Abstract
Insulin-dependent (type 1) diabetes mellitus (T1D) onset is mediated by individual human genetics as well as undefined environmental influences such as viral infections. The group B coxsackieviruses (CVB) are commonly named as putative T1D-inducing agents. We studied CVB replication in nonobese diabetic (NOD) mice to assess how infection by diverse CVB strains affected T1D incidence in a model of human T1D. Inoculation of 4- or 8-week-old NOD mice with any of nine different CVB strains significantly reduced the incidence of T1D by 2- to 10-fold over a 10-month period relative to T1D incidences in mock-infected control mice. Greater protection was conferred by more-pathogenic CVB strains relative to less-virulent or avirulent strains. Two CVB3 strains were employed to further explore the relationship of CVB virulence phenotypes to T1D onset and incidence: a pathogenic strain (CVB3/M) and a nonvirulent strain (CVB3/GA). CVB3/M replicated to four- to fivefold-higher titers than CVB3/GA in the pancreas and induced widespread pancreatitis, whereas CVB3/GA induced no pancreatitis. Apoptotic nuclei were detected by TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assay in CVB3/M-infected pancreata but not in CVB3/GA-infected pancreata. In situ hybridization detected CVB3 RNA in acinar tissue but not in pancreatic islets. Although islets demonstrated inflammatory infiltrates in CVB3-protected mice, insulin remained detectable by immunohistochemistry in these islets but not in those from diabetic mice. Enzyme-linked immunosorbent assay-based examination of murine sera for immunoglobulin G1 (IgG1) and IgG2a immunoreactivity against diabetic autoantigens insulin and HSP60 revealed no statistically significant relationship between CVB3-protected mice or diabetic mice and specific autoimmunity. However, when pooled sera from CVB3/M-protected mice were used to probe a Western blot of pancreatic proteins, numerous proteins were detected, whereas only one band was detected by sera from CVB3/GA-protected mice. No proteins were detected by sera from diabetic or normal mice. Cumulatively, these data do not support the hypothesis that CVB are causative agents of T1D. To the contrary, CVB infections provide significant protection from T1D onset in NOD mice. Possible mechanisms by which this virus-induced protection may occur are discussed.
The group B coxsackieviruses (CVB; family Picornaviridae, genus Enterovirus, species group B coxsackievirus; six serotypes, CVB1 to -6) are among the best studied of human enteroviruses (102). The CVB genome is a single strand of positive sense RNA 7,400 nucleotides in length that encodes 11 proteins in a single open reading frame (89). The CVB have been associated with diverse human diseases, among the more serious of which are myocarditis, pancreatitis, and aseptic meningitis. The CVB have been soundly implicated as causes of human myocarditis (1, 26, 42, 60-62, 73, 74, 108, 109) and pancreatitis (2, 41, 54, 58, 66, 107) and, furthermore, cause these diseases readily in mice (9, 40, 43, 85, 86, 105). Although CVB have been suggested as infectious triggers of human insulin-dependent (type 1) diabetes mellitus (T1D) (7, 22, 34, 53, 116), there is no consensus as to the etiologic role for CVB in T1D development (25, 32, 33, 47, 52, 63, 64, 110). Unlike the readiness with which CVB cause pancreatitis and myocarditis in mice, very few CVB strains have been characterized that induce even transient glucose abnormalities or diabetes in inbred strains of mice (56, 87, 99).
T1D results from the destruction of insulin-producing beta cells in the pancreatic islets of Langerhans through an autoimmune process (5, 17, 30). The mechanism(s) that determine when and why T1D occurs is not well defined (6, 64, 71, 95). The etiology of human T1D has a genetic component that serves to explain many but not all cases of T1D (114); in identical twins, T1D concordance varies at or <40% (8, 12, 30, 69, 88). Consequently, an environmental contribution to the etiology has been proposed to account for the overall observed T1D incidence (37, 63, 111), although the mechanism(s) by which this occurs is not known. Various putative environmental agents have been suggested, among the most common of which are viral infections (55). In utero rubella virus infections have been associated with higher incidences of T1D in these children than in uninfected controls (76). This has been attributed to antigenic mimicry because T cells of diabetic humans that recognize similar antigenic epitopes in glutamic acid decarboxylase (GAD65), a pancreatic autoantigen, can also recognize rubella virus (81). Diabetic autoantigens such as GAD65, HSP60, and tyrosine phosphatases have been linked to common antigenic epitopes in coxsackieviral and enteroviral proteins (3, 44). Activation of B cells with Escherichia coli lipopolysaccharide suppresses Th1 immunity in nonobese diabetic (NOD) mice (97), suggesting a mechanism by which an infectious event could impact B cells and so contribute to the intricate etiology of T1D (92). Although CVB or enteroviral RNA has been associated with some cases of diabetes (7, 22, 35, 53, 90), it remains unresolved whether antigenic mimicry between enteroviral and pancreatic proteins is involved in the pancreatic islet and insulin producing beta-cell destruction that occurs in human T1D.
The NOD mouse mimics many aspects of human T1D (4, 72, 115). The highly diabetes-prone NOD mouse begins to shed glucose in the urine and becomes hyperglycemic at ca. 15 weeks of age. Insulitis, characterized by inflammatory infiltrates in the insulin-producing beta-cell containing islets, also occurs. T1D can be suppressed or postponed in this model by exposure to a wide variety of agents (reviewed in reference 4). Infection of NOD mice with different rodent viruses (lymphocytic choriomeningitis virus [LCMV] [79], murine hepatitis virus [113], encephalomyocarditis virus [46], and lactate dehydrogenase virus [96]) suppresses T1D incidence in NOD mice to various extents, although the mechanisms by which these diverse viruses suppress T1D have not been elucidated. Oldstone et al. proceeded to map T1D suppressive activity in NOD mice by LCMV, strain Pasteur, to the S RNA genome segment (80), thereby demonstrating that specific viral genetics can play a role in T1D suppression.
