Abstract
Neonates are thought to mount less vigorous adaptive immune responses than adults to antigens and infectious agents. This concept has led to a delay in the administration of many currently available vaccines until late infancy or early childhood. It has recently been shown that vaccines composed of plasmid DNA can induce both humoral and cell-mediated antimicrobial immunity when administered within hours of birth. In most of these studies, immune responses were measured weeks or months after the initial vaccination, and it is therefore questionable whether the observed responses were actually the result of priming of splenocytes within the neonatal period. Here we show that DNA vaccination at birth results in the rapid induction of antigen-specific CD8+ T cells within neonatal life. Analyses of T-cell effector functions critical for the resolution of many viral infections revealed that neonatal and adult CD8+ T cells produce similar arrays of cytokines. Furthermore, the avidities of neonatal and adult CD8+ T cells for peptide and the rapidity with which they upregulate cytokine production after recall encounters with antigen are similar. Protective immunity against the arenavirus lymphocytic choriomeningitis virus, which is mediated by CD8+ cytotoxic T cells, is also rapidly acquired within the neonatal period. Collectively these data imply that, at least in the case of CD8+ T cells, neonates are not as immunodeficient as previously supposed and that DNA vaccines may be an effective and safe means of providing critical cell-mediated antiviral immunity extremely early in life.
Despite major successes in the development and worldwide distribution of effective antiviral vaccines, viral infections are still responsible for significant global morbidity and mortality, particularly among infants and young children. Cell-mediated immune responses play an essential role in the clearance of many viruses in vivo, and numerous in vitro studies in both humans and mice have uncovered specific age-related functional defects in the responses of neonatal T and B cells (for a review, see reference 1). Following antigen exposure early in life, both murine and human CD4+ T cells preferentially develop into interleukin-4-secreting Th2 effector cells, a situation thought to limit their ability to promote the development of strong cytotoxic CD8+-T-cell responses (20). Despite these observations, evidence exists for the development early in life of CD8+-T-cell responses to microbial infections in both human and murine infants (8, 21, 24, 25, 30).
To successfully combat viral infections in vivo, primary and memory CD8+ T cells express a variety of effector functions, including the production of proinflammatory cytokines, such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), and the direct killing of virus-infected cells via perforin- or Fas-dependent pathways (15). All of these processes operate in concert to limit viral spread within the infected host. However, in the case of the very young, it has not yet been determined if antigen-experienced CD8+ T cells induced by vaccination can express the vigorous effector functions associated with adult CD8+ T cells. If neonatal CD8+ T cells are functionally competent at birth, then vaccines that rapidly mobilize these responses in vivo could provide the neonate with a significant level of protective immunity early in life, potentially reducing the severity of common childhood viral infections. If, on the other hand, the neonatal CD8+-T-cell compartment is less able to respond functionally to antigens than adult cells, prudence may dictate a delay in vaccination until more mature adult-like responses can develop. Using a highly sensitive intracellular-cytokine-staining assay for effector T-cell function, we have examined both the ability of DNA vaccines to prime CD8+-T-cell responses within the neonatal period and the expression of effector functions by recently primed neonatal and adult CD8+ T cells.
DNA vaccination early in life has been shown to be an effective means of priming antiviral immunity against a variety of viruses in animal models (5-7, 22, 23, 27, 31, 35, 36). A single intramuscular inoculation of plasmid DNA expressing the nucleoprotein gene from lymphocytic choriomeningitis virus (LCMV) has previously been shown to result in the rapid priming of virus-specific CD8+ T cells in adults and the associated acquisition of protective immunity against both systemic and intracranial LCMV infections (16). It has also been previously shown that CD8+ memory T cells primed in response to a DNA-encoded antigen administered either in adulthood or within hours of birth remain detectable directly ex vivo for at least 1 year postvaccination. These cells contain perforin and produce both IFN-γ and TNF-α upon peptide stimulation, a phenotype reminiscent of T cells induced by LCMV infection (32). In addition, protective immunity against LCMV, which is mediated by antigen-specific CD8+ T cells, can be maintained in adults vaccinated as neonates for up to 1 year in vivo in the absence of boosting (32). Thus, DNA vaccination early in life primes a biologically active population of CD8+ T cells that are functionally similar to CD8+ T cells primed by viral infection in adulthood. However, plasmid DNA persists for weeks or months in vivo (9, 38), and it is possible that the CD8+-T-cell responses observed in adults vaccinated as neonates were primed after the neonatal period. Here, we have addressed this issue by examining antigen-specific CD8+-T-cell responses in neonatal mice within days of vaccination in order to identify what functional similarities exist between cells primed during neonatal life and those primed in adulthood. Lastly, and more importantly, we also wished to determine if DNA-vaccinated neonates acquire protective immunity within the neonatal period.
MATERIALS AND METHODS
Mice.
BALB/c and CB6 (C57BL/6 female × BALB/c male) mice were purchased from the Scripps Research Institute animal facility. Pregnant females from timed matings were monitored from 19 to 22 days postinsemination, and the date of birth was recorded as day zero. All neonates were immunized within 48 h of birth. The animals were housed in specific-pathogen-free environments and used in accordance with institutional and National Institutes of Health guidelines governing the humane care and use of laboratory animals.
Viruses and viral infections.
Stocks of the Armstrong strain of LCMV were grown on BHK cells in RPMI containing 10% fetal bovine serum (FBS), 50 U of penicillin G/liter, 50 μg of streptomycin/liter, and 20 mM l-glutamine (all from Gibco BRL, Rockville, Md.). To analyze cytotoxic-T-lymphocyte (CTL) responses or the acquisition of protective immunity against systemic infection, mice were infected intraperitoneally 14 days post-DNA vaccination with 2.5 × 105 PFU of LCMV. Four days postinfection, the spleens were removed and either processed as described below for analysis of CTL activity or snap frozen in liquid nitrogen for later virus titration. LCMV titers were determined by plaque assay on Vero cell monolayers as previously described (18).
CTL assays.
Single-cell suspensions of splenocytes depleted of red blood cells by ammonium chloride lysis were washed in complete RPMI (RPMI containing 50 U of penicillin-G/liter, 50 μg of streptomycin sulfate/liter, and 20 mM l-glutamine) supplemented with 5% bovine serum and resuspended at a final concentration of 107 cells/ml in complete RPMI containing 10% FBS. Serial twofold dilutions were prepared in the same medium, and 100 μl of these effector cells was incubated in triplicate at 37°C with 2 × 104 peptide-pulsed or unpulsed 51Cr-radiolabeled major histocompatibility complex (MHC)-matched (BALB/c17; H-2d) or MHC-mismatched (MC57; H-2b) target cells. After a 5-h incubation, 100 μl of supernatant was removed from each well and assayed for radioactivity. The percent specific lysis was calculated as (experimental release − spontaneous release) × 100/(total release − spontaneous release). Values for total release and spontaneous release were obtained by incubating triplicate wells of target cells alone in the presence (total) or absence (spontaneous) of 1% Nonidet P-40 (Sigma, St. Louis, Mo.).
