postchallenge on 2 days. In contrast, in the mock-immunized group, the challenge infection produced mild to severe clinical disease (Table 2), showing that the chosen challenge procedure was suited to reproducing a clinical BRSV infection. One animal was euthanatized on day 7 after challenge because of heavy respiratory distress.
On day 8 after challenge, the calves were bled under deep anesthesia, and postmortem examinations were done. Protection was evaluated from a comparison of challenge-induced gross lesions, histological lesions, and immunohistological results in the respiratory tracts of calves from the immunized groups versus the mock-immunized group. Tissue samples were taken for virological, histological, and bacteriological examinations. In the immunized groups, singular atelectatic lung lobuli were found in the lungs of several calves (Table 3). However, histological analysis revealed that in all cases, the lesions found in previously immunized animals were of chronic character, with atelectasis and beginning induration, resembling those which are found in conventionally raised calves under field conditions. In the mock-immunized group, two calves showed extensive pneumonic gross lesions, with more than 60% of affected lung tissue in one animal and in one calf several lung lobes with up to 50% of the lobes consisting of acute pneumonic lesions. In neither of the animals were secondary bacterial pathogens present in amounts that can be regarded as causative for clinical disease. Taken together, two out of three animals of the mock control group were heavily affected by the challenge infection, with pathological and histological findings typical for a BRSV infection (1, 4, 23, 26, 36).
TABLE 3.
Pathological and histological results postchallenge
| Groupa | Calf no. | Pneu- monic lesionsb | Histopathological findings
|
||
|---|---|---|---|---|---|
| Description | Lung lobec | IFAd | |||
| rBRSV | 29 | 1 | |||
| 2 | − | ||||
| 35 | (+) | 2 | − | ||
| 4 | − | ||||
| 74 | (+) | 2 | − | ||
| 3 | − | ||||
| 79 | 2 | − | |||
| 3 | − | ||||
| rBRSV G | 28 | (+) | 3 | − | |
| 41 | ++ | Chronic | 2 | − | |
| 76 | ++ | Chronic | 3 | − | |
| 4 | − | ||||
| 77 | 2 | − | |||
| 3 | − | ||||
| Mock | 26 | ++++ | Acute pneumonia, syncytia | 3 | ++ |
| 5 | ++++ | ||||
| 7 | + | ||||
| 36 | + | Chronic | 2 | - | |
| 5 | − | ||||
| 6 | − | ||||
| 37 | ++ | Acute alveolar pneumonia | 2 | ++ | |
| 3 | - | ||||
| 4 | +++ | ||||
Six weeks after intranasal immunization with the indicated virus, calves were challenged with 5 × 104 PFU of virulent BRSV. Eight days after challenge (7 days in the case of calf no. 26), calves were necropsied and gross and histologic lung lesions were evaluated.
Total percentage of pneumonic lung area: (+), <1; +, 1 to 10; ++, 10 to 20; +++, 21 to 50; ++++, >50.
Lung lobes: 1, left apical cranial; 2, left apical caudal; 3, left diaphragmaticus; 4, right apical cranial; 5, right apical caudal; 6, medius; 7, right diaphragmaticus.
An indirect immunofluorescence assay (IFA) was performed to detect BRSV antigen in lung cryosections. ++++, antigen in more than 80% of cells; +++, antigen in more than 50%; ++, antigen in more than 10%; +, antigen in less than 10% of cells; −, not detectable.
The immunohistological results clearly confirmed that the gross lesions in the mock-immunized group were caused by the BRSV challenge (Table 3). Tissue samples were snap-frozen in n-heptane, and cryosections were fixed with acetone for 10 to 15 min at −20°C. Sections were incubated with monoclonal antibody F9 directed against the F protein of BRSV (a gift of Geraldine Taylor) and fluorescein isothiocyanate-labeled secondary goat F(ab) anti-mouse immunoglobulin (DAKO, Glostrup, Denmark). Large areas of bronchiolar and alveolar epithelial cells with BRSV-specific immunofluorescence were present in several lung lobes, whereas in the vaccinated groups, no BRSV-specific immunofluorescent staining was found in either of the gross lesions examined immunohistologically, indicating that in the immunized groups, the lower respiratory tract was protected from challenge virus replication.
