Abstract
Dengue virus (DV) replication, antibody-enhanced viral infection, and cytokine responses of human primary B lymphocytes (cells) were characterized and compared with those of monocytes. The presence of a replication template (negative-strand RNA intermediate), viral antigens including core and nonstructural proteins, and increasing amounts of virus with time postinfection indicated that DV actively replicated in B cells. Virus infection also induced B cells to produce interleukin-6 and tumor necrosis factor alpha, which have been previously implicated in virus pathogenesis. In addition, a heterologous antibody was able to enhance both virus and cytokine production in B cells. Furthermore, the levels of virus replication, antibody-enhanced virus replication, and cytokine responses observed in B cells were not statistically different from those in monocytes. These results suggest that B cells may play an important role in DV pathogenesis.
Infection with any of the four serotypes of dengue virus (DV1, -2, -3, and -4), a mosquito-borne flavivirus, can cause self-limiting dengue fever or severe dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). The incidence of fatal DHF cases has increased sharply in Asia over the last 2 decades, making it a leading cause of morbidity (16, 30). Several mechanisms have been proposed to explain the pathogenesis of DV infection. A long-standing hypothesis, antibody-dependent enhancement (ADE), proposes that preexisting nonneutralizing antibodies enhance DV infection of monocytes via the Fc receptor (14). More recently, immunopathogenesis theories suggest that ADE results in increased T-cell activation and cytokine production, which subsequently activate complement to damage endothelial cells (reviewed in references 23 and 32). We have found that, besides monocytes and T cells, B lymphocytes (B cells) contribute to pathogenesis by producing high titers of antiplatelet and anti-endothelial cell autoantibodies, particularly in DHF and DSS patients, which could induce coagulopathy and vasculopathy (24-26), two major pathologies of DHF and DSS.
Throughout DV infection, virus and cytokines are detected in patient blood (2, 13, 17, 18, 28, 36, 39, 40), and peripheral blood mononuclear cells (PBMC) are found to be one of the most common recovery sites of virus (21, 34). In PBMC, virus is detected frequently in the adherent monocytes (34). In addition, a study previously established that only B cells and monocytes in human PBMC support virus replication and that monocytes produce more viruses than B cells (37). Based on these observations, and particularly based on ADE, monocytes are thought to be the major cell target for DV. However, two clinical observations of infected patients with evident syndromes revealed that viral antigen appeared only on B cells (4) and that B cells, not monocytes, are infected with the virus (21). Whether DV replicates actively in B cells is still an open question. Because B cells and monocytes have Fc receptors (29), they are both potential targets for antibody-enhanced infection. B cells could also secrete cytokines such as interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) (15, 38), which are found to be elevated specifically in DHF and DSS patients (2, 17, 18, 40) and affect endothelial cells (1, 3, 27). Additionally, increased levels of IL-6 have been shown to correlate with a deficiency in coagulation factor XII and with elevated levels of antiplatelet and anti-endothelial cell autoantibodies as well as fibrinolytic components, such as tissue-type plasminogen activator, in patients with DV infection (10; Y.-H. Huang, H.-Y. Lei, H.-S. Liu, Y.-S. Lin, C.-C. Liu, S.-H. Chen, and T.-M. Yeh, submitted for publication; reviewed in reference 24). In this report, we characterized DV replication, antibody-enhanced virus infection, and cytokine responses in human B cells and compared them to the corresponding responses of monocytes.
MATERIALS AND METHODS
Cells, viruses, and reagents.
The B-cell line Raji was maintained in RPMI medium containing 10% fetal bovine serum according to American Type Culture Collection instructions. A DV3 strain isolated from a patient in Taiwan and DV2 strains PL046 and M16681 were propagated in C6/36 mosquito cells and titrated on BHK cells as previously described (18). Unless otherwise specified, strain PL046 was used as the source of DV to infect cells. The same batch of each virus stock was used throughout the experiments. Human DV3 immune serum was obtained with consent from an infected patient. The titer of this serum was 1:12,000 against DV3 and 1:3,200 against DV2 strain PL046, as determined by measuring 50% plaque reduction in a neutralization assay using BHK cell monolayers (14). Control serum was collected from a healthy blood donor without DV-specific antibodies in serum as determined by an enzyme-linked immunosorbent assay (ELISA) modified from a previous report (19). A monoclonal antibody (MAb) against the viral envelope protein was obtained from Chemicon (Temecula, Calif.), and a MAb against the viral core protein with high specificity was purified from supernatant of hybridoma cells as described previously (S.-H. Wang, W.-J. Suy, K.-J. Huang, H.-Y. Lei, C.-W. Yao, C.-C. King, and S.-T. Hu, submitted for publication). The immunoglobulin G (IgG) fractions of mouse normal serum and mouse hyperimmune serum of viral nonstructural protein 1 (NS1) used for flow-cytometric analyses were prepared and purified as described previously (26).
Isolation of primary B cells and monocytes from human PBMC.
Peripheral venous blood obtained from healthy blood donors was kindly provided by the Tainan Blood Center. Sera were tested for DV-specific antibodies by an ELISA as described above. PBMC were separated from plasma and granulocytes by Ficoll-Hypaque gradient centrifugation, washed three times with Hanks basal salt solution by centrifugation for 10 min, and then counted by the trypan blue exclusion method. Afterward, PBMC were resuspended in RPMI medium to 2 × 106 cells/ml and seeded to 10-cm-diameter culture dishes for 3 days at 37°C as described in the study of Theofilopoulos et al. (37). The adherent monocytes contained 92% CD14+ cells as determined by fluorescence-activated cell sorter (FACS) analysis using an anti-human CD14 antibody (BD Biosciences Clontech, Palo Alto, Calif.). B cells were purified from nonadherent cells by magnetic cell sorting superparamagnetic microbeads conjugated with a anti-human CD19 antibody (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocol. Consistent with the manufacturer's protocol and a previous study (33), the purified cells contained 90% CD19+ B cells and less than 3% CD14+ monocytes as determined by FACS analysis using anti-human CD19 (BD Biosciences Clontech) or CD14 antibodies.