DNA vaccinations and plasmid DNA.
The plasmid pCMVNP encodes the full-length LCMV nucleoprotein (Armstrong strain); pCMV, the vector control, contains no LCMV sequences. The construction of these plasmids has been previously described (39). The plasmids were propagated in Escherichia coli and purified using an Endofree plasmid purification kit (Qiagen, Chatsworth, Calif.) according to the manufacturer's instructions. Adult mice received bilateral 50-μl injections of plasmid DNA in 1 N saline (1 to 2 μg/μl; 100 to 200 μg/mouse) into the anterior tibialis muscles. Neonatal mice (<48 h old) were injected with 25 to 50 μl of DNA (2 μg/μl; 100 to 200 μg/mouse) in each upper thigh.
Intracellular cytokine staining and flow cytometric analysis of antigen-specific T-cell responses.
Intracellular-cytokine staining was done essentially as previously described (34) on 2 × 106 to 4 × 106 splenocytes/well in 96-well plates after a 5.5-h incubation in 200 μl of RPMI containing 10% FBS, 20 mM HEPES, and 2 μg of brefeldin A/ml in the presence of a peptide corresponding to the immunodominant H-2d-restricted CD8+-T-cell epitope in the LCMV nucleoprotein (RPQASGVYM; amino acids 118 to 126; final concentration [unless otherwise stated], 10−7 M). Background cytokine staining was determined by incubating cells in the same medium in the absence of peptide. After stimulation, the cells were washed with phosphate-buffered saline (PBS) plus 5% FBS, stained overnight with an anti-mouse CD8 cychrome-conjugated antibody (clone 53-6.7), fixed in cold PBS plus 2% formaldehyde, and permeabilized with 0.1% saponin in PBS plus 1% FBS. Intracellular cytokines were detected with anti-mouse IFN-γ-fluorescein isothiocyanate (clone XMG1.2) and anti-mouse TNF-α-phycoerythrin (clone MP6-XT22) antibodies. All antibodies were purchased from either BD PharMingen (San Diego, Calif.) or Caltag Laboratories (Burlingame, Calif.). The stained cells were collected using a FACScan flow cytometer (Becton Dickinson, Oxnard, Calif.) and analyzed with CellQuest version 3.1f software. Only the responses among the live CD8+ population are shown. The percentage of peptide-specific CD8+ T cells was calculated by subtracting the percentage of cytokine-positive CD8+ T cells detected in the absence of peptide (<0.2%) from the percentage of cytokine-positive CD8+ T cells detected in the presence of peptide.
RESULTS
The T-cell compartment of neonatal mice develops slowly after birth.
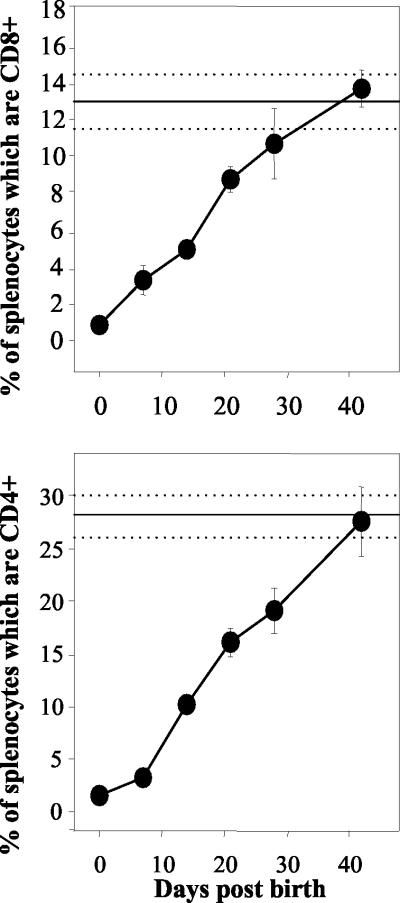
The limited number of mature peripheral T cells and antigen-presenting cells present at birth has been postulated to account for the poor ability of newborns to respond to vaccination and infections (12, 13, 28). Our analyses of the percentages of CD4+ and CD8+ lymphocytes in the murine spleen as a function of age (Fig. 1) shows that the splenic T-cell compartment in neonatal mice develops very slowly after birth. To take into account the considerable differences between the total numbers of splenocytes in adults and neonates, these analyses have focused on the percentages of individual subpopulations of T cells rather than their absolute numbers. Splenocytes from CB6 mice expressing CD8 (Fig. 1, upper panel) or CD4 (Fig. 1, lower panel) were quantified by flow cytometry at 0, 7, 14, 21, 27, and 42 days after birth. The average percentages of CD8 and CD4 lymphocytes in adult spleens, measured concurrently, are represented in each panel with standard deviations.
FIG. 1.
The T-cell compartment of neonatal mice develops slowly after birth. Splenocytes (106) from individual mice were stained with anti-CD8-cychrome (upper panel) and anti-CD4-phycoerythrin (lower panel) antibodies and analyzed by flow cytometry at 0, 7, 14, 21, 28, and 42 days after birth. The solid and dotted horizontal lines represent the combined averages and standard deviations for three adult mice (>6 weeks old) analyzed concurrently at each time point. The error bars indicate standard deviations.
Among naïve adults, CD8+ and CD4+ T cells constituted 13 and 30% of total splenocytes, respectively. In contrast, <1% of spleen cells expressed the CD8 T-cell marker at birth. Over time, a steady increase in the percentage of CD8+ T cells was observed: 3% at week 1, 5% at week 2, and 9% by week 3. The percentage of splenocytes expressing detectable CD8 did not reach the adult level until the time of weaning at 4 weeks after birth. Analysis of neonatal CD4+ cells revealed a similar pattern. At birth, only 1.4% of splenocytes expressed CD4. Thereafter, the percentage of CD4+ lymphocytes increased to 19% by week 4 and reached adult levels between 5 and 6 weeks after birth. It is therefore evident from these data that at the time of DNA vaccination, 0 to 48 h after birth, and for a considerable period afterward, there are clear age-related differences in the makeup of neonatal and adult murine spleens. The relative scarcity of naïve neonatal CD8+ and CD4+ T cells in the spleen could have a profound effect on how neonatal mice respond to vaccination or infection.