Virus differentiation by serology and RT-PCR.
To test the specificity of the antibody response induced by the immunization, a further characterization of the serological response was done by using an indirect immunofluorescence assay with cells transiently expressing singular BRSV glycoproteins. For this purpose, BHK-BSR T7/5 cells were transfected with plasmids expressing the BRSV G or F protein under control of the T7 RNA polymerase promoter. After 24 h, the cells were fixed with acetone and incubated with 1:100 dilutions of heat-inactivated calf sera and subsequently stained with fluorescein isothiocyanate-conjugated goat anti-bovine immunoglobulin G secondary antibody. Monoclonal antibodies directed against the BRSV F or G protein served as controls. In the mock-immunized group, specific antibodies were not detectable at any time point. Calves primed with rBRSV showed positive reactions against BRSV G and F on day 35 after immunization and on day 8 after the challenge infection. The sera of calves primed with rBRSVΔG showed specific antibodies against the BRSV F protein but not against BRSV G on day 8 after challenge, showing that they were efficiently primed for BRSV F. The absence of a serological response to BRSV G after immunization with rBRSVΔG might be useful with respect to the development of a marker vaccine, allowing the diagnosis of a field virus infection.
To further differentiate the BRSV isolates from nasal swabs, RT-PCR was performed, using genomic RNA as a template. The amplified region spans an rBRSV marker restriction site contained in the F/M2 intergenic region which is absent in wild-type BRSV. As shown in Fig. 2, due to the presence of synthetic marker restriction sites (3), recombinant BRSV which was reisolated from nasal swabs can be distinguished from the BRSV challenge strain CA-1 by restriction analysis.
FIG. 2.
Differentiation of rBRSV and wild-type BRSV isolated from nasal swabs using a synthetic marker restriction site. Top, RT-PCR was performed with total RNA of cell cultures inoculated with nasal swab material (RNeasy; Qiagen). RNAs from recombinant viruses or from strain CA-1 were reverse transcribed using a positive-sense primer which was complementary to the BRSV F gene (ATue51908; nt 7218 to 7240). An aliquot of the first-strand cDNA was used for PCR with the first-strand primer and a BRSV M2-specific primer (ATue51908; nt 7852 to 7832, negative sense). RT-PCR products were analyzed on 3% agarose gels. The RT-PCR products were consistent with the predicted sizes. Digestion with XhoI yielded the expected fragments for rBRSV. As expected, the RT-PCR products resulting from the challenge BRSV, strain CA-1, remained uncleaved. The bottom panel gives a schematic overview of the locations of primers and XhoI marker restriction site, with the sizes of the resulting fragments indicated. control, RT-PCR performed on total RNA of cells infected with rBRSV.
rBRSVΔG was chosen for an immunization-challenge experiment because glycoprotein G seems to be linked to RSV immunopathogenesis (10, 11, 12). To date, there are several animal studies aiming at a deeper understanding of the HRSV immunopathogenesis induced by a formalin-inactivated vaccine (9, 24, 34, 37; also reviewed in reference 22), which was first dramatically revealed during clinical trials about 30 years ago (13, 16). With respect to RSV immunopathogenesis studies, the rBRSV model system might represent an alternative to the mouse model, which is somewhat artificial due to the low level of susceptibility of mice to HRSV (27). In the experiment presented here, the protection induced by rBRSV was relatively complete, with the challenge virus replication being below the level of further antibody induction. Due to the low level or absence of challenge virus replication and clinical signs in both immunized groups, immunological parameters were not further studied in this experiment.