Despite observations that rodent viruses suppressed T1D incidence in the widely accepted NOD mouse model of human T1D (4), few reports explored the impact which different strains of CVB, the human enterovirus most associated with an etiologic role in human T1D, have upon diabetes development in these mice. Whereas the experiments reported here were in progress, CVB4/Edwards (59, 112) was reported to increase the rate of diabetes onset in 61.5% of older (8-week-old) NOD mice but not in younger (6-week-old) mice (94); these mice were maintained through 23 weeks of age. It was suggested that diabetes was accelerated only in older mice due to ongoing insulitis and a growing pool of autoimmune lymphocytes, a result subsequently confirmed by another group (49). It has been shown by using a variety of transgenic NOD and other mouse strains that infection by CVB4/Edwards alone is insufficient to induce diabetes in mice (50), a finding consistent with the general inability of most CVB strains to induce diabetes or glucose abnormalities in mice. Recently, the action of interferons has been demonstrated to be key in preventing productive replication of CVB4/Edwards in NOD mouse pancreatic islets (31).
Although often cited as putative inducers of human T1D, the actual impact of CVB infection on T1D incidence in NOD mice—the best experimental model for human T1D—has not been adequately examined. To study the effect of CVB infection in NOD mice, we examined CVB3 replication in young (4-week-old) mice, in which islet inflammation is not detectable, and in older (8-week-old), prediabetic mice in which islet inflammation has begun. We demonstrate here that inoculation of NOD mice with any of nine different CVB strains results in 2- to 10-fold-lower T1D incidence than in mock-infected groups. Greater extents of protection were provided by more pathogenic CVB strains and correlated with the presence of higher virus titers, virus-induced pancreatitis, and the induction of an antipancreas autoimmunity.
MATERIALS AND METHODS
Viruses.
The CVB type 3 (CVB3) and CVB4 strains used in the present study have been previously described (105). Table 1 lists the viruses used in the present study with the known phenotypes as measured in inbred lines of mice. CVB4, strain Edwards, was generously provided as a virus stock from Charles Gauntt (University of Texas Health Sciences Center, San Antonio, Tex.); this strain has been reported to induce glucose abnormalities in some inbred mice (59, 112). CVB4, strain JVB/Benschoten, was purchased from the American Type Culture Collection (Manassas, Va.) as a virus stock. The RNA genomes of coxsackievirus type B3, strains M and 20, have been cloned as infectious cDNA copies (67, 103). The genome of CVB3/28 (pCVB3-28 [106]) was derived from the infectious cDNA clone of CVB3 strain 0 (CVB3/0 [19]) by alteration of nucleotide 234 from C to T; the resultant virulence phenotype is that of the CVB3/20 virus strain. All CVB were propagated in HeLa cell monolayers from preexisting virus stocks or from infectious cDNA transfection (CVB3/M and CVB3/28) as described previously (106). Lysates of CVB inoculated HeLa cell cultures were frozen and thawed three times (−75 and 37°C), after which cellular debris was removed by centrifugation at 120,000 × g for 30 min. Viruses were divided into aliquots and stored at −75°C. Virus titers were determined as described previously on HeLa cell monolayers and expressed as the 50% tissue culture infective dose (TCID50) per ml (48).
TABLE 1.
Naturally occurring CVB strains used in this studya
| Virus strain | Heart phenotypeb | Pancreas phenotypeb | Reference(s) |
|---|---|---|---|
| CVB3/M | Myocarditic | Pancreovirulent | 67, 104, 105 |
| CVB3/20 | Myocarditic | Pancreovirulent | 103 |
| CVB3/ZU | Myocarditic | Pancreovirulent | 104, 105 |
| CVB3/AS | Myocarditic | Pancreovirulent | 104, 105 |
| CVB3/OL | Not virulent | Pancreovirulent | 105 |
| CVB3/CO | Not virulent | Pancreovirulent | 104 |
| CVB3/GA | Not virulent | Not virulent | 104, 105 |
| CVB4/Edwards | Not known | Pancreovirulent | 59, 75, 94, 112 |
| CVB4/JVB Benschoten | Not known | Not known | 24 |
References direct the reader to data pertaining to the characterization of the phenotypes.
“Myocarditic” is defined as the ability to induce inflammatory lesions in mouse heart muscle within 5 to 8 days of inoculation in all or the majority of mice. “Pancreovirulent” is defined as the ability to induce inflammation of mouse pancreatic acinar tissue (pancreatitis) within 2 to 4 days of inoculation in all or many mice. “Not virulent” is defined as inducing no observable inflammatory disease or tissue destruction in any of the mice.
Mice.
NOD female mice (NODMrk/Tac) were purchased from Taconic Laboratories (Germantown, Pa.) at 4 or 8 weeks of age. The weights of randomly chosen mice from each shipment were consistent with the weight for age specified by the supplier. Five mice were housed per cage and provided steam-sterilized water and food (Teklad 7012 Mouse/Rat Sterilizable Diet; Harlan-Teklad, Madison, Wis.) ad libitum. All cage components and bedding were steam sterilized. Mice were rested for 3 days before inoculation, and then mice of the same age were randomized into groups of 10 and inoculated with virus (5 × 105 TCID50 in 0.1 ml of sterile unsupplemented RPMI tissue culture medium) or virus diluent (mock-infected controls) at 4 and again at 6 weeks of age or at 8 and again at 10 weeks of age unless noted. Mice were euthanized by carbon dioxide inhalation, followed by cervical dislocation.
Determination of diabetes onset.
Due to the number of mice involved and the length of time mice were maintained, timely evaluation of individual blood sugar levels on a weekly basis was prohibitive. Diabetes onset was therefore assayed weekly by determining glucose content in the urine by using dipsticks (Diastix; Bayer, Elkhart, Ind.). A mouse was considered diabetic when the glucose content measured ≥2,000 mg of glucose per ml of urine, the highest concentration measurable on the dipsticks. Mice developed glycosuria rapidly within a week; rarely did we observe glucose levels lower than the maximum measurable. Repeat measurement of numerous glycosuric mice over several weeks showed that glycosuria was not reversible. Diabetic mice were killed within 1 week of developing diabetes. At sacrifice, assay of blood for glucose content (Accu-Check Advantage; Roche Diagnostics Corp., Indianopolis, Ind.) confirmed that glycosuric mice were also hyperglycemic. The average glucose levels in the sera of 36 normal, nondiabetic (nonglycosuric) control mice between 4 and 6 weeks of age were 140 ± 20 mg/dl, whereas the glucose levels of diabetic (glycosuric) mice were all greater than 600 mg/dl (data not shown); a value of >300 mg/ml can be considered diabetic (79). All nondiabetic mice surviving to the end of the study were healthy, with sleek fur, normal weight for age, normal body shape (diabetic mice become pear-shaped), and normal grooming and nesting behaviors (diabetic mice fail to groom or nest normally). The statistical significance of the results was evaluated by using Fisher exact test.