Neonatally vaccinated mice mount rapid antigen-specific T-cell responses.
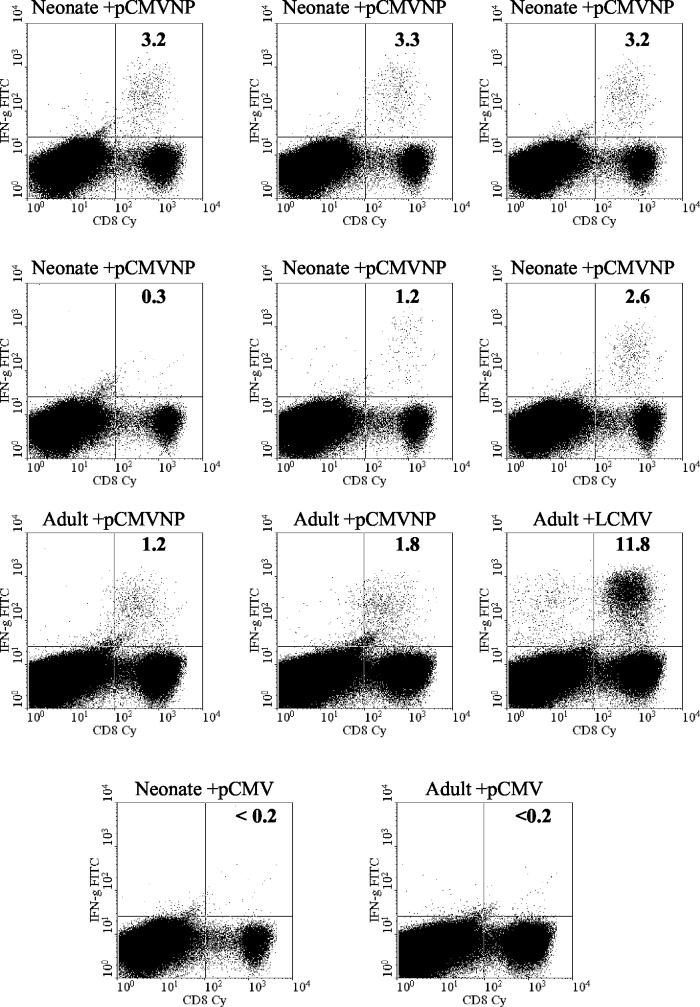
Using a highly sensitive intracellular-cytokine-staining assay (ICCS) to identify antigen-specific T cells, it was previously shown that CD8+-T-cell responses in DNA-immunized adults peak between 11 and 15 days postvaccination, in contrast to the maximal response to viral infection, which is reached 7 to 8 days postinfection (3, 16). To determine if antigen-specific neonatal CD8+ T cells induced by DNA vaccination could be detected directly ex vivo in the spleen within the neonatal period (from 0 to 2 weeks after birth), groups of neonates were vaccinated on the day of birth or at 6 weeks of age with 100 μg of the LCMV nucleoprotein-expressing plasmid pCMVNP or the vector control pCMV, respectively. Two weeks later (14 days after birth), splenic CD8+ T cells were analyzed by ICCS for IFN-γ production using an H-2d-restricted CD8+-T-cell epitope peptide corresponding to amino acids 118 to 126 within the LCMV nucleoprotein (NP118-126) (37). As a control, adult mice (6 weeks of age) received the same DNAs. The peptide-specific responses of six representative pCMVNP-vaccinated neonates, two adults, an adult infected with LCMV 360 days before, and one neonate and one adult immunized with the vector pCMV are shown in Fig. 2. One year after LCMV infection, >10% of splenic CD8+ T cells produced IFN-γ when stimulated by NP118-126 peptide. As expected from our previously published data, peptide-specific responses were also detectable in pCMVNP-immunized adults 2 weeks postimmunization and, in this experiment, constituted 1.2 and 1.8% of the total CD8+-T-cell pool. CD8+ T cells specific for NP118-126 were also detectable in five of the six vaccinated neonates within the first 2 weeks of life. The responses among the neonates were quantitatively similar to those observed in the adults, ranging from 1.2 to 3.3% of total CD8+ T cells. This indicates that neonates can mount a robust primary response to a single injection of plasmid DNA shortly after birth. Only background levels of cytokine staining (<0.2% of CD8+ T cells) were observed in neonates or adults vaccinated with the vector control plasmid pCMV.
FIG. 2.
Neonatally vaccinated mice mount rapid antigen-specific T-cell responses. Splenocytes (4 × 106) from individual pCMVNP- or pCMV-immunized BALB/c neonates or adults were assayed by ICCS 14 days postimmunization for the presence of IFN-γ after 5.5 h of stimulation with the immunodominant H-2d-specific NP peptide NP118-126 as described in Materials and Methods. An adult infected with LCMV 360 days before was included as a positive control. The percentage of CD8+ T cells producing IFN-γ is shown in each upper right quadrant. FITC, fluorescein isothiocyanate; Cy, cychrome.
Thus, neonatal CD8+ T cells present within the spleen during the first 2 weeks of life are capable of responding to DNA vaccines and express at least one of the effector functions associated with adult antigen-specific T cells, production of IFN-γ upon peptide stimulation. Our ability to detect these cells so early in life provides us with the unique opportunity to examine their functional competence directly ex vivo.
Neonatal and adult T cells undergo similar maturations in cytokine expression patterns.
Cytokines such as IFN-γ and TNF-α have potent antiviral effects (reviewed in reference 33). It has recently been established that, during the course of a systemic LCMV infection in adult mice, the pattern of cytokines produced by the virus-specific CD8+-T-cell pool changes as the response matures from the acute to the memory stage (32). Prior to, and at the peak of, the acute response to LCMV, two discrete populations of antigen-specific CD8+ T cells can be observed; roughly half of the virus-specific CD8+ T cells secrete only IFN-γ upon in vitro stimulation with viral peptides, whereas the other half produce both IFN-γ and TNF-α. By day 11, over 80% of antigen-specific CD8+ T cells are secreting both cytokines, and at 30 days postinfection, ∼95% of the antigen-specific cells are double positive. A similar change in cytokine expression patterns has also been observed in adult T cells primed either by DNA vaccination or infection with a recombinant vaccinia virus expressing the LCMV nucleoprotein (16).