It may be assumed that, if not replication, at least mucosal antigen expression was achieved after intranasal immunization with rBRSVΔG, at an expression level which is sufficient to induce an immune response. By intranasal immunization of calves with large amounts of UV-inactivated BRSV, it is not possible to induce a serum antibody response, nor is it possible to reduce shedding of challenge virus (17). For HRSV, there are two reports of recombinant HRSV with a similar deletion of the G gene (29, 30), which both describe a reduced in vitro growth of the deletion mutants in HEp-2 cells. One of the studies also describes a strong attenuation of the G deletion mutant in the mouse model (30). Moreover, an HRSV SH/G deletion mutant of serotype B (HRSV B1 cp-52) was similarly found not to replicate well in vivo nor to induce a neutralizing antibody response in human volunteers after intranasal administration (15). This HRSV mutant was generated by cold passage, and it contains several additional mutations with putative additional phenotypic effects. Since rBRSV and rBRSVΔG differ exclusively in the deletion of the G gene, this work gives evidence that BRSV G is essential for BRSV replication in vivo. Using the BRSV reverse-genetics system, we currently determine which of the several known functional features of the G protein (8, 30, 31) play an important in vivo role.
For HRSV, the antibody response to G was found to be significantly related to protection in young infants under 2 months of age (38). In light of these findings, a preferable approach to developing a recombinant live vaccine against BRSV would be to preserve the antigenic epitopes of the G protein and to remove any functional features linked to immunopathogenesis. However, with regard to protection, it has to be taken into account that the antigenic epitopes of RSV G proteins are highly variable. For BRSV, G escape mutants were found to circulate in the field after a period of broadly applied vaccines (32). The work presented here shows that by mucosal application of a live vaccine, protection can also be achieved using a deletion mutant lacking the G gene, which emphasizes the role of mucosal immunity and of neutralizing antibodies directed to BRSV F in calves and might be beneficial with respect to the suspected role of the G protein for vaccine-induced immunopathogenesis. Taken together, this work represents the first characterization of recombinant BRSV in cattle, and the results imply that it will be possible to develop a live-attenuated vaccine based on rBRSV.
Acknowledgments
We thank Geraldine Taylor, Compton, United Kingdom, for a gift of monoclonal antibodies. We thank Axel Karger for helpful comments on the manuscript. We also thank Kathrin Schuldt for perfect technical assistance.
This work was supported by Intervet International B.V., The Netherlands.
REFERENCES
- 1.Bryson, D. G., M. S. McNulty, E. F. Logan, and P. F. Cush. 1983. Respiratory syncytial virus pneumonia in young calves: clinical and pathologic findings. Am. J. Vet. Res. 44:1648-1655. [PubMed] [Google Scholar]
- 2.Buchholz, U. J., S. Finke, and K.-K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchholz, U. J., H. Granzow, K. Schuldt, S. S. Whitehead, B. R. Murphy, and P. L. Collins. 2000. Chimeric bovine respiratory syncytial virus with glycoprotein gene substitution from human respiratory syncytial virus (HRSV): effects on host range and evaluation as a live-attenuated HRSV vaccine. J. Virol. 74:1187-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castleman, W. L., J. C. Lay, E. J. Dubovi, and D. O. Slauson. 1985. Experimental bovine respiratory syncytial virus infection in conventional calves. Light microscopic lesions, microbiology, and studies on lavaged lung cells. Am. J. Vet. Res. 46:547-553. [PubMed] [Google Scholar]
- 5.Collins, J. K., R. M. Teegarden, D. W. MacVean, Salman, G. H. Smith, and G. R. Frank. 1988. Prevalence and specificity of antibodies to bovine respiratory syncytial virus in sera from feedlot and range cattle. Am. J. Vet. Res. 49:1316-1319. [PubMed] [Google Scholar]