Measurement of virus titers in tissue.
Pancreas and heart tissue samples were obtained and frozen under dry ice and then stored at −75°C until the time of assay. Virus titers in pancreas and heart samples were determined as previously described (105). Briefly, frozen tissue was homogenized in complete tissue culture medium, frozen and thawed, and centrifugally cleared of debris, and titers were determined on HeLa cell monolayers. All titers were expressed as TCID50/gram of tissue.
Evaluation of pathological changes in tissue.
Pancreatic tissue obtained at sacrifice was placed in buffered formalin and then paraffin embedded and sectioned at a 6-μm thickness for staining with hemotoxylin and eosin (105). Light microscopic examination determined whether pancreatitis was present (105).
Immunohistochemistry.
Immunohistochemical detection of insulin and glucagon was performed essentially as described previously (27). Pancreas sections were deparaffinized in xylene and then rehydrated in an ethanol series. Sections were blocked in normal goat or rabbit sera in phosphate-buffered saline (PBS) for 20 min at room temperature (RT). Antigen staining was performed with either a guinea pig anti-pig antibody to insulin (Sigma, St. Louis, Mo.) or a polyclonal rabbit anti-human antisera to glucagon (BD Pharmingen, San Diego, Calif.). Slides were incubated 1 h at RT with the primary antibody at a dilution of 1:250. After a 1-h incubation with a secondary biotinylated antibody (rabbit anti-guinea pig or goat anti-rabbit; Vector Laboratories, Burlingame, Calif.), immunoreactivity was detected by using the avidin-biotin immunoperoxidase technique (Vector Labs). The reaction product was visualized by using Hanker Yates reagent (Polysciences, Warrington, Pa.) with hydrogen peroxide as the substrate. Slides were counterstained with Mayer's hematoxylin. A dark brown reaction product is indicative of positive staining. For determination of apoptosis, TUNEL was carried out on paraffin-embedded pancreas sections by using the ApopTag kit (Intergen, Purchase, N.Y.) according to the suggested protocol (27).
In situ hybridization.
The CVB3/20 subclone pGP51 (101) containing nucleotides 1 to 534 of the CVB3 genome in pGEM1 (Promega, Madison, Wis.) was employed for the in situ detection of CVB3 RNA in pancreatic tissue. These DNAs were used to generate RNA probes in a runoff synthesis reaction that incorporated digoxigenin-labeled dUTP per the manufacturer's instructions (Roche Molecular Biochemicals, Elkhardt, Ind.). Synthesis from the T7 promoter of HindIII-digested DNA resulted in the generation of a negative-stranded probe (for detection of positive-strand viral RNA). In situ hybridization was carried out essentially as described previously (28, 100). At the time of assay, formalin-fixed tissue sections, 6-μm thick, were deparaffinized and rehydrated in a 100 to 50% ethanol series. Slides were briefly treated with proteinase K, acetylated in 0.1 M triethanolamine-acetic anhydride, and prehybridized 2 h at 37°C. Slides were hybridized for 16 to 20 h at 37°C, washed, RNase treated, blocked, and incubated with the alkaline phosphatase-conjugated anti-digoxigenin antibody (diluted 1:1,000; Roche Biochemicals). Slides were developed with nitroblue tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate; Roche Biochemicals) for 18 h in the dark and then washed and counterstained with 1% eosin.
Enzyme linked immunosorbent assay.
Enzyme-linked immunosorbent assay were used to detect immunoglobulins in murine sera that bind pancreatic autoantigens insulin (recombinant human; Sigma) and HSP60 (StressGen, Vancouver, Canada). Insulin and HSP60 were used at 0.5 μg/ml. Capture antigen (50 μl/well) was used to coat 96-well flat-bottom plates (Costar 3590; Costar, Corning, N.Y.) for 24 h at 4°C. After a washing step in 160 mM NaCl containing 0.05% Tween 20 (Sigma), the plates were blocked for 2 h in PBS containing 0.1% (wt/vol) bovine serum albumin and 0.05% (vol/vol) Tween 20 (PBS-BT) at RT. Murine sera were centrifuged, diluted 1/100 in PBS-BT, applied at 50 μl per well, and then incubated at 37°C for 1 h. After the plate was washed, murine immunoglobulin were detected by using either horseradish peroxidase (HRP)-labeled rat anti-mouse immunoglobulin G1 (IgG1) or IgG2a (BD Pharmingen, San Diego, Calif.), 50 μl per well, and diluted 1/1,000 in PBS-BT for 1 h at 37°C. Color was developed by using 3,3′,5,5′-tetramethylbenzidine and hydrogen peroxide (TMB substrate; BD Pharmingen). Color development was stopped with the addition of 2 N sulfuric acid. Samples were assayed in triplicate, and the statistical significance was evaluated by using the Student t test.
Western blot analysis.
Pancreata excised from 4-week-old female NOD mice were rinsed in ice-cold Tris-buffered saline (TBS)-EDTA (0.05 M Tris; 0.1 M NaCl, pH 7.6; 0.01% NaN3; 10 mM EDTA) containing 2 mM phenylmethylsulfonyl fluoride and 5 mM N-ethylmaleimide and then processed in a Tenbroeck homogenizer until dispersed. Pancreas was lysed by adding n-octyl glucoside (Sigma) to a final concentration of 2% (wt/vol). The sample was vortexed, placed on ice for 15 min, and then centrifuged at 4°C at 1,800 × g for 15 min. The supernatant was collected and added to 5 mg of soybean trypsin inhibitor (Sigma), whereas the pellet was reextracted with 2.5 ml of TBS-EDTA-phenylmethylsulfonyl fluoride-N-ethylmaleimide and 2% n-octyl glucoside. This supernatant was combined with the first lysate and stored in aliquots at −20°C. Individual lysate aliquots were thawed at 37°C and vortexed to suspend the precipitate that formed on freezing. Samples of the suspension were combined with 0.5 volumes of Laemmli-sodium dodecyl sulfate (SDS) sample solvent (65) without reducing agents and then boiled for 10 min. Samples were applied to 10 to 15% polyacrylamide gradient gels containing SDS (65), and proteins were resolved by electrophoresis. Proteins were transferred to Immobilon-P membranes (Millipore, Bedford, Mass.) and blocked overnight at 4C in TBS containing 0.02% (vol/vol) Tween 20 and 6% (wt/vol) nonfat dried milk. Membranes were rinsed in TBS-Tween, sliced into strips, and incubated with mouse sera diluted 1/500 in TBS-Tween-nonfat milk. The membranes were washed with TBS-Tween and incubated with HRP-conjugated rabbit anti-mouse immunoglobulins (Dako, Carpenteria, Calif.) diluted 1/1,000 in TBS-Tween. After a washing step, blots were developed with ECL+Plus (Amersham, Piscataway, N.J.) by using Kodak BioMax film. Films were digitally scanned and printed from Adobe PhotoShop.