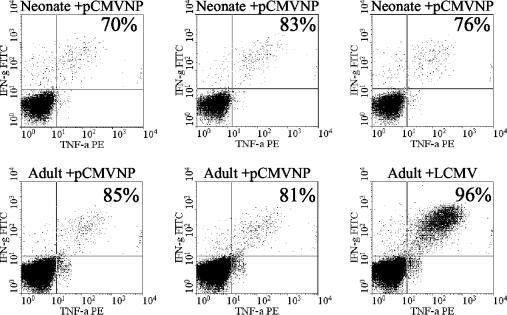
In light of our previous demonstration of the pattern of cytokine synthesis by adult memory T cells induced by DNA vaccination, we wished to determine if neonatal T cells primed by DNA vaccination showed the same pattern of cytokine expression as adult cells. Therefore, the IFN-γ and TNF-α expression profiles of antigen-specific CD8+ T cells present in the spleens of neonates and adults 14 days post-vaccination with pCMVNP were compared. Splenocytes from three of the neonates and two of the pCMVNP-vaccinated adults and the LCMV-infected adult control shown in Fig. 2 were subjected to ICCS and stained with antibodies specific for murine CD8, IFN-γ, and TNF-α. Live cells were then gated for CD8 expression and analyzed for intracellular IFN-γ and TNF-α (Fig. 3). In pCMVNP-immunized adult mice, 81 to 85% of the peptide-specific CD8+ T cells were double positive for cytokine secretion, as were 96% of the responding cells in the LCMV-infected adult 360 days postinfection (Fig. 3, upper right quadrants). These data are consistent with previously published results (16). The majority (70 to 83%) of antigen-specific neonatal CD8+ T cells were also capable of producing both IFN-γ and TNF-α 14 days after birth. Thus, the neonatal environment does not restrict the ability of antigen-specific CD8+ T cells to rapidly develop into effector cells that express two of the cytokines associated with adult, virus-specific memory T cells, IFN-γ and TNF-α.
FIG. 3.
Neonatal and adult T cells undergo similar maturations in cytokine expression patterns. Splenocytes (4 × 106/well) were stimulated with peptide NP118-126 and subjected to ICCS as described in Materials and Methods. Live cells were gated for the expression of CD8 and analyzed for the presence of TNF-α and IFN-γ (x and y axis, respectively). The TNF-α-IFN-γ double-positive cells in each sample are all shown within the upper right quadrant. The percentage in each panel represents the fraction of cells within the upper right quadrant as a function of the sum of the number of cells within the upper left and upper right quadrants. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Antigen-specific neonatal and adult T cells have similar avidities for peptide.
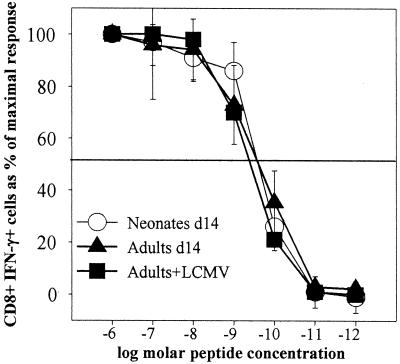
Neonatal mice do not express the enzyme terminal deoxynucleotidyl transferase (TdT) until approximately 7 to 9 days after birth (4). TdT is responsible for the untemplated addition of nucleotides to germ line T-cell and B-cell receptor sequences during their recombination and is therefore necessary for maximizing the diversity of the T- and B-cell repertoires. These untemplated nucleotides are confined to the CDR3 loop and are predicted to make important contacts with the peptide MHC complex (11). Using a TdT knockout mouse model as a surrogate for neonatal T cells, Gavin and Bevan have previously shown that T-cell clones maturing in the absence of TdT have altered peptide specificities but similar affinities for peptide MHC ligands compared to clones derived from wild-type mice (14). However, a direct comparison of the relative avidities of nontransgenic polyclonal neonatal and adult antigen-specific T cells has not yet been done. The ICCS assay now permits us to analyze the relative avidity of the antigen-specific T-cell pool among adult and neonatal DNA-vaccinated mice directly ex vivo. To determine if the antigen-specific CD8+ cells present in neonates and adults at the peak of the DNA vaccine response have similar avidities, three neonatal and three adult mice were immunized with 200 μg of pCMVNP, and 14 days later, splenocytes from individual mice were analyzed for IFN-γ production by ICCS in the presence of different concentrations of NP118-126 peptide. An adult mouse infected with LCMV 14 days before served as a positive control. The percentage of cytokine secreting CD8+ T cells observed at the highest concentration of peptide used (10−6 M) was defined as 100%, and responses observed at lower concentrations of peptide were expressed as percentages of this maximal response. The average responses in groups of adults and neonates are shown in Fig. 4. The avidity profiles of the three DNA-immunized neonates are similar to the avidity profiles observed in both the DNA-immunized and the LCMV-infected adult mice, with a half-maximal response at about 10−9 M. Under the conditions employed in this assay, the pool of antigen-specific CD8+ T cells present in adult and neonatal spleens 14 days postimmunization do not differ in their relative abilities to produce IFN-γ in the presence of limiting amounts of peptide antigen.
FIG. 4.
Antigen-specific neonatal and adult T cells have similar avidities for peptide. Splenocytes from individual BALB/c mice were obtained 14 days postvaccination with pCMVNP or postinfection with LCMV, stimulated for 5.5 h in vitro in the presence of various concentrations of NP118-126, stained for CD8, and then subjected to ICCS for intracellular IFN-γ as described in Materials and Methods. The average responses (±1 standard deviation) of groups of three neonates (Neonates d14), three adults (Adults d14), and one adult infected with LCMV (Adults+LCMV), each measured in triplicate at each peptide concentration, are shown. For each group, the response at each peptide concentration is expressed as the maximal response (measured at 1,000 nM peptide). The half-maximal response is shown as a solid horizontal line.
Neonatal and adult T cells rapidly mobilize cytokine production in response to peptide stimulation.
The ability to rapidly upregulate cytokine production after T-cell receptor contact with the appropriate MHC-peptide combination is one of the hallmarks of both acute effector and memory CD8+ T cells in adults. Neither TNF-α nor IFN-γ is produced constitutively; T-cell receptor triggering by a peptide-MHC complex is required to initiate and maintain cytokine production in an antigen-specific manner (32). To determine if neonatal CD8+ T cells can initiate the production of proinflammatory cytokines as quickly as adult antigen-specific CD8+ T cells, splenocytes from pCMVNP-vaccinated neonates and adults were stimulated with NP118-126 peptide for various lengths of time and then were analyzed for intracellular IFN-γ by ICCS. The results were normalized for each mouse and are expressed in Fig. 5 as the percentage of the maximal response 8 h poststimulation.