Infection and preparation of virus-antibody complexes for infection of cells.
Viruses were incubated with 2 × 106 cells for 90 min at 37°C for infection. To prepare virus-antibody complexes, viruses were mixed with diluted DV3 immune serum or control serum. These mixtures were incubated for 15 min at 37°C before infecting cells. After infection, cells were washed two times and resuspended in 4 ml of RPMI medium or medium containing a dilution of human serum to achieve the same final concentration of antibodies used for virus infection. Infected cells were then divided into four aliquots and cultured in 5-ml tubes. At various times ranging from 4 to 120 h after infection, cultures were centrifuged. Supernatants were assayed for virus by plaque assay on BHK cell monolayers.
RNA isolation and RT-PCR analyses.
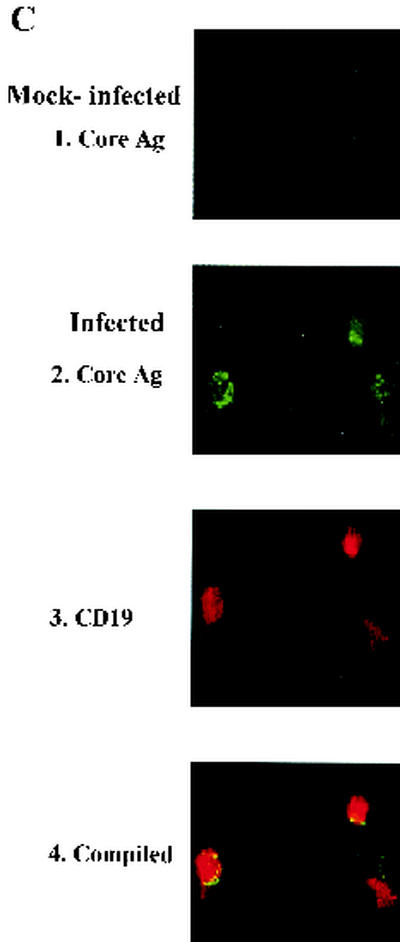
Total RNA was isolated from cells 1 day after infection with TRI reagent (Sigma, St. Louis, Mo.) according to the manufacturer's protocol. To analyze positive- and negative-stand RNA genomes as well as β-actin transcripts, total RNA was split into two portions. One portion was annealed with 2 μM reverse primer for human β-actin (β2 [5′-CAGGGTACATGGTGGTGCC-3′) and virus forward primer D1 (5′-TATGCTGAAACGCGCGAGAAA-3′; genomic positions 138 to 161). The other portion was annealed with reverse primers for β-actin and virus reverse primer D2 (5′-TTGCACCAACAGTCAATGTC-3′; genomic positions 616 to 635). Mixtures were subjected to thermal cycling for 7 min at 70°C and then cooled down to 4°C for the RNA to denature and anneal with primers. Half of each sample was added to a reverse transcriptase (RT) reaction mixture containing 32 U of RNase inhibitor, 0.5 mM deoxynucleoside triphosphates (dNTPs), and buffer with or without avian myeloblastosis virus RT (10 U; Promega, Madison, Wis.) and reverse transcribed at 42°C for 90 min and then at 94°C for 10 min to inactivate the RT. Aliquots of cDNA were combined with a PCR mixture containing 2.5 U of Taq polymerase, buffer, 0.25 mM dNTPs, and 2 mM D1 and D2 primers and then subjected to thermocycles to amplify 498-bp DNA products. The PCR conditions were 94°C for 4 min, 55°C for 1 min, and 72°C for 1 min followed by 39 cycles of 94°C for 30 s, 55°C for 35 s, and 72°C for 35 s. To analyze β-actin transcripts, aliquots of cDNA were combined with the PCR mixture as described above except that forward (β1 [5′-AGCGGGAAATCGTGCGTG-3′]) and reverse primers for β-actin were used and then 26 PCR cycles (1 min each at 94, 58, and 72°C) to amplify 309-bp DNA products were performed. Reaction products were separated by electrophoresis on agarose gels containing ethidium bromide. Gels were scanned with a FluorImager FLA3000 (Fuji, Tokyo, Japan) according to the manufacturer's protocol. The intensity of each band was quantified, and the ratio of negative-strand virus genome to β-actin was calculated for each sample. Next, the ratio of negative-strand virus genome to β-actin of the DV3 immune serum-treated sample was divided by the ratio of negative-strand virus genome to β-actin of the control serum-treated sample to determine the fold increase of negative-strand virus genome under ADE conditions.
Immunofluorescence assay.
Forty-eight hours after infection, B cells were harvested and immobilized on glass slides coated with 1% poly-l-lysine by cytospin. Next, the cells were fixed with 1% paraformaldehyde and stained with an anti-DV core or envelope protein MAb. After being washed, slides were incubated with diluted fluorescein isothiocyanate-conjugated anti-mouse IgG (ICN Pharmaceuticals, Costa Mesa, Calif.) for 1 h. After being washed, slides were stained with a phycoerythrin-conjugated anti-human CD19 antibody (Pharmingen, San Diego, Calif.). Slides were viewed and photographed by using fluorescence microscopy (Olympus).
Flow cytometry.
Infected B cells were washed with phosphate-buffered saline (PBS) twice, fixed, and permeabilized simultaneously with a solution containing 1% paraformaldehyde and 0.3% saponin. The permeabilized cells were washed and incubated with the IgG fraction of NS1 hyperimmune serum or normal mouse serum for 30 min at 4°C. Cells were then washed and incubated with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (ICN Pharmaceuticals) for 30 min at 4°C. After incubation, cells were washed twice with PBS, resuspended in PBS, and then analyzed with a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, Calif.).