RESULTS
Effect on diabetes onset by inoculation of NOD mice with CVB.
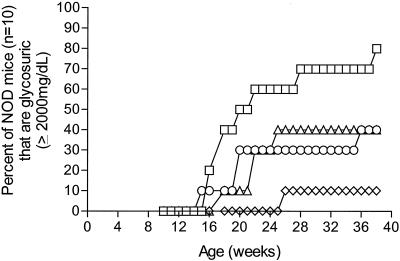
The effect of CVB3 infection upon T1D onset in NOD mice was initially evaluated by using three CVB3 strains that had been selected for specific virulence phenotypes (105). Strain CVB3/M causes both pancreatitis and myocarditis in various inbred mice, strain CVB3/OL causes variable levels of pancreatitis but not myocarditis, and strain CVB3/GA is nonpathogenic (Table 1); all three strains replicate well and induce immune responses in mice. Groups of 10 female NOD mice were inoculated twice, first at 4 and again at 6 weeks of age. Examination of NOD mouse pancreata taken at 4 weeks of age prior to virus inoculation showed no evidence of inflammatory infiltrates in the pancreatic islets (insulitis) (data not shown). The group of mock-infected control mice rapidly developed T1D; 70% were diabetic by 28 weeks of age (Fig. 1). In contrast, only 40% of CVB3/GA mice, 30% of CVB3/OL mice, and 10% of CVB3/M mice were diabetic at this age. The experiment was halted when surviving mice were 10 months old (38 week old). At this age, just one mouse inoculated with CVB3/M had developed diabetes; the other nine remained healthy and normoglycemic. The other two CVB3-inoculated groups experienced a 40% T1D incidence, twofold-lower than mock-infected control mice at 80%. The results of this experiment with three biologically diverse CVB3 strains suggested that CVB3, instead of promoting faster T1D onset or higher T1D incidences in NOD mice, significantly suppressed T1D incidence (P = 0.0073). In mice that developed diabetes, initial disease onset was slowed relative to controls by 2 to 5 weeks.
FIG. 1.
CVB3 inoculation of young NOD mice suppresses diabetes incidence. Female NOD mice were inoculated intraperitoneally with 5 × 105 TCID50 of the CVB3 strains shown at 4 and 6 weeks of age. Control mice received virus diluent only. Suppression of T1D incidence by CVB3 was statistically significant (P = 0.0073). Symbols: □, control; ◊, CVB3/M; ○, CVB3/OL; ▵, CVB3/GA.
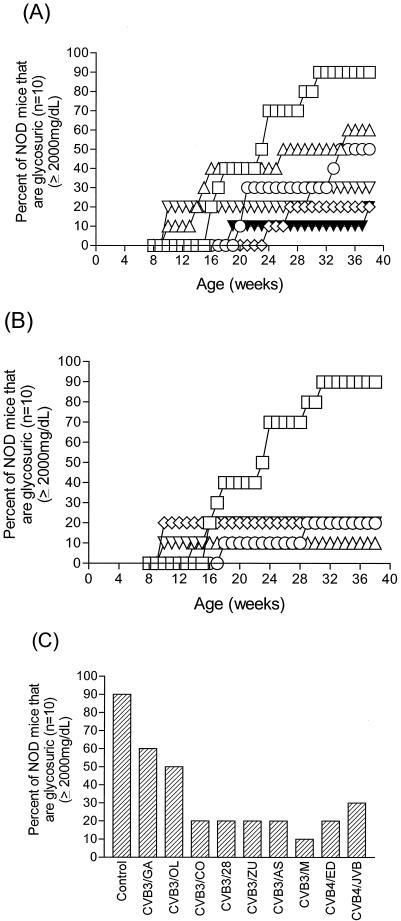
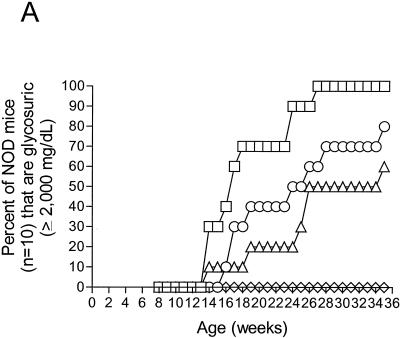
While this work was in progress, other researchers demonstrated that a CVB4 strain accelerated T1D in some mice when they were were inoculated at 8 weeks of age (94). To determine whether our findings were limited to a small number of CVB3 strains or were a general property of CVB infections in NOD mice, we expanded our studies to include more CVB strains. We used seven CVB3 strains (the previous three used in Fig. 1 and four additional ones), as well as two CVB4 strains (Table 1). Since the possibility existed that only younger mice could be protected from T1D onset by CVB inoculation, we used 8-week-old mice to test whether this protection was an age-related phenomenon. We inoculated groups (n = 10) of female NOD mice at 8 weeks of age and then again at 10 weeks of age. Untreated mice at 8 weeks of age and prior to virus inoculation showed developing insulitis in each of five mice examined (data not shown). Mice were assayed weekly starting at 8 weeks of age through 39 weeks of age for glycosuria (Fig. 2). The data have been arbitrarily separated for ease of examination into cardiovirulent CVB3 strains and controls (Fig. 2B) and all other viruses (Fig. 2A), whereas the T1D incidences in all nine virus-inoculated groups at the end of the experiment are shown in Fig. 2C. At the end of the experiment, 90% (9 of 10) mock-infected control mice had developed diabetes. Similar to results observed in the first experiment, all seven CVB3 and two CVB4 strains significantly suppressed T1D incidences relative to control, mock-infected mice by 2- to 10-fold.