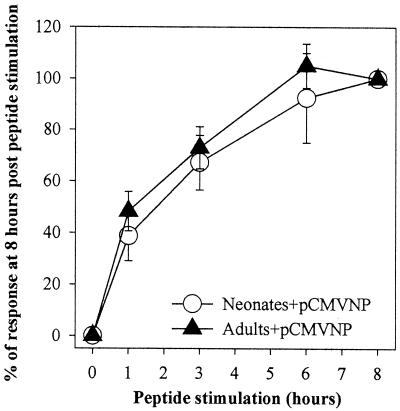
FIG. 5.
Neonatal and adult T cells rapidly mobilize cytokine production in response to peptide stimulation. Splenocytes from individual neonatal (Neonates+pCMVNP; n = 3) or adult (Adults+pCMVNP; n = 2) BALB/c mice were obtained 14 days after immunization with 100 μg of pCMVNP. Cells from each individual were stimulated in vitro for 0 to 8 h. Cells (4 × 106) were harvested at each time point, stained with anti-CD8 antibody, and subjected to ICCS as described in Materials and Methods. The average response among groups at each time point is expressed as a percentage of the average response (±1 standard deviation) observed among individuals within that group after 8 h of peptide stimulation.
As expected, the lack of detectable IFN-γ in either neonate or adult splenocytes directly ex vivo (hour zero) indicates that neither population produces this cytokine constitutively. However, within 1 h of peptide stimulation, IFN-γ was clearly detectable in a subpopulation of peptide-stimulated CD8+ T cells. At this time 40% of the antigen-specific cells in both neonates and adults produced detectable levels of IFN-γ. This was followed by a steady linear increase in the percentage of detectable cytokine-producing cells in both neonates and adults for at least the first 6 h of peptide stimulation. Moreover, at each time point examined, the relative percentages of IFN-γ-positive CD8+ T cells among pCMVNP-vaccinated neonates and adults were similar, implying similar kinetics of IFN-γ induction among antigen-specific CD8+ neonatal and adult T cells.
Protective immunity against viral challenge is acquired during the neonatal period.
We have demonstrated that neonatal mice vaccinated with plasmid DNA can mount robust adult-like CD8+-T-cell responses which can be detected directly ex vivo within the neonatal period. To determine if these cells can also confer protective immunity against viral challenge, mice were immunized on the day after birth with 100 μg of either pCMVNP or the vector control pCMV and 14 days later were challenged intraperitoneally with 2.5 × 105 PFU of LCMV. Infectious virus was then measured in the spleens of these mice 4 days later. Two pCMVNP-vaccinated adult mice and groups of two unvaccinated adults analyzed 8 and 4 days postinfection were included as positive and negative controls. The results of this experiment are shown in Fig. 6.
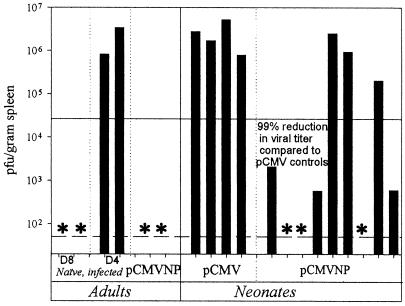
FIG. 6.
Protective immunity against viral challenge is acquired during the neonatal period. Groups of BALB/c neonates were immunized within 48 h of birth with 100 μg of pCMV or pCMVNP. To compare neonatal and adult responses, two adults were immunized concurrently with 100 μg of pCMVNP. All mice were challenged intraperitoneally 14 days later with 2.5 × 105 PFU of LCMV Armstrong. Spleen samples were titered for infectious virus on Vero cell monolayers 4 days postinfection as described in Materials and Methods. To evaluate viral clearance, two adults infected 8 and 4 days prior to the assay were included as positive and negative controls, respectively. The solid horizontal line represents a 99.9% reduction in splenic viral titers 4 days postinfection compared to the titers among pCMV-vaccinated neonates. The dashed line indicates the sensitivity of the plaque assay, and the asterisks represent single mice for which no recoverable virus could be demonstrated in the spleen.
In naïve adult mice, intraperitoneal injection of the Armstrong strain of LCMV leads to a disseminated systemic infection and high titers of virus in the spleen 4 days postinjection (Fig. 6, lane D4). The induction of lytic CD8+-T-cell responses within immunocompetent adults allows them to largely clear the virus from their spleens 4 days later, by 8 days postinfection (Fig. 6, lane D8). Confirming previous results, the vaccination of adult mice with a plasmid expressing the nucleoprotein gene from LCMV prior to infection primes a population of adult CD8+ T cells that can accelerate the clearance of LCMV from the spleen (16, 39). This resulted in a decrease in splenic titers at 4 days postinfection in both of the adults that received pCMVNP compared to the unvaccinated day 4 adult controls. As expected, high titers of LCMV were observed in the spleens of all four neonates that received pCMV; the titers were similar to those observed in the two unvaccinated, infected day 4 controls. In contrast, six of nine neonates (66%) vaccinated at birth with pCMVNP showed evidence of a 99% or greater reduction in viral titers at 4 days postinfection (Fig. 6) compared to the neonates vaccinated with pCMV. Thus, the ability of these six neonates to rapidly resolve systemic LCMV infection indicates that DNA vaccination at birth has the capacity to confer protective CD8+-T-cell-mediated immunity within neonatal life.
Rapid acquisition of CTL activity following infection during neonatal life.