Cytokine measurement.
Cytokines were measured by ELISA kits according to the manufacturer's protocols. The detection limits for the cytokines (Endogen, Woburn, Mass.) were as follows: TNF-α, 16 pg/ml; IL-6, 11 pg/ml.
Statistics.
Data were analyzed or plotted, and statistics were calculated with Microsoft Excel. When necessary, the results were expressed as the means ± standard errors of the means (SEM). Student's t test was used to determine the significance, which was taken as a value of P ≤0.05.
RESULTS
Active DV replication in human primary B cells.
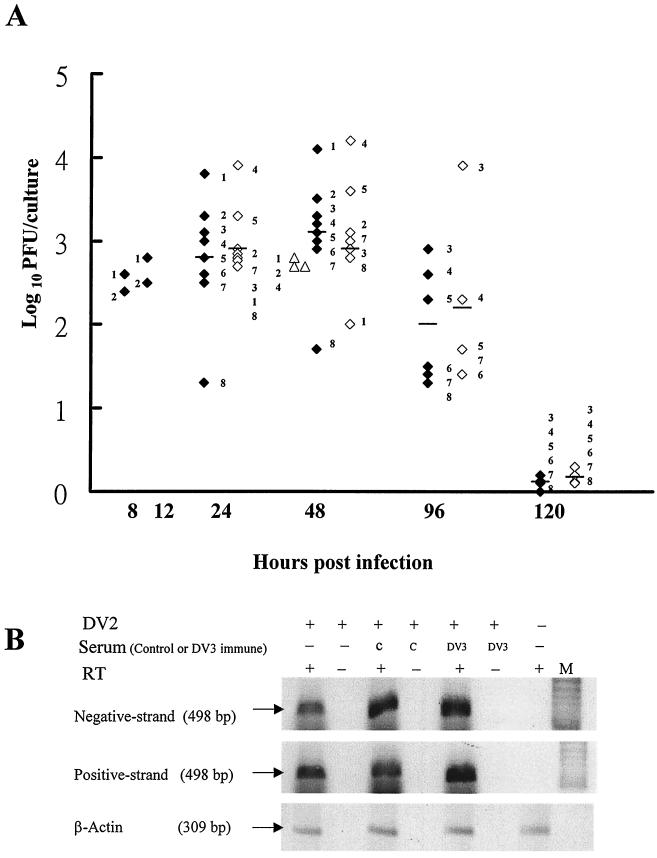
Purified B cells were infected with DV2 strain PL046 at a multiplicity of infection (MOI) of 10. Supernatants were harvested after infection to determine virus titers by plaque assay on BHK cells (Fig. 1A). The amount of virus detected in B cells of donors 1 and 2 increased from 8 to 48 h postinfection (p.i.), indicating that DV actively replicated in B cells. DV3 was also able to infect B cells with similar growth kinetics although the peak virus titer at 48 h p.i. was about 10-fold less than that of DV2 in the three donors that we tested.
FIG. 1.
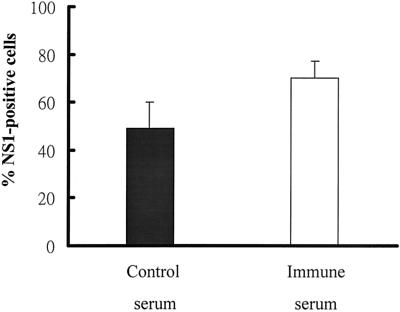
Active DV replication in human B cells. (A) B cells were infected with DV2 (solid diamonds) or DV3 (open triangles) and monocytes were infected with DV2 (open diamonds) at an MOI of 10. Culture supernatants were collected at the indicated times to determine virus titers. Numbers adjacent to symbols designate individual donors, and bars represent the mean values for each group. (B) After infection or mock infection of B cells of donor 4 with DV2 at an MOI of 10 in the absence or presence of control or DV3 immune serum, total RNA was isolated from B cells at 24 h p.i. Portions of each sample were hybridized with β2 and D1 primers (top) and the D2 primer (middle) to analyze negative- and positive-strand RNA genomes. Afterward, half of each sample was incubated with (+) or without (−) RT, amplified by PCR, and run in adjacent lanes. β-Actin (bottom) served as an internal control. M, DNA marker. (C) Staining of DV2-infected B cells. After mock treatment (1) or DV2 infection (2 to 4) at an MOI of 10, B cells of donor 6 were collected 48 h later and stained for intracellular viral core protein (1 and 2) and cell surface CD19 molecules (3). Panel 4 is the compiled image of panels 2 and 3.
During DV replication, the negative-strand RNA genome is synthesized from the positive-strand virus genome and serves as a template for replication. Therefore, the presence of negative-strand RNA could be used as an index to determine if virus is replicating in and not simply being absorbed on or sequestered by B cells. The replication of DV2 in B cells was confirmed by detecting the negative-strand virus RNA in infected cells, but not in mock-infected cells, at 24 h p.i. by using RT-PCR analyses (Fig. 1B, lanes 1 and 7). Additionally, the replication of DV2 in B cells was further confirmed by staining cells with both B-cell marker CD19 and intracellular viral core protein and then analyzing them with a fluorescence microscope (Fig. 1C). DV2-infected B cells strongly coexpressed both the viral core protein and CD19 at 48 h p.i. Mock-infected cells were only weakly stained by the antibody to the viral core antigen, and this is probably due to the interaction of the Fc portion of the antibody with the Fc receptors on B cells. Infected B cells also expressed the viral NS1 protein (see Fig. 4); 17 and 49% of the infected B cells expressed viral core and NS1 proteins, respectively.
FIG. 4.