FIG. 2.
CVB3 and CVB4 inoculation of older, prediabetic NOD mice suppresses diabetes incidence. Female NOD mice were inoculated intraperitoneally with 5 × 105 TCID50 of the CVB3 and CVB4 strains shown at 8 and 10 weeks of age. (A and B) Kinetics of diabetes onset for three of the less pathogenic CVB3 strains and two CVB4 strains (A) and for the pathogenic CVB3 strains (B). Symbols in panel A: □, control; ▵, CVB3/GA; ○, CVB3/OL; ◊, CVB3/CO; ▾, CVB4/ED; ▿, CVB4/JVB. Symbols in panel B: □, control; ○, B3/28; ◊, B3/ZU; ▿, B3/AS; ▵, B3/M. (C) Bar graph showing the final T1D incidences in each group when the experiment was ended at 10 months of age. Suppression of T1D by the less-pathogenic strains CVB3/OL, CVB3/CO, and CVB3/GA and the two CVB4 strains (in panel A) or by the pathogenic strains CVB3/M, CVB3/20, CVB3/AS, and CVB3/ZU (in panel B) were statistically significant (P = 0.028 and P = 0.000039, respectively).
It is apparent that certain strains (Fig. 2B) were more efficient in protecting NOD mice from developing diabetes than other strains (Fig. 2A). The CVB3 strains have been characterized previously (105) and, with the exception of CVB3/GA, cause pancreatitis in mice; the specific strains shown in Fig. 2B also induce myocarditis in many inbred mice, although we have never observed myocarditis in NOD mice. In all groups for which protection from T1D was high (T1D incidence ranged between 10 to 20%), pancreatitis was observed in pancreas tissue sections (see Fig. 5). CVB3 strains with established cardiovirulent phenotypes in other mice (CVB3/20, CVB3/ZU, CVB3/AS, and CVB3/M; Fig. 2B) protected 8 to 9 of 10 mice from T1D. As in the previous experiment, CVB3/M protected 90% of mice from T1D onset through 10 months of age. The less-pathogenic CVB3/CO and CVB3/OL strains and the avirulent strain CVB3/GA, as well as the two CVB4 strains, protected between 50 and 80% of mice from T1D onset. We observed that four CVB3 strains and one CVB4 strain accelerated T1D onset in 10 to 40% of these older mice by as much as 6 weeks ahead of control mock-infected mice (Fig. 2A and B); T1D acceleration was not induced by all CVB strains in this experiment. With the exception of CVB3/GA, the T1D incidences in groups of mice in which accelerated T1D was observed did not increase beyond the original levels (Fig. 2C). In no case, did T1D incidence in groups of mice in which accelerated disease onset occurred approach the incidence in mock-infected control mice. Acceleration of disease onset would have been predicted in all groups were preexisting autoimmune T cells the only arbiter (94), suggesting that both mouse age and virus strain must play roles in the mechanism of early T1D onset in NOD mice. Cumulatively, the results of inoculating both young and older NOD mice demonstrated that inoculation with CVB is beneficial to the group as a whole, reducing the normal incidence of T1D from 80 to 90% in mock-infected control mice to between 10 and 50% in CVB-inoculated mice. This occurred even in older mice with evidence of the developing insulitis that precedes frank diabetes. The inclusion and comparison of two CVB4 strains alongside CVB3 strains did not demonstrate a CVB4 type-specific predisposition for inducing more cases of T1D, demonstrating that the generally beneficial effect of CVB inoculation in NOD mice for suppressing T1D incidence is not limited to a single CVB species.
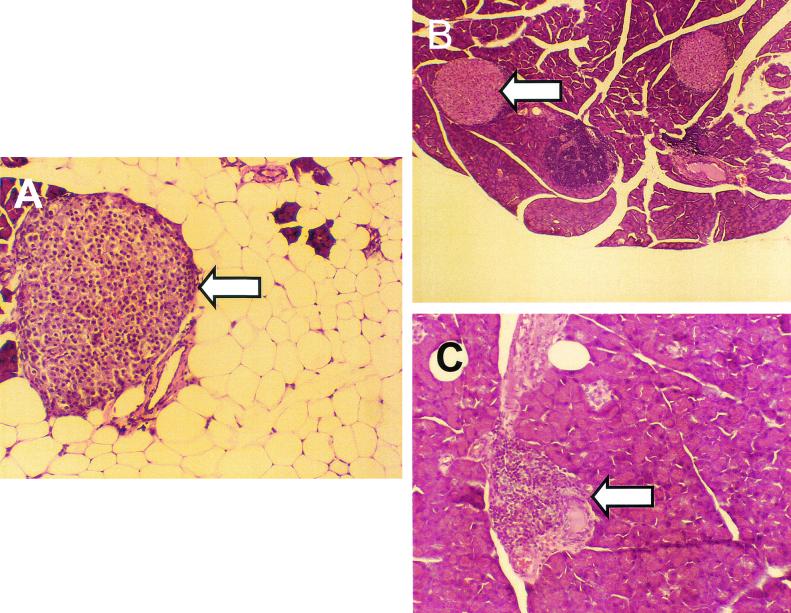
FIG. 5.