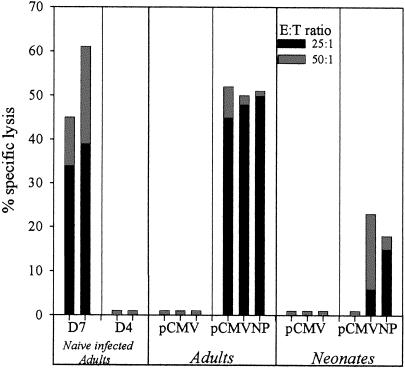
Clearance of LCMV in vivo is accomplished by the lysis of virus-infected cells by CD8+ CTLs. Lysis by CTLs in this model is MHC class I restricted and dependent upon production of the pore-forming protein perforin by CD8+ T cells (10, 19). Although the rapid clearance of virus from the spleens of DNA-immunized, virus-infected neonatal mice observed in Fig. 6 is most likely accomplished by the cytolytic activity of CD8+ CTLs, we wished to determine directly if neonatal T cells induced by DNA vaccination at birth can rapidly acquire lytic effector function following a viral challenge. Therefore, groups of three neonates and three adults were immunized with pCMVNP or pCMV and 14 days later were infected with LCMV. Four days postinfection, splenocytes from each mouse were assayed in vitro for lytic activity against H-2d- and H-2b-restricted target cells in the absence or presence of the H-2d CD8+-T-cell epitope peptide NP118-126. The results of this experiment are shown in Fig. 7. Although CD8+ T cells are rapidly primed and expanded in vivo in naïve mice after LCMV infection, their limited numbers 4 days postinfection prohibit their detection in a standard chromium release assay at this time point (Fig. 7, lane D4). Three days later, vigorous lytic responses are detectable in nonvaccinated, virus-infected, mice (Fig. 7, lane D7). In contrast to naïve mice, peptide-specific lytic activity was detectable in all three of the adults, and two out of three neonates which had received pCMVNP and which should therefore have had a preexisting CD8+-T-cell response to the LCMV nucleoprotein at the time of virus infection showed clear evidence of an anamnestic CTL response against H-2d target cells displaying a CD8+-T-cell epitope peptide 4 days postinfection. As expected, lytic responses were measurably lower in the responding pCMVNP-vaccinated neonates than in the adults. These results are consistent with the results shown in Fig. 1 and 2, which demonstrate that although similar percentages of the CD8+-T-cell pool respond to pCMVNP vaccination in both adults and neonates, the lower percentage of CD8+ T cells in the neonatal spleen results in a much lower total number of antigen-specific cells in the neonate at the time of infection. Neonatal and adult mice that received the negative control plasmid pCMV had no detectable LCMV-specific lytic responses 4 days postinfection. Likewise, only background levels of lysis (<1%) were observed when splenocytes from these same mice were incubated with H-2b target cells in the presence of NP118-126 peptide or H-2d target cells that did not present the NP118-126 peptide (data not shown). Therefore, antigen-specific CD8+ neonatal T cells primed by DNA vaccination at birth can rapidly acquire virus-specific lytic effector function shortly after virus infection.
FIG. 7.
Rapid acquisition of CTL activity following infection during neonatal life. Groups of three neonatal and three adult BALB/c mice were immunized intramuscularly with pCMVNP or pCMV and challenged intraperitoneally 2 weeks later with LCMV. Four days postchallenge, splenocytes were assayed for LCMV-specific lytic responses in a standard 5-h chromium release assay at effector-to-target ratios of 50:1 and 25:1 on BALB/cl7 (H-2d) NP118-126 peptide-pulsed target cells as described in Materials and Methods. Naïve adult mice infected with LCMV 7 (D7) or 4 (D4) days prior to the assay were included as positive and negative controls. Each bar reflects the results for an individual mouse.
DISCUSSION
Neonates are at heightened risk for a variety of viral diseases. Historically, this has been thought to be due to the relative immaturity of the neonatal immune system and the predisposition of neonates to the induction of tolerance (12). Recently, this view has been challenged by observations that demonstrate that neonates do mount both CD4+- and CD8+-T-cell responses after exposure to antigens and infections early in life (25, 28, 30). However, it is presently unclear if the enhanced susceptibility of neonates to viral infections may be due to subtle functional deficiencies in neonatal effector CD8+ T cells. In light of the fact that CD8+ T cells are essential for the rapid clearance of many viral pathogens, and for the maintenance of long-lived immune memory, a greater understanding of how rapidly these cells can develop in neonates, what functional properties they possess, and how effective they are in limiting viral infections in vivo could lead to improvements in virus control efforts in this critical age group.
This report examined the aforementioned issues using DNA vaccination in a well-studied host-pathogen model system, murine infection with lymphocytic choriomeningitis virus. The major findings of this work are that a single dose of plasmid DNA applied at birth can rapidly prime both protective antiviral immunity and a population of antigen-specific CD8+ T cells that exhibit functional characteristics similar to those of adult CD8+ T cells. In order to assess the functional competence of neonatal CD8+ T cells, we have used intracellular cytokine-staining and chromium release assays to directly examine a number of critical functions of recently primed neonatal CD8+ T cells. It has previously been shown by others using the LCMV system that the frequency of antigen-specific CD8+ T cells identified by intracellular cytokine staining closely matches the frequency of cells detected using MHC class I tetramers (26). Therefore, it is likely that our experiments identify most if not all neonatal CD8+ T cells specific for the immunodominant NP118-126 epitope. As shown in a previous report, which documented the rapid acquisition of antigen-specific T-cell responses among DNA-immunized adults (16), naïve neonatal CD8+ T cells can also respond quite rapidly to DNA immunization. Interestingly, these antigen-experienced cells quickly become a dominant part of the CD8+-T-cell pool during neonatal life, exceeding frequencies in most mice of >1 in 100 CD8+ T cells. These results are consistent with those noted in DNA-vaccinated adults and adults primed by DNA vaccination in infancy (17). While the initial frequency of antigen-specific CD8+ T cells can have a significant impact on the clinical severity of an infection, the expression of appropriate effector functions by these cells may be equally important in determining its outcome. At the initiation of this study, it was unclear whether neonatal CD8+ T cells induced by DNA vaccination could express the vigorous effector functions necessary to effectively deal with viral infections early in life. Therefore, we focused on the production by CD8+ T cells of two important soluble proinflammatory mediators, IFN-γ and TNF-α. Analysis of these cytokines revealed that, like adult CD8+ T cells induced either by virus infection or DNA immunization, the majority of peptide-specific neonatal T cells produced both IFN-γ and TNF-α after brief exposure to peptide antigen in vitro. Further studies aimed at determining the kinetics and the avidity of the peptide-specific CD8+ T cells also revealed similarities between neonatal and adult T cells induced by vaccination and adult T cells primed by virus infection. These two studies are relevant because they uncovered neither functional delays in cytokine production nor any requirement for higher concentrations of peptide for the expression of functional activity among neonatal CD8+ T cells. Therefore, it is likely that recently activated neonatal CD8+ T cells are capable of responding rapidly and appropriately to antigenic stimulation in vivo, at least with respect to the synthesis of these two important cytokines. Furthermore, these studies also imply that antigen presentation by neonatal antigen-presenting cells must also be operating effectively in vivo, as we did not add any exogenous adult antigen-presenting cells to our in vitro assays. In contrast to previous reports examining the functionality of neonatal T cells (2, 8, 24), we were able to assess the responses of antigen-specific neonatal CD8+ T cells during the neonatal period directly without resorting to in vivo virus infection, prolonged antigenic stimulation or nonspecific stimulation in vitro, or the addition of exogenous adult antigen-presenting cells or cytokines, all of which could potentially alter the phenotype of the cells under study. Therefore, we believe that our results are more reflective of the functional capacity of neonatal CD8+ T cells in vivo than previous studies, which relied on either nonspecific stimulation in vitro or prolonged periods of in vitro expansion prior to analysis of effector functions.