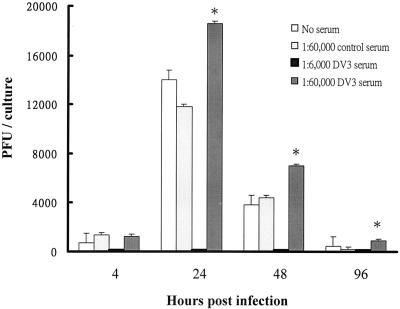
Antibody-enhanced NS1 expression in infected B cells. DV2 was incubated with normal human serum or DV3 immune serum before infecting B cells of donors 4, 5, and 8 at an MOI of 1. At 48 h p.i., infected cells were collected and subjected to flow-cytometric analysis of the viral NS1 protein. The results are expressed as the mean percentages of cells that express the NS1 protein of DV ± SEM.
Comparison of DV2 replication in B cells with that in monocytes.
We next investigated DV2 replication in B cells and compared our results with those for monocytes. B cells obtained from eight donors were all able to support DV2 replication (Fig. 1A), but the amounts of virus produced by different donors varied. B cells of donor 1 yielded the highest virus titer (4.09 log units), and those of donor 8 yielded the lowest virus titer (1.65 log units), with a 274-fold difference between these two donors at 48 h p.i. The amounts of virus produced by infected monocytes also varied among donors, with a 128-fold difference between donor 4 (4.10 log units) and donor 1 (2.0 log units) at 48 h p.i. Not only different individuals but also different types of cells from the same individual differ in their abilities to support DV2 infection. B cells of donors 1, 2, and 3 produced 17-, 3-, and 2-fold more viruses, respectively, than monocytes at 48 h p.i. However, B cells of donors 4, 5, 7, and 8 produced 1.3- to 8-fold less viruses than monocytes after infection. A modified ELISA performed on sera of donors 1, 2, 4, and 8 could not detect a DV-specific IgG or IgM antibody, suggesting that these four donors were not previously infected (data not shown). Surprisingly, we found that not only did the ranges of virus produced by infected B cells and monocytes overlap but also the mean values for these two cell types were not statistically different (P > 0.6) at any time point in the donors we tested.
Antibody-enhanced DV2 replication in a B-cell line, primary B cells, and monocytes.
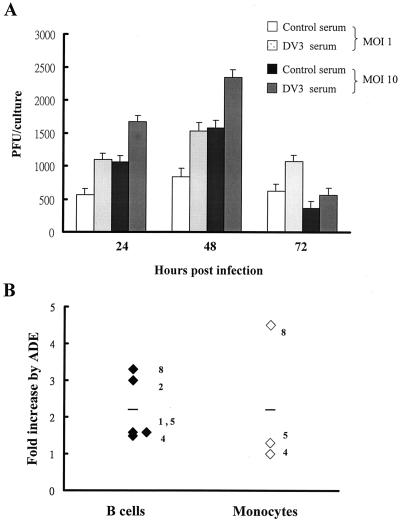
To investigate the ADE effect on virus replication in human B cells, the B-cell line Raji was used for pilot studies and for comparison to primary cultured B cells. DV2 was mixed with human serum collected from a healthy individual and a DV3-infected patient with a neutralizing titer of 1:12,000, and then the mixture was added to Raji cells. The virus was not able to infect Raji cells when mixed with a neutralizing dose (1: 6,000) of DV3 immune serum (Fig. 2). A fivefold dilution beyond the neutralizing titer has been used by Brandt et al. (6) to achieve optimal ADE. Therefore a 1:60,000 dilution of serum was used to investigate ADE. Using this dilution, we did not detect any apparent effect of control serum on virus replication in B cells. However, this nonneutralizing dose of DV3 immune serum was able to enhance DV2 replication significantly by 1.6-, 2-, and 4.5-fold at 24, 48, and 96 h p.i. (P ≤ 0.05), respectively, compared to control serum. The heterologous DV serum enhanced virus infection but did not alter the growth kinetics of virus in Raji cells.
FIG. 2.
Antibody-enhanced DV infection of B-cell line Raji. Viruses were incubated with control or DV3 immune serum diluted as indicated before infecting cells at an MOI of 10. Culture supernatants were collected at the indicated times, and virus titers were determined. The means ± SEM of duplicate samples are shown. ∗, P < 0.05 when immune serum-treated samples are compared to control serum-treated samples.
In primary cultured B cells, DV3 immune serum was also able to enhance viral replication (Fig. 3A). The levels of ADE on viral replication at two different MOIs were comparable (1.9-, 1.8-, and 1.7-fold increases for an MOI of 1 and 1.6-, 1.5-, and 1.6-fold increases for an MOI of 10 at 24, 48, and 72 h p.i., respectively). The effects of ADE on viral replication in infected B cells and monocytes in additional donors (donors 1, 2, 4, 5, and 8) were investigated. The ranges of ADE of viral replication in B cells and monocytes overlapped (Fig. 3B). DV immune serum slightly increased viral replication by an average of 2.2-fold in both infected B cells and monocytes (P > 0.05). RT-PCR assays detected a 1.3-fold increase of the negative-strand RNA genome in infected B cells of donor 4 with DV3 immune serum compared to that with normal serum, further confirming that ADE increases virus replication (Fig. 1B). Flow-cytometric analyses of NS1 expression were performed for donors 4, 5, and 8 (Fig. 4). We found that, in the presence of DV3 immune serum, not only was the percentage of cells positive for the viral NS1 protein increased significantly (P < 0.05) but also the mean fluorescence intensity of NS1 expression in infected B cells was augmented about 2.8-fold (78 ± 32 [control serum] versus 221 ± 92 [immune serum]).
FIG. 3.
Antibody-enhanced DV infection of primary mononuclear cells. (A) Viruses were incubated with a 1:60,000 dilution of control serum or DV3 immune serum before infecting B cells of donor 4 at MOIs of 1 and 10. Culture supernatants were collected at the indicated times, and virus titers were determined. The means ± SEM of duplicate samples are shown. (B) B cells (solid diamonds) and monocytes (open diamonds) of donors were infected with DV2 at an MOI of 10 in the presence of control serum or DV3 serum. Culture supernatants were collected at 48 h p.i., and virus titers were determined. The fold increase of virus titer due to ADE was calculated as follows: fold increase = virus titer of DV3 immune serum sample/virus titer of control serum sample. Numbers adjacent to symbols designate individual donors, and bars represent the mean values for each group.
Comparison of cytokine responses in DV-infected B cells and monocytes.
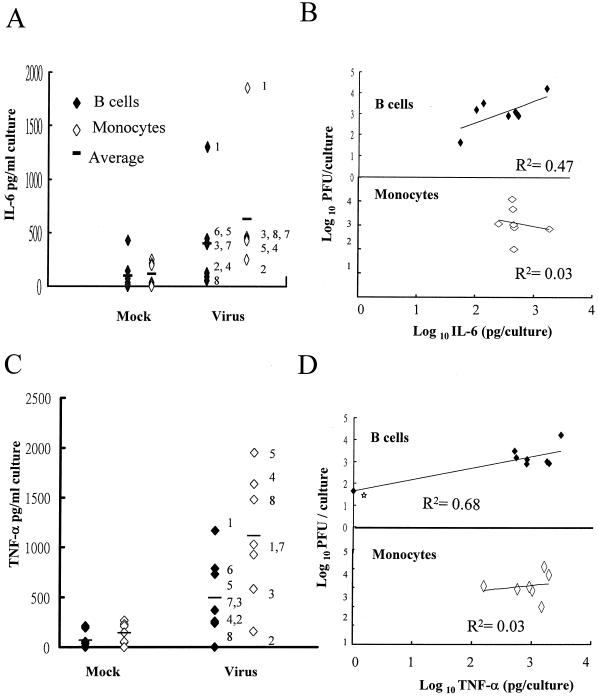
Both B cells and monocytes can produce IL-6 and TNF-α (15, 38), so the cytokine responses of these two cell types after infection were examined. DV2 infection significantly increased the levels of IL-6 in both B cells (P < 0.001) and monocytes (P < 0.02) 48 h after infection (Fig. 5A). Similar to the results of virus yield, the ranges of IL-6 produced by infected B cells and monocytes overlapped. Although the average amount of IL-6 secreted by infected B cells was 1.6-fold less than that secreted by infected monocytes, this difference did not attain statistical significance (P = 0.37). By a comparison of Fig. 1A and 5A, a correlation between the amount of virus and IL-6 produced by infected B cells in some donors can be seen, so linear regression analyses were applied to investigate the correlation between these two values. We found that the correlation was strong (r2 = 0.47) for B cells but not apparent (r2 = 0.03) for monocytes (Fig. 5B). In the presence of DV3 immune serum, IL-6 secretion was slightly increased: about 1.6-fold in infected B cells and 1.3-fold in infected monocytes of donors 4, 5, and 8 (P > 0.05).
FIG. 5.
Cytokine production in DV-infected human mononuclear cells. The same 48-h supernatant samples used for virus titration in Fig. 1A and their corresponding mock-treated samples of B cells (solid diamonds) and monocytes (open diamonds) were assayed for IL-6 (A) and TNF-α (C) by using ELISA kits. Numbers adjacent to symbols represent individual donors, and bars represent mean values for each group. (B and D) Correlation of IL-6 and virus production (B) and correlation of TNF-α and virus production (D) in infected B cells (top) and monocytes (bottom). The amounts of the indicated cytokines produced in the infected samples were plotted against the amounts of virus produced in the same samples. The best-fit lines were generated by linear regression of log10 values. Star, virus production in B cells for donor 8.
Similar to the IL-6 response, the average amounts of TNF-α in both B cells (P < 0.001) and monocytes (P < 0.001) were also significantly increased, by about eightfold, after infection (Fig. 5C). The ranges of TNF-α produced by the two cell types overlapped. Although the average amount of TNF-α secreted by infected B cells was 2.7-fold less than that secreted by infected monocytes, this difference was not statistically significant (P = 0.14) and, actually, was very close to the difference between the amounts secreted by infected B cells and uninfected cells (2.2-fold). Therefore, the levels of induction of TNF-α after infection in B cells and monocytes were equivalent. We also found that the correlation between virus and TNF-α responses in infected B cells with (r2 = 0.68) or without (r2 = 0.22) donor 8 was greater than that between these responses in infected monocytes (r2 = 0.03; Fig. 5D). In the presence of DV3 immune serum, TNF-α secretion was only slightly increased: about 1.5-fold in infected B cells and 1.3-fold in infected monocytes of donors 4, 5, and 8 (P > 0.05).
DISCUSSION
We initiated these studies to determine the contribution of B cells to DV amplification and cytokine secretion in the triggering of pathological responses. Our results demonstrate that DV replication, antibody-enhanced DV replication, and cytokine responses in infected B cells and monocytes are similar. We discuss these findings below.
B cells actively support DV replication.
Only one report documented DV replication in human B cells by detecting increasing amounts of virus after infection (37). In this study, we found more substantial evidence to indicate that DV actively replicates in B cells. Our expanded study has demonstrated the following: (i) the negative-strand RNA genome, which is the replication template present only during virus replication, was found in infected cells; (ii) viral antigens, including core and NS1 proteins, were detected in infected cells; (iii) amounts of virus detected in infected cells increased over time; (iv) cytokines were secreted from infected cells; and (v) the heterologous antibody was able to enhance both DV replication and cytokine secretion in infected cells. The replication of DV observed in B cells was not due to the contamination of monocytes for the following reasons: (i) B-cell cultures used for studies contained a high percentage of B cells (≥90%); (ii) infected cells coexpressed the B-cell marker CD19 and viral antigens; and (iii) in some donors, the levels of virus, cytokines, and ADE produced by B cells were higher than those produced by monocytes after infection. We also made sure that the viruses used for this study were never grown or passaged in any B-cell line or primary cultured B cells previously, because one earlier report has shown that only a Raji cell-adapted strain of DV2 could replicate in lymphocytes (5).
Consistent with the previous study (37), our initial results showed that primary mononuclear cells, including PBMC, B cells, and monocytes, inoculated with virus immediately (4 h) after isolation from most donors were not permissive for DV infection. One possible explanation for this phenomenon is that mononuclear cells are affected during the process of blood collection and cell isolation and it may take time for the cells to recover. For example, the use of heparin for anticoagulation during blood collection may affect the heparin sulfate on the cell surface, which has been identified as the receptor for virus entry into cells (9). Indeed, experiments set up to test this hypothesis demonstrated that the permissiveness of B cells to DV, as determined by measuring virus production, gradually increased with cultivation time in the 3-day test period. Additional studies are needed to examine whether the increased permissiveness of mononuclear cells to DV after culture may be due to the recovery of the viral receptor.
The role of B cells in virus amplification and spread during infection.
We found that DV actively replicates in B cells, indicating that the virus detected in or recovered from B cells of symptomatic patients in two clinical studies (4, 21) most likely replicates in B cells. These two clinical studies also suggested that B cells are a major site of DV replication. However, our results suggest that DV replicates equally well in both B cells and monocytes. It is possible that our infection conditions were not optimal to observe maximum growth of DV in B cells. As described earlier, the cultivation time and MOI (Fig. 3) used for infection dramatically affected DV replication in B cells. Therefore, conditions other than 3-day culture or an MOI of 10 may be needed to observe a growth profile closer to in vivo situations. Another possibility is that only selected individuals such as donors 1, 2, and 3, whose B cells support DV replication better than monocytes, develop clinically evident syndromes. There is support for this notion in two clinical studies (4, 21). Alternatively, another unknown factor(s) might be able to promote DV replication of B cells in vivo.
Our report is the first one to demonstrate that a heterologous antibody is able to enhance DV replication in both primary B cells and a B-cell line. The antibody is able to enhance the number of B cells infected. This is similar to results seen in monocytes (11, 12, 22). Additionally, we observed that antibodies also enhanced the level of viral replication in individual cells, as determined by measuring the mean fluorescence intensity of NS1 expression. This observation has not been previously documented. The enhancing effect of the antibody could possibly result from the antibody increasing virus entry into cells or transducing a signal to activate B cells (20) or from another unknown mechanism. The levels of ADE in B cells were not much different from those in monocytes. However, the overall observed levels were minimal compared to those in monocytic cell line U937 (6, 11, 12). This was not due to the MOI we used because the levels of ADE at two different MOIs (1 and 10) were comparable and because similar MOIs have been used to observe optimal ADE in U937 cells (6, 11, 12, 22). Although the donors from whom we obtained mononuclear cells for studies were mostly nonimmune, the levels of ADE in both their B cells and monocytes were comparable to those in monocytes obtained from immune donors (35).
In the blood circulation, the numbers of B cells and monocytes and their abilities to support DV amplification are similar. Because B cells circulate between lymphoid tissue and blood, while monocytes transit from bone marrow to peripheral tissues (reviewed in the discussion of reference 21), it is suggested that infected B cells may be more efficient than infected monocytes in spreading the virus between lymph nodes and the circulation, which are the two most common sites of virus recovery in infected patients (21, 31, 34). Taken together, these data suggest that B cells play an important role in virus amplification and spread during infection.
Role of B cells in the secretion of cytokines and autoantibodies to trigger pathological responses during infection.
This report is also the first one to show that DV infection is able to significantly induce cytokine responses in B cells and that cytokine responses of infected B cells and monocytes are comparable. RT-PCR analysis demonstrated the mRNAs of both IL-6 and TNF-α in infected cells were elevated (unpublished results). Therefore DV infection could increase synthesis of these two cytokines, either at the transcriptional level or by the enhancement of mRNA stability. In addition, we found a greater correlation between virus and cytokine production in B cells than in monocytes. This suggests that DV might be able to induce cytokine synthesis in B cells directly. In monocytes, DV infection increases the production of IL-1 (8). IL-1 is a well-known inducer of both IL-6 and TNF-α and may act to stimulate the synthesis of these two cytokines (15, 38). Future studies investigating how DV induces IL-6 and TNF-α production are warranted in order to address why the correlation between virus and cytokine production is greater in B cells than in monocytes. Interestingly, the antibody also slightly increased cytokine responses in both infected B cells and infected monocytes. This may explain why the maximum activation of endothelial cells mediated by TNF-α was obtained with culture fluids from monocytes in which virus infection was achieved by the addition of DV immune serum (1).
In addition to the activities described above, B cells could also produce autoantibodies directed to human platelets and endothelial cells to trigger pathological responses during infection (25, 26). Mechanisms including molecular mimicry and a polyclonal, nonspecific B-cell activation could cause this phenomenon. A number of viruses are able to induce polyclonal B-cell activation once they infect or interact with B cells (reviewed in reference 7). Our results show that DV not only is able to replicate in B cells but also induces IL-6, which is a growth factor as well as a differentiation factor for B cells (15). This raises the possibility of polyclonal B-cell activation after DV infection. Preliminary results have shown that B cells remained viable 1 week after infection. Studies to examine whether DV could induce polyclonal B-cell activation are in progress. Taken together, our results suggest that B cells may be an important component in DV pathogenesis.
Acknowledgments
We thank Robert Lausch and Christopher Cubitt for review of the manuscript and J.-H. Huang for analyzing DV-specific antibodies in sera.
This research was supported by grant NSC 90-2320-B-006-074 from the National Science Council (S.-H.C) and by NHRI program project grant NHRI-CN-CL8901P (S.-H.C., H.-Y.L., and T.-M.Y.) from the National Health Research Institute of the Department of Health of the Republic of China.
REFERENCES
- 1.Anderson, R., S. Wang, C. Osiowy, and A. C. Issekutz. 1997. Activation of endothelial cells via antibody-enhanced dengue virus infection of peripheral blood monocytes. J. Virol. 71:4226-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bethell, D. B., K. Flobbe, X. T. Cao, N. P. Day, T. P. Pham, W. A. Buurman, M. J. Cardosa, N. J. White, and D. Kwiatkowski. 1998. Pathological and progonostic role of cytokines in dengue hemorrhagic fever. J. Infect. Dis. 177:778-782. [DOI] [PubMed] [Google Scholar]
- 3.Boehme, M. W., Y. Deng, U. Raeth, A. Bierhaus, R. Ziegler, W. Stremmel, and P. P. Nawroth. 1996. Release of thrombomodulin from endothelial cells by concerted action of TNF-α and neutrophils: in vivo and in vitro studies. Immunology 87:134-140. [PMC free article] [PubMed] [Google Scholar]
- 4.Boonpucknavig, S., N. Bhamarapravati, S. Nimmannitya, A. Phalavadhtana, and J. Siripont. 1976. Immunofluorescent staining of the surfaces of lymphocytes in suspension from patients with dengue hemorrhagic fever. Am. J. Pathol. 85:37-48. [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt, W. E., J. M. McCown, F. H. J. Top, W. H. Bancroft, and P. K. Russell. 1979. Effect of passage history on dengue-2 virus replication in subpopulations of human leukocytes. Infect. Immun. 26:534-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandt, W. E., J. M. McCown, M. K. Gentry, and P. K. Russell. 1982. Infection enhancement of dengue type 2 virus in the U-937 human monocyte cell line by antibodies to flavivirus cross-reactive determinants. Infect. Immun. 36:1036-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cash, E., J. Charreire, and O. Rott. 1996. B-cell activation by superstimulatory influenza virus hemagglutinin: a pathogenesis for autoimmunity? Immunol. Rev. 152:67-88. [DOI] [PubMed] [Google Scholar]
- 8.Chang, D.-M., and M.-F. Shaio. 1994. Production of interleukin-1 (IL-1) and IL-1 inhibitor by human monocytes exposed to dengue virus. J. Infect. Dis. 170:811-817. [DOI] [PubMed] [Google Scholar]
- 9.Chen, Y., T. Maguire, R. E. Hileman, J. R. Fromm, J. D. Esko, R. J. Linhardt, and R. M. Marks. 1997. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 3:866-871. [DOI] [PubMed] [Google Scholar]
- 10.Citarella, F., A. Felici, M. Brouwer, J. Wagstaff, A. Fantoni, and C. E. Hack. 1997. Interleukin-6 downregulates factor XII production by human hepatoma cell line (HepG2). Blood 90:1501-1507. [PubMed] [Google Scholar]
- 11.Diamond, M. S., D. Edgil, T. G. Roberts, B. Lu, and E. Harris. 2000. Infection of human cells by dengue virus is modulated by different cell types and viral strains. J. Virol. 74:7814-7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond, M. S., T. G. Roberts, D. Edgil, B. Lu, J. Ernst, and E. Harris. 2000. Modulation of dengue virus infection in human cells by alpha, beta, and gamma interferons. J. Virol. 74:4957-4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gubler, D. J., W. Suharyono, R. Tan, M. Abidin, and A. Sie. 1981. Viraemia in patients with naturally acquired dengue infection. Bull. W. H. O. 59:623-630. [PMC free article] [PubMed] [Google Scholar]
- 14.Halstead, S. B., and E. J. O'Rourke. 1977. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J. Exp. Med. 146:201-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirano, T. 1994. Interleukin-6, p. 145-168. In A. W. Thomson (ed.), The cytokine handbook, 2nd ed. Academic Press Inc., San Diego, Calif.
- 16.Ho, L.-J., J.-J. Wang, M.-F. Shaio, C.-L. Kao, D.-M. Chang, S.-W. Han, and J.-H. Lai. 2001. Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J. Immunol. 166:1499-1506. [DOI] [PubMed] [Google Scholar]
- 17.Hober, H., L. Poli, B. Roblin, P. Gestas, E. Chungue, G. Granic, P. Imbert, J. L. Pecarere, R. Vergez-Pascal, P. Wattre, and M. Maniez-Montreuil. 1993. Serum level of tumor necrosis factor (TNF-alpha), interleukin-6 (IL-6) and interleukin-1 beta (IL-1 beta) in dengue-infected patients. Am. J. Trop. Med. Hyg. 48:324-331. [DOI] [PubMed] [Google Scholar]
- 18.Huang, Y.-H., H.-Y. Lei, H.-S. Liu, Y.-S. Lin, C.-C. Liu, and T.-M. Yeh. 2000. Dengue virus infects human endothelial cells and induces IL-6 and IL-8 production. Am. J. Trop. Med. Hyg. 63:71-75. [DOI] [PubMed] [Google Scholar]
- 19.Innis, B. L., A. Nisalak, S. Nimmannitya, S. Kusalerdchariya, V. Chongswasdi, S. Suntayakorn, P. Puttisri, and C. H. Hoke. 1989. An enzyme-linked immunosorbent assay to characterize dengue and Japanese encephalitis co-circulate. Am. J. Trop. Med. Hyg. 40:418-427. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs, M. G., P. J. Robinson, C. Bletchly, J. M. Mackenzie, and P. R. Young. 2000. Dengue virus nonstructural protein 1 is expressed in a glycosyl-phosphatidylinositol-linked form that is capable of signal transduction. FASEB J. 14:1603-1610. [DOI] [PubMed] [Google Scholar]
- 21.King, A. D., A. Nisalak, S. Kalayanrooj, K. S. A. Myint, K. Pattanapanyasat, S. Nimmannitya, and B. L. Innis. 1999. B cells are the principal circulating mononuclear cells infected by dengue virus. Southeast Asian J. Trop. Med. Public Health 30:718-728. [PubMed] [Google Scholar]
- 22.Kontny, U., I. Kurane, and F. A. Ennis. 1988. Gamma interferon augments Fcγ receptor-mediated dengue virus infection of human monocytic cells. J. Virol. 62:3928-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurane, I., and F. A. Ennis. 1992. Immunity and immunopathogenesis in dengue virus infections. Semin. Immunol. 4:121-127. [PubMed] [Google Scholar]
- 24.Lei, H.-Y., T.-M. Yeh, H.-S. Liu, Y.-S. Lin, S.-H. Chen, and C.-C. Liu. 2001. Immunopathogenesis of dengue virus infection. J. Biomed. Sci. 8:377-388. [DOI] [PubMed] [Google Scholar]
- 25.Lin, C.-F., H.-Y. Lei, C.-C. Liu, H.-S. Liu, T.-M. Yeh, S.-T. Wang, T.-I. Yang, F.-C. Shen, C.-F. Kuo, and Y.-S. Lin. 2001. Generation of IgM anti-platelet autoantibody in dengue patients. J. Med. Virol. 63:143-149. [PubMed] [Google Scholar]
- 26.Lin, C.-F., H.-Y. Lei, A.-L. Shiau, H.-S. Liu, T.-M. Yeh, S.-H. Chen, C.-C. Liu, S.-C. Chiu, and Y.-S. Lin. 2002. Endothelial cell apoptosis induced by antibodies against dengue virus nonstructural protein 1 via production of NO. J. Immunol. 169:657-664. [DOI] [PubMed] [Google Scholar]
- 27.Mauro, N., I. Morita, M. Shirao, and S. I. Murota. 1992. IL-6 increases endothelial permeability in vitro. Endocrinology 131:710-714. [DOI] [PubMed] [Google Scholar]
- 28.Murgue, B., C. Roche, E. Chungue, and X. Deparis. 2000. Prospective study of the duration and magnitude of viraemia in children hospitalised during the 1996-1997 dengue-2 outbreak in French Polynesia. J. Med. Virol. 60:432-438. [DOI] [PubMed] [Google Scholar]
- 29.Ravetch, J. V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275-290. [DOI] [PubMed] [Google Scholar]
- 30.Rigau-Perez, J. G., G. G. Clark, D. J. Gubler, P. Reiter, E. J. Sanders, and A. V. Vorndam. 1998. Dengue and dengue haemorrhagic fever. Lancet 352:971-977. [DOI] [PubMed] [Google Scholar]
- 31.Rosen, L., M. T. Drouet, and V. Deubel. 1999. Detection of dengue virus RNA by reverse transcription-polymerase chain reaction in the liver and lymphoid organs but not in the brain in fatal human infection. Am. J. Trop. Med. Hyg. 61:720-724. [DOI] [PubMed] [Google Scholar]
- 32.Rothman, A. L., and F. A. Ennis. 1999. Immunopathogenesis of dengue hemorrhagic fever. Virology 257:1-6. [DOI] [PubMed] [Google Scholar]
- 33.Schratzberger, P., N. Reinisch, W. M. Prodinger, C. M. Kahler, B. A. Sitte, R. Bellmann, R. Fisher-Colbrie, H. Winkler, and C. J. Wiedermann. 1997. Different chemotactic activities of sensory neuropeptides for human peripheral blood mononuclear cells. J. Immunol. 158:3895-3901. [PubMed] [Google Scholar]
- 34.Scott, R. M., A. Nisalak, U. Cheamudon, S. Seridhoranakul, and S. Nimmannitya. 1980. Isolation of dengue viruses from peripheral blood leukocytes of patients with hemorrhagic fever. J. Infect. Dis. 141:1-6. [DOI] [PubMed] [Google Scholar]
- 35.Sittisombut, N., N. Maneekarn, A. Kanjanahaluethai, W. Kasinrerk, K. Viputtikul, and J. Supawadee. 1995. Lack of augmenting effect of interferon-γ on dengue virus multiplication in human peripheral blood monocytes. J. Med. Virol. 45:43-49. [DOI] [PubMed] [Google Scholar]
- 36.Sudiro, T. M., J. Zivny, H. Ishiko, S. Green, D. W. Vaughn, S. Kalayanarooj, A. Nisalak, J. E. Norman, F. A. Ennis, and A. L. Rothman. 2001. Analysis of plasma viral RNA levels during acute dengue virus infection using quantitative competitor reverse transcription-polymerase chain reaction. J. Med. Virol. 63:29-34. [PubMed] [Google Scholar]
- 37.Theofilopoulos, A. N., W. E. Brandt, P. K. Russell, and F. T. Dixon. 1976. Replication of dengue-2 virus in cultured human lymphoblastoid cells and subpopulations of human peripheral leukocytes. J. Immunol. 117:953-961. [PubMed] [Google Scholar]
- 38.Tracey, K. J. 1994. Tumour necrosis factor, p. 289-318. In A. W. Thomson (ed.), The cytokine handbook, 2nd ed. Academic Press Inc., San Diego, Calif.
- 39.Vaughn, D. W., S. Green, S. Kalayanarooj, B. L. Innis, S. Nimmannitya, S. Suntayakorn, T. P. Endy, B. Raengsakulrach, A. L. Rothman, F. A. Ennis, and A. Nisalak. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 181:2-9. [DOI] [PubMed] [Google Scholar]
- 40.Vitarana, T., H. de Sliva, N. Withana, and C. Gunasekera. 1991. Elevated tumor necrosis factor in dengue fever and dengue haemorrhagic fever. Ceylon Med. J. 36:63-65. [PubMed] [Google Scholar]