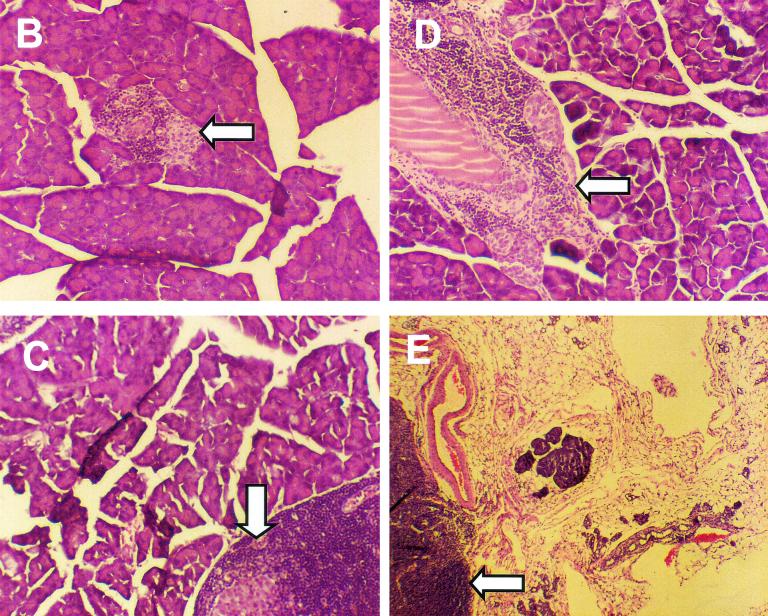
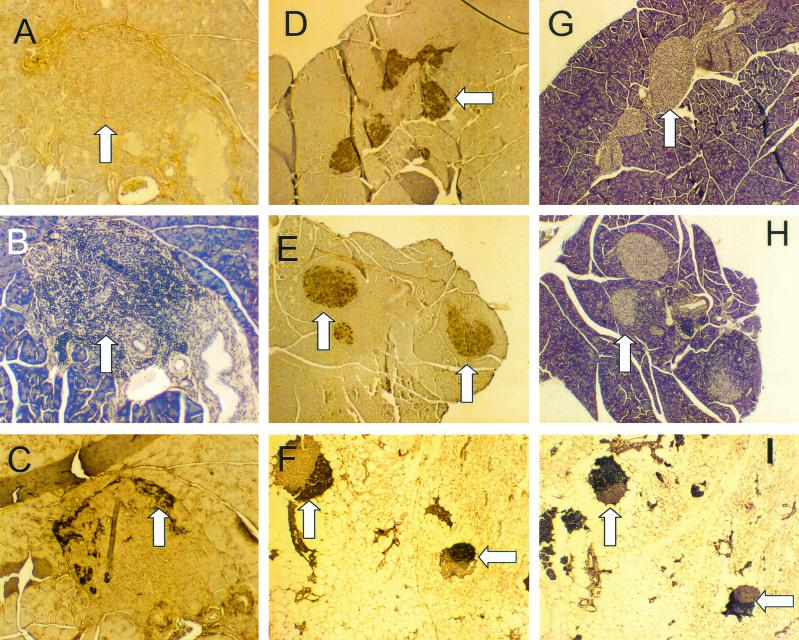
CVB3/M inoculation induced pancreatitis, which resulted in widespread acinar tissue damage and fat replacement. Sections from formalin-fixed and embedded pancreata of mice inoculated at 4 and 6 weeks of age with CVB3/M (A), CVB3/GA (B), or virus diluent only (control; C) and sacrificed at 38 weeks of age were stained with hemotoxylin and eosin. Loss of acinar tissue subsequent to CVB3/M replication is evident in panel A, whereas exocrine tissue appears to be normal in pancreata from mice inoculated with CVB3/GA (panel B compared to panel C). All images captured at ×75 magnification. Arrowheads indicate islets.
Replication of CVB3 in NOD mice.
The foregoing work demonstrated fewer cases of T1D occurred in groups of NOD mice inoculated with the more pathogenic CVB3 strains compared to mice inoculated with less pathogenic or avirulent strains. To characterize this system in greater detail, we selected two strains of CVB3 for further analysis. The pathogenic strain CVB3/M (which is capable of inducing both pancreatitis and myocarditis in inbred mice [19, 36, 105]) and the avirulent strain CVB3/GA (which replicates well in mice but induces no apparent disease [105]) represent polar ends of the established CVB pathogenicity spectrum (105).
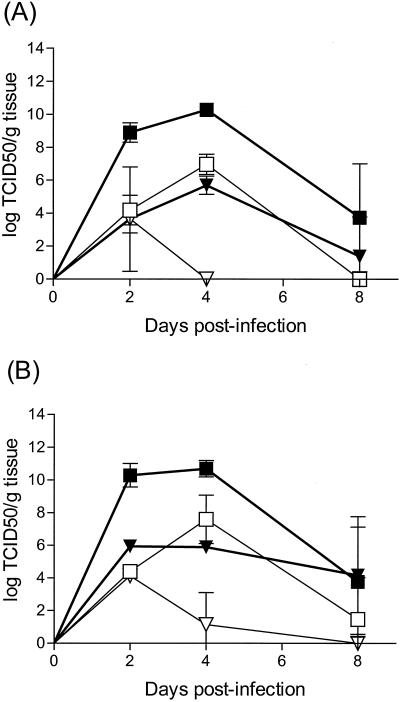
Earlier work had demonstrated CVB3/M replicates to higher titers in pancreas and heart than does CVB3/GA (105). We hypothesized that the greater protection afforded NOD mice from T1D onset by CVB3/M might be due to a greater virus load and/or pancreatitis induction. We therefore measured the extent of replication of CVB3/M and CVB3/GA in NOD mouse pancreas as a function of age at the time of inoculation (6 versus 9 weeks old; Fig. 3A and B, respectively). The results were equivalent as a function of age, suggesting no modifying factor due to different ages. Virulent CVB3/M replicated to 4- to 5-log-higher titers in pancreata than did avirulent CVB3/GA and persisted longer in the pancreas at higher titers than did CVB3/GA. Similar results were obtained when virus titers were assayed in mouse heart tissue, demonstrating that the difference in pancreatic titers was not a pancreas-specific occurrence.
FIG. 3.
Pathogenic CVB3/M replicates to higher titers than does avirulent CVB3/GA in NOD mouse pancreata and heart tissues. Female NOD mice were inoculated once intraperitoneally with 5 × 105 TCID50 CVB3/M or CVB3/GA at 6 weeks of age (A) or 9 weeks of age (B). Symbols: □, GA pancreas; ▪, M pancreas; ▿, GA heart; ▾, M heart. Tissues were excised when mice were sacrificed and homogenized in tissue culture medium, and the titer of replicating virus was determined on HeLa cell monolayers.
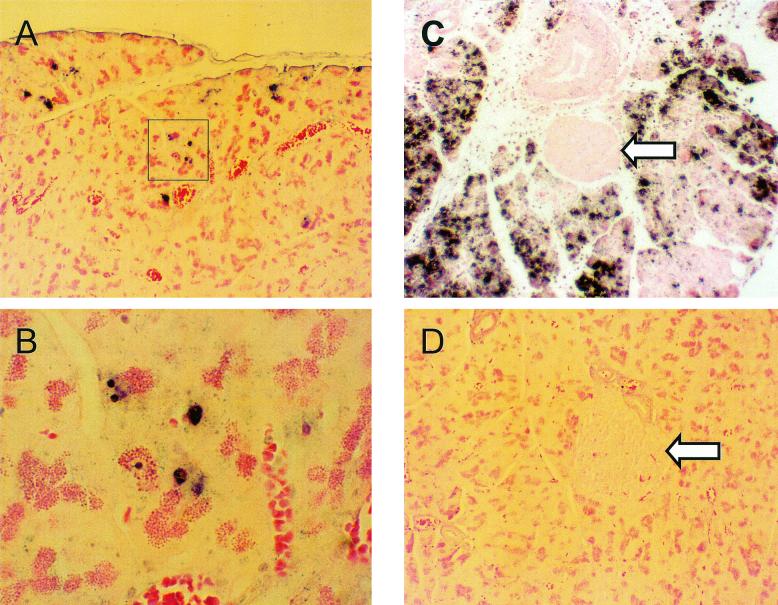
The primary receptor used by the CVB (coxsackievirus adenovirus receptor) has been identified (10, 11, 15, 16, 98) and, although it is expressed prevalently in pancreatic acinar tissue, it has been reported to be present at low levels in pancreatic islets (75). Studies with the CVB4 strain Edwards demonstrated that viral RNA is detected poorly, if at all, in pancreatic islets of NOD mice, although viral RNA is readily detected in acinar tissue (31, 75). A recent study showed that CVB4/Edwards replicated productively in islets only in the absence of interferons, suggesting that the block to replication is not at the level of the virus receptor but elsewhere (31). Due to the disparity observed between the extents of protection from T1D and in virus titers in pancreas in CVB3/M versus CVB3/GA infected mice, we hypothesized that CVB3/GA might be able to replicate in islets, thereby inducing damage which would lead to insulin deficiency and thus to lower extents of protection from T1D. We therefore assayed formalin-fixed, paraffin-embedded sections taken at days 2, 4, and 8 postinoculation by in situ hybridization to localize viral positive-strand RNA (Fig. 4). Viral RNA was detected only in pancreatic acinar tissue of mice inoculated with either CVB3/GA (Fig. 4A and B) or CVB3/M (Fig. 4C) on all 3 days with signal becoming most intense on day 4 postinfection (p.i.) (shown are representative fields from mice at day 4 p.i.). Figure 4D shows results with the same probe on sections from an unifected NOD mouse heart; no hybridization was observed. Consistent with greater titers observed in pancreas tissue (Fig. 3), the extent of hybridization in sections for CVB3/M was significantly more widespread than the very focal and fewer sites of replication of CVB3/GA (Fig. 4B shows the same hybridization foci at higher magnification that are within the box in Fig. 4A). We detected no evidence of viral RNA in pancreatic islets (Fig. 4C is a representative example). We conclude that the reduced extent of protection from T1D provided by CVB3/GA versus CVB3/M cannot be due to widespread replication of virus in islets but may be related to the extent of virus replication in the pancreatic exocrine tissue. The data strongly suggest that neither productive replication of CVB in islets of experimentally infected NOD mice nor direct CVB-mediated islet destruction occurs and that the differential ability of CVB strains to reduce T1D incidence does not correlate with the ability to infect islets.
FIG. 4.
In situ hybridization demonstrates that CVB3/M and CVB3/GA do not replicate productively in pancreatic islets. Female NOD mice were inoculated once with CVB3/M or CVB3/GA and then sacrificed 4 days later. Pancreatic tissue was formalin fixed and paraffin embedded, and 6-μm were sections cut. In situ hybridization was carried out with a negative-strand digoxigenin-labeled riboprobe to detect the positive-stranded CVB3 genome as described in Materials and Methods. (A) CVB/GA replication was detected in discrete and sparse foci (highlighted foci are shown at ×300 magnification in panel B). (C) CVB3/M replicated throughout the acinar tissue but was not observed in islets. (D) A section of a normal uninfected mouse pancreas similarly probed shows no hybridization. All images are shown at ×75 magnification unless otherwise noted. Arrows indicate islets.
Pathological changes in the NOD mouse pancreas as a function of CVB3 virulence phenotype.
Previous work has shown that CVB3/M and other virulent strains such as CVB3/20 induce widespread pancreatitis in inbred mice, whereas CVB3/GA induces no obvious pathological changes in the pancreas (39, 105). Strains of other CVB serotypes are similar in their ability to induce pancreatitis in mice (83, 84, 105). Inoculation of 6- or 9-week-old female NOD mice with strains CVB3/M and CVB3/GA demonstrated that pathogenic CVB3/M strain-induced pancreatitis that resulted in the widespread loss of acinar tissue with subsequent fat replacement that remained throughout the life of the mouse (Fig. 5A). Pancreata of mice inoculated with the nonvirulent CVB3/GA did not show pathological changes in the pancreatic tissue (Fig. 5B), being indistinguishable from uninfected control mouse pancreata (Fig. 5C). Histological examination of pancreatic tissue 21 to 26 days p.i. with CVB3/GA also showed no evidence of CVB3/GA-induced pathological changes (data not shown), demonstrating that CVB3/GA did not induce pancreatic pathology at a later time than CVB3/M. An examination of pancreata from mice inoculated with the pathogenic strains CVB3/M, CVB3/20, CVB3/ZU, and CVB3/AS similarly showed widespread exocrine tissue destruction both in diabetic and disease protected mice (data not shown). If we assume that pancreatic acinar tissue damage occurred within 1 to 2 weeks after inoculation with virus (as has been noted by ourselves and others [14, 39, 105]), the mice survived with a fraction of intact and functional exocrine pancreas (see also Fig. 6E). In no instance did we detect myocarditis (data not shown), classically observed in many inbred strains of mice inoculated with virulent strains such as these (36, 103). Greater extents of protection from T1D by pathogenic CVB3 strains were regularly associated with CVB3-induced pancreatitis.
FIG. 6.