The primary role of antigen-experienced CD8+ T cells is thought to be the recognition and destruction of host cells expressing foreign peptides on their surfaces in association with MHC class I molecules. The cytolytic activity of CD8+ T lymphocytes is indispensable for the rapid clearance of many viruses in vivo, including LCMV (10). While previous studies have shown that neonatal T cells can acquire a lytic effector function during acute virus infections, to our knowledge this is the first study which has addressed the ability of a DNA vaccine to prime such responses so early in life (25, 31). Previous studies from our laboratory and others using the LCMV model have shown that, on average, 50% of adult mice which have received a single dose of an LCMV-expressing plasmid DNA are capable of rapidly clearing virus from the spleen after a peripheral challenge or surviving a lethal intracranial challenge (18, 29). In both cases, the ability to successfully control LCMV replication in vivo is directly correlated with the rapid amplification of virus-specific, MHC class I-restricted CD8+ CTL. While the small number of cells in the spleens of neonates 4 days after peripheral virus challenge (18 days after birth) precluded us from analyzing virus clearance and cytotoxicity in the same animal, separate experiments have revealed that neonatal vaccinees can successfully limit virus replication in the spleen and acquire LCMV-specific lytic activity against cells presenting an LCMV CD8+-T-cell epitope peptide. However, the currently available assays used to measure antigen-specific lysis by CTL are not quantitative, making it difficult to directly compare the lytic capacities of neonatal and adult CD8+ T cells. It therefore still remains to be determined if neonatal CD8+ T cells are as effective at lysing virus-infected cells on a per-cell basis as their adult counterparts.
These data clearly show that naïve murine T cells present in the periphery early in life can rapidly mature into adult-like antigen-specific effector cells following DNA vaccination on the day of birth. If these results are reflective of the situation which occurs in humans, early prophylactic interventions using plasmid DNA vaccines may eventually allow us to mobilize effective CD8+-T-cell responses very early in life, thus limiting the pathogenic effects of commonly acquired childhood viral illnesses. While clinical evidence of the effectiveness of vaccination shortly after birth exists, most industrialized nations begin prophylactic antiviral immunizations in early infancy, weeks or months after birth. This delay is thought to be required to allow the neonatal immune system to mature to a functionally competent adult-like state and to allow the infant to eliminate maternal antibodies that could interfere with effective vaccination. Unfortunately these delays may have resulted in the deaths of many infants due to infections that could have been prevented by early antiviral vaccination. A new generation of vaccines specifically tailored to the unique needs of infants is clearly necessary to protect children at risk. However, in order to rationally design more effective vaccines for neonates and infants, detailed information regarding the functional competence of human neonatal T cells is necessary. The assays we have used here are easily applicable to humans and should provide us with a wealth of new data regarding the function of the immune system early in life.
Acknowledgments
We thank Annette Lord for excellent secretarial assistance.
This study was supported by NIH grants AI37186 to J.L.W. and AI45981 to D.E.H.
Footnotes
This is manuscript no. 14962-NP from the Scripps Research Institute.
REFERENCES
- 1.Adkins, B. 1999. T-cell function in newborn mice and humans. Immunol. Today 20:330-335. [DOI] [PubMed] [Google Scholar]
- 2.Adkins, B., and K. Hamilton. 1992. Freshly isolated, murine neonatal T cells produce IL-4 in response to anti-CD3 stimulation. J. Immunol. 149:3448-3455. [PubMed] [Google Scholar]
- 3.Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science 272:54-60. [DOI] [PubMed] [Google Scholar]
- 4.Benedict, C. L., S. Gilfillan, T. H. Thai, and J. F. Kearney. 2000. Terminal deoxynucleotidyl transferase and repertoire development. Immunol. Rev. 175:150-157. [PubMed] [Google Scholar]
- 5.Bot, A., S. Bot, and C. Bona. 1998. Enhanced protection against influenza virus of mice immunized as newborns with a mixture of plasmids expressing hemagglutinin and nucleoprotein. Vaccine 16:1675-1682. [DOI] [PubMed] [Google Scholar]
- 6.Bot, A., S. Bot, A. Garcia-Sastre, and C. Bona. 1996. DNA immunization of newborn mice with a plasmid-expressing nucleoprotein of influenza virus. Viral Immunol. 9:207-210. [DOI] [PubMed] [Google Scholar]
- 7.Bot, A., M. Shearer, S. Bot, C. Woods, J. Limmer, R. Kennedy, S. Casares, and C. Bona. 1999. Induction of antibody response by DNA immunization of newborn baboons against influenza virus. Viral Immunol. 12:91-96. [DOI] [PubMed] [Google Scholar]
- 8.Buseyne, F., S. Blanche, D. Schmitt, C. Griscelli, and Y. Riviere. 1993. Detection of HIV-specific cell-mediated cytotoxicity in the peripheral blood from infected children. J. Immunol. 150:3569-3581. [PubMed] [Google Scholar]
- 9.Chun, S., M. Daheshia, S. Lee, S. K. Eo, and B. T. Rouse. 1999. Distribution fate and mechanism of immune modulation following mucosal delivery of plasmid DNA encoding IL-10. J. Immunol. 163:2393-2402. [PubMed] [Google Scholar]
- 10.Clark, W. R., C. M. Walsh, A. A. Glass, M. T. Huang, R. Ahmed, and M. Matloubian. 1995. Cell-mediated cytotoxicity in perforin-less mice. Int. Rev. Immunol. 13:1-14. [DOI] [PubMed] [Google Scholar]
- 11.Davis, M. M., and P. J. Bjorkman. 1988. T-cell antigen receptor genes and T-cell recognition. Nature 334:395-402. [DOI] [PubMed] [Google Scholar]
- 12.Fadel, S., and M. Sarzotti. 2000. Cellular immune responses in neonates. Int. Rev. Immunol. 19:173-193. [DOI] [PubMed] [Google Scholar]
- 13.Garcia, A. M., S. A. Fadel, S. Cao, and M. Sarzotti. 2000. T cell immunity in neonates. Immunol. Res. 22:177-190. [DOI] [PubMed] [Google Scholar]
- 14.Gavin, M. A., and M. J. Bevan. 1995. Increased peptide promiscuity provides a rationale for the lack of N regions in the neonatal T cell repertoire. Immunity 3:793-800. [DOI] [PubMed] [Google Scholar]
- 15.Harty, J. T., A. R. Tvinnereim, and D. W. White. 2000. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18:275-308. [DOI] [PubMed] [Google Scholar]
- 16.Hassett, D. E., M. K. Slifka, J. Zhang, and J. L. Whitton. 2000. Direct ex vivo kinetic and phenotypic analyses of CD8+ T-cell responses induced by DNA immunization. J. Virol. 74:8286-8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassett, D. E., J. Zhang, M. Slifka, and J. L. Whitton. 2000. Immune responses following neonatal DNA vaccination are long-lived, abundant, and qualitatively similar to those induced by conventional immunization. J. Virol. 74:2620-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassett, D. E., J. Zhang, and J. L. Whitton. 1997. Neonatal DNA immunization with a plasmid encoding an internal viral protein is effective in the presence of maternal antibodies and protects against subsequent viral challenge. J. Virol. 71:7881-7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hildeman, D., M. Salvato, J. L. Whitton, and D. Muller. 1996. Vaccination protects beta 2 microglobulin deficient mice from immune mediated mortality but not from persisting viral infection. Vaccine 14:1223-1229. [DOI] [PubMed] [Google Scholar]
- 20.Janeway, C., and P. Travers. 1997. Immunobiology: the immune system in health and disease, 3rd ed., vol. 3. Garland Publishing Inc., New York, N.Y.