- 6.Collins, P. L., R. M. Chanock, and B. R. Murphy. 2001. Respiratory syncytial virus, p. 1433-1485. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 7.Ellis, J. A., K. West, C. Konoby, T. Leard, G. Gallo, J. Conlon, and N. Fitzgerald. 2001. Efficacy of an inactivated respiratory syncytial virus vaccine in calves. J. Am. Vet. Med. Assoc. 218:1973-1980. [DOI] [PubMed] [Google Scholar]
- 8.Feldman, S. A., R. M. Hendry, and J. A. Beeler. 1999. Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G. J. Virol. 73:6610-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gershwin, L. J., E. S. Schelegle, R. A. Gunther, M. L. Anderson, A. R. Woolums, D. R. Larochelle, G. A. Boyle, K. E. Friebertshauser, and R. S. Singer. 1998. A bovine model of vaccine enhanced respiratory syncytial virus pathophysiology. Vaccine 16:1225-1236. [DOI] [PubMed] [Google Scholar]
- 10.Hancock, G. E., D. J. Speelman, K. Heers, E. Bortell, J. Smith, and C. Cosco. 1996. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J. Virol. 70:7783-7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson, T. R., J. E. Johnson, S. R. Roberts, G. W. Wertz, R. A. Parker, and B. S. Graham. 1998. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J. Virol. 72:2871-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, T. R., and B. S. Graham. 1999. Secreted respiratory syncytial virus G glycoprotein induces interleukin-5 (IL-5), IL-13, and eosinophilia by an IL-4-independent mechanism. J. Virol. 73:8485-8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapikian, A. Z., R. H. Mitchell, R. M. Chanock, R. A. Shevdoff, and C. E. Stewart. 1968. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 89:405-413. [DOI] [PubMed] [Google Scholar]
- 14.Karger, A., U. Schmidt, and U. J. Buchholz. 2001. Recombinant bovine respiratory syncytial virus with deletions of the G or SH genes: G and F proteins bind heparin. J. Gen. Virol. 82:631-640. [DOI] [PubMed] [Google Scholar]
- 15.Karron, R. A., D. A. Buonagurio, A. F. Georgiu, S. S. Whitehead, J. E. Adamus, M. L. Clements-Mann, D. O. Harris, V. E. Randolph, S. A. Udem, B. R. Murphy, and M. S. Sidhu. 1997. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc. Natl. Acad. Sci. USA 94:13961-13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, H. W., J. G. Canchola, C. D. Brandt, G. Pyles, R. M. Chanock, K. Jensen, and R. H. Parrott. 1969. Respiratory synctial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422-434. [DOI] [PubMed] [Google Scholar]
- 17.Kimman, T. G., F. Westenbrink, and P. J. Straver. 1989. Priming for local and systemic antibody memory responses to bovine respiratory syncytial virus: effect of amount of virus, virus replication, route of administration and maternal antibodies. Vet. Immunol. Immunopathol. 22:145-160. [DOI] [PubMed] [Google Scholar]
- 18.Langedijk, J. P. M., W. M. M. Schaaper, R. H. Meloen, and J. T. van Oirschot. 1996. Proposed three-dimensional model for the attachment protein G of respiratory syncytial virus. J. Gen. Virol. 77:1249-1257. [DOI] [PubMed] [Google Scholar]
- 19.Larsen, L. E., C. Tegtmeier, and E. Pedersen. 2001. Bovine Respiratory Syncytial Virus (BRSV) pneumonia in beef calf herds despite vaccination. Acta Vet. Scand. 42:113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lerch, R. A., K. Anderson, and G. W. Wertz. 1990. Nucleotide sequence analysis and expression from recombinant vectors demonstrate that the attachment protein G of bovine respiratory syncytial virus is distinct from that of human respiratory syncytial virus. J. Virol. 64:5559-5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohanty, S. B., M. G. Lillie, and A. L. Ingling. 1976. Effect of serum and nasal neutralizing antibodies on bovine respiratory syncytial virus infection in calves. J. Infect. Dis. 134:409-413. [DOI] [PubMed] [Google Scholar]
- 22.Openshaw, P. J. M., F. J. Culley, and W. Olszewska. 2002. Immunopathogenesis of vaccine-enhanced RSV disease. Vaccine 20:S27-S31. [DOI] [PubMed] [Google Scholar]
- 23.Paccaud, M. F., and C. Jacquier. 1970. A respiratory syncytial virus of bovine origin. Arch. Gesamte Virusforsch. 30:327-342. [DOI] [PubMed] [Google Scholar]
- 24.Prince, G. A., S. J. Curtis, K. C. Yim, and D. D. Porter. 2001. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J. Gen. Virol. 82:2881-2888. [DOI] [PubMed] [Google Scholar]
- 25.Stope, M. B., A. Karger, U. Schmidt, and U. J. Buchholz. 2001. Chimeric bovine respiratory syncytial virus with attachment and fusion glycoproteins replaced by bovine parainfluenza virus type 3 hemagglutinin-neuraminidase and fusion proteins. J. Virol. 75:9367-9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stott, E. J., and G. Taylor. 1985. Respiratory syncytial virus. Arch. Virol. 84:1-52. [DOI] [PubMed] [Google Scholar]
- 27.Taylor, G., E. J. Stott, M. Hughes, and A. P. Collins. 1984. Respiratory syncytial virus infection in mice. Infect. Immun. 43:649-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor, G., L. H. Thomas, J. M. Furze, R. S. Cook, S. G. Wyld, R. Lerch, R. Hardy, and G. W. Wertz. 1997. Recombinant vaccinia viruses expressing the F, G, or N, but not the M2, protein of bovine respiratory syncytial virus (BRSV) induce resistance to BRSV challenge in the calf and protect against the development of pneumonic lesions. J. Gen. Virol. 78:3195-3206. [DOI] [PubMed] [Google Scholar]
- 29.Techaarpornkul, S., N. Barretto, and M. E. Peeples. 2001. Functional analysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene. J. Virol. 75:6825-6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teng, M. N., S. S. Whitehead, and P. L. Collins. 2001. Contribution of the respiratory syncytial virus G glycoprotein and its secreted and membrane-bound forms to virus replication in vitro and in vivo. Virology 289:283-296. [DOI] [PubMed] [Google Scholar]
- 31.Tripp, R. A., L. P. Jones, L. M. Haynes, H. Q. Zheng, P. M. Murphy, and L. J. Anderson. 2001. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat. Immunol. 8:732-738. [DOI] [PubMed] [Google Scholar]
- 32.Valarcher, J. F., F. Schelcher, and H. Bourhy. 2000. Evolution of bovine respiratory syncytial virus. J. Virol. 74:10714-10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van der Poel, W. H. M., A. Brand, J. A. Kramps, and J. T. van Oirschot. 1994. Respiratory syncytial virus infections in human beings and in cattle. J. Infect. 29:215-228. [DOI] [PubMed] [Google Scholar]
- 34.West, K., L. Petrie, D. M. Haines, C. Konoby, E. G. Clark, K. Martin, and J. A. Ellis. 1999. The effect of formalin-inactivated vaccine on respiratory disease associated with bovine respiratory syncytial virus infection in calves. Vaccine 17:809-820. [DOI] [PubMed] [Google Scholar]
- 35.West, K., L. Petrie, C. Konoby, D. M. Haines, V. S. Cortese, and J. A. Ellis. 2000. The efficacy of modified-live bovine respiratory syncytial virus vaccines in experimentally infected calves. Vaccine 18:907-919. [DOI] [PubMed] [Google Scholar]
- 36.Woolums, A. R., M. L. Anderson, R. A. Gunther, E. S. Schelegle, D. R. Larochelle, R. S. Singer, G. A. Boyle, K. E. Friebertshauser, and L. J. Gershwin. 1999. Evaluation of severe disease induced by aerosol inoculation of calves with bovine respiratory syncytial virus. Am. J. Vet. Res. 60:473-480. [PubMed] [Google Scholar]
- 37.Woolums, A. R., R. S. Singer, G. A. Boyle, and L. J. Gershwin. 1999. Interferon gamma production during bovine respiratory syncytial virus (BRSV) infection is diminished in calves vaccinated with formalin-inactivated BRSV. Vaccine 17:1293-1297. [DOI] [PubMed] [Google Scholar]
- 38.Wright, P. F., R. A. Karron, R. B. Belshe, J. Thompson, J. E. Crowe, Jr., T. G. Boyce, L. L. Halburnt, G. W. Reed, S. S. Whitehead, E. L. Anderson, A. E. Wittek, R. Casey, M. Eichelberger, B. Thumar, V. B. Randolph, S. A. Udem, R. M. Chanock, and B. R. Murphy. 2000. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J. Infect. Dis. 182:1331-1342. [DOI] [PubMed] [Google Scholar]