Induction of anti-CVB3 immunity inhibits CVB3/M-induced pancreatitis. (A) Female NOD mice were inoculated intraperitoneally with 5 × 105 TCID50 of CVB3/GA (CVB3/GA) or virus diluent (control) at 4 weeks of age. Symbols: □, control; ○, CVB3/GA-M; ▵, CVB3/GA; ◊, medium-CVB3/M. One group of mice previously inoculated with CVB3/GA was then inoculated with CVB3/M at 6 weeks of age (CVB3/GA-M). A group of age-matched mice that received only virus diluent at 4 weeks of age was inoculated with CVB3/M at 6 weeks (medium-CVB3/M). The experiment was terminated when surviving mice were 35 weeks old. Acinar tissue destruction was not observed in any mouse inoculated with CVB3/GA alone (B) or CVB3/GA followed by CVB3/M (C) or in control mice (D). Fat replacement due to pancreatitis was observed only in mice after inoculation with CVB3/M (E). Images were captured at ×75 magnification. Arrowheads indicate islets. the suppression of T1D incidence by CVB3/M was highly significant (P = 0.0000055), whereas suppression by CVB3/GA or CVB3/GA, followed by that cause by CVB3/M, was not statistically significant (P = 0.064).
The pancreatitis observed in NOD mice infected with the pathogenic CVB strains may be subsequent to some other key event, associating only indirectly with protection from T1D onset. We theorized that specific antigenic epitopes found in the more pathogenic CVB strains such as CVB3/M might be involved in this resistance to T1D. As a test of this hypothesis, we inoculated NOD mice with CVB3/GA at 4 weeks of age and then inoculated the mice again at 6 weeks with CVB3/M. We established that mice developed neutralizing anti-CVB3 serum antibody titers of 64 to 128 and that this immunity was protective as subsequent inoculation of CVB3/M induced no pancreatitis (data not shown). Groups (n = 10) of age-matched control mice were inoculated only with CVB3/GA at 4 weeks or CVB3/M at 6 weeks. Mice were maintained through 35 weeks of age. All mock-infected mice developed diabetes by 27 weeks of age (Fig. 6A). Seventy percent of the mice that had been inoculated with first with CVB3/GA and two weeks later, with CVB3/M, developed diabetes; 40% of mice inoculated with CVB3/GA alone developed diabetes. None of the control mice nor any of the CVB3/GA strain- or CVB3/GA-CVB3/M strain-inoculated mice showed pathological evidence of previous or ongoing pancreatitis (Fig. 6B to D). In contrast, none of the mice inoculated once with CVB3/M at 6 weeks of age developed T1D by 35 weeks of age; when sacrificed, all mice in this group showed acinar tissue destruction with fat replacement (85) due to previous extensive pancreatitis (see Fig. 8C) with small islands of surviving acinar tissue (Fig. 6E). These data illustrate that the suppression of active CVB3/M replication by prior induction of anti-CVB3 protective immunity was associated with both the elimination of CVB3/M-induced pancreatitis and a concomitant increased risk for T1D onset.
FIG. 8.
Apoptosis detected by TUNEL staining only in CVB3-infected pancreata. Four-week-old female NOD mice were inoculated with CVB3/GA or CVB3/M. Pancreas sections taken from paraffin-embedded, formalin-fixed pancreata were probed for apoptosis by the TUNEL technique. Pancreata of CVB3/GA infected mice showed no pancreatitis (A) and no apoptotic nuclei (B). Pancreas sections from CVB3/M-infected mice showed active pancreatitis (C) and acinar tissue that stained widely for apoptotic nuclei by TUNEL (D; representative examples are shown at ×300 magnification in E and F). Arrowheads indicate islets.
This hypothesis was tested by using another approach. For these studies, we used CVB3 strains that varied phenotypically yet expressed the same proteins. In earlier work, we demonstrated that differences in the 5′ nontranslated region (NTR) of CVB3 strains could account for observed cardiovirulent phenotypes in mice (29). We therefore used five different strains from the present study that varied only in the 5′ NTR; all strains held the proteins in the CVB3/20 genome constant. Like CVB3/M, CVB3/20 inoculation had been shown to prevent T1D in most mice (Fig. 2). Inoculation of 4-week-old female NOD mice demonstrated that these strains replicated to equivalent titers in the pancreas by 4 days postinoculation (ranging between 1 × 109 and 3 × 109 TCID50/g of pancreas tissue), which were 10- to 50-fold lower than those observed with CVB3/M strain but higher than those observed for CVB3/GA strain (see, for example, Fig. 3). Unlike the parental virus strain CVB3/20, however, none of the chimeric CVB3 strains induced pancreatitis in any of the mice. The incidence of T1D in these mice ranged between 40 and 80%, resembling results obtained with less-pathogenic or nonpathogenic CVB strains (see Fig. 1 and 2). Since the same proteins were expressed by all recombinant strains and the pancreovirulent CVB3/20 strain, these results also argued against the hypothesis that differences in antigenic epitopes are responsible for differences in extents of protection from T1D onset provided by pathogenic CVB3 strains.
Immunohistochemical analyses of pancreata from diabetic and CVB3-protected mice.
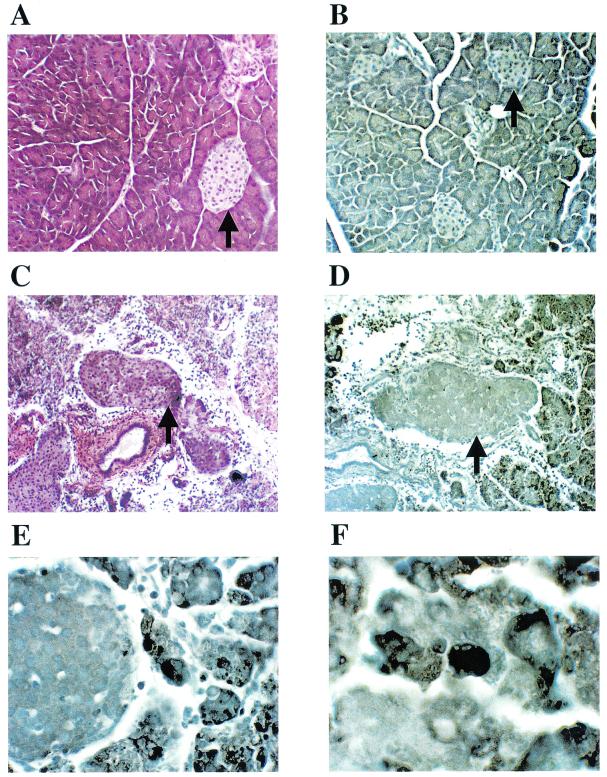
Diabetes in NOD mice