- 21.Luzuriaga, K., D. Holmes, A. Hereema, J. Wong, D. L. Panicali, and J. L. Sullivan. 1995. HIV-1-specific cytotoxic T lymphocyte responses in the first year of life. J. Immunol. 154:433-443. [PubMed] [Google Scholar]
- 22.Manickan, E., Z. Yu, and B. T. Rouse. 1997. DNA immunization of neonates induces immunity despite the presence of maternal antibody. J. Clin. Investig. 100:2371-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez, X., C. Brandt, F. Saddallah, C. Tougne, C. Barrios, F. Wild, G. Dougan, P. H. Lambert, and C. A. Siegrist. 1997. DNA immunization circumvents deficient induction of T helper type 1 and cytotoxic T lymphocyte responses in neonates and during early life. Proc. Natl. Acad. Sci. USA 94:8726-8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McFarland, E. J., P. A. Harding, D. Luckey, B. Conway, R. K. Young, and D. R. Kuritzkes. 1994. High frequency of Gag- and envelope-specific cytotoxic T lymphocyte precursors in children with vertically acquired human immunodeficiency virus type 1 infection. J. Infect. Dis. 170:766-774. [DOI] [PubMed] [Google Scholar]
- 25.Moser, J. M., J. D. Altman, and A. E. Lukacher. 2001. Antiviral CD8+ T cell responses in neonatal mice: susceptibility to polyoma virus-induced tumors is associated with lack of cytotoxic function by viral antigen-specific T cells. J. Exp. Med. 193:595-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 27.Pertmer, T. M., and H. L. Robinson. 1999. Studies on antibody responses following neonatal immunization with influenza hemagglutinin DNA or protein. Virology 257:406-414. [DOI] [PubMed] [Google Scholar]
- 28.Ridge, J. P., E. J. Fuchs, and P. Matzinger. 1996. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science 271:1723-1726. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez, F., J. Zhang, and J. L. Whitton. 1997. DNA immunization: ubiquitination of a viral protein enhances cytotoxic T-lymphocyte induction and antiviral protection but abrogates antibody induction. J. Virol. 71:8497-8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarzotti, M., T. A. Dean, M. Remington, and P. M. Hoffman. 1994. Ultraviolet-light-inactivated Cas-Br-M murine leukemia virus induces a protective CD8+ cytotoxic T lymphocyte response in newborn mice. AIDS Res. Hum. Retrovir. 10:1695-1702. [DOI] [PubMed] [Google Scholar]
- 31.Sarzotti, M., T. A. Dean, M. P. Remington, C. D. Ly, P. A. Furth, and D. S. Robbins. 1997. Induction of cytotoxic T cell responses in newborn mice by DNA immunization. Vaccine 15:795-797. [DOI] [PubMed] [Google Scholar]
- 32.Slifka, M. K., and J. L. Whitton. 2000. Activated and memory CD8+ T cells can be distinguished by their cytokine profiles and phenotypic markers. J. Immunol. 164:208-216. [DOI] [PubMed] [Google Scholar]
- 33.Slifka, M. K., and J. L. Whitton. 2000. Clinical implications of dysregulated cytokine production. J. Mol. Med. 78:74-80. [DOI] [PubMed] [Google Scholar]
- 34.Sourdive, D. J., K. Murali-Krishna, J. D. Altman, A. J. Zajac, J. K. Whitmire, C. Pannetier, P. Kourilsky, B. Evavold, A. Sette, and R. Ahmed. 1998. Conserved T cell receptor repertoire in primary and memory CD8 T cell responses to an acute viral infection. J. Exp. Med. 188:71-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Drunen Littel-van den Hurk, S., R. P. Braun, P. J. Lewis, B. C. Karvonen, L. A. Babiuk, and P. J. Griebel. 1999. Immunization of neonates with DNA encoding a bovine herpesvirus glycoprotein is effective in the presence of maternal antibodies. Viral Immunol. 12:67-77. [DOI] [PubMed] [Google Scholar]
- 36.Wang, Y., Z. Xiang, S. Pasquini, and H. C. Ertl. 1997. Immune response to neonatal genetic immunization. Virology 228:278-284. [DOI] [PubMed] [Google Scholar]
- 37.Whitton, J. L., A. Tishon, H. Lewicki, J. Gebhard, T. Cook, M. Salvato, E. Joly, and M. B. Oldstone. 1989. Molecular analyses of a five-amino-acid cytotoxic T-lymphocyte (CTL) epitope: an immunodominant region which induces nonreciprocal CTL cross-reactivity. J. Virol. 63:4303-4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolff, J. A., R. W. Malone, P. Williams, W. Chong, G. Acsadi, A. Jani, and P. L. Felgner. 1990. Direct gene transfer into mouse muscle in vivo. Science 247:1465-1468. [DOI] [PubMed] [Google Scholar]
- 39.Yokoyama, M., J. Zhang, and J. L. Whitton. 1995. DNA immunization confers protection against lethal lymphocytic choriomeningitis virus infection. J. Virol. 69:2684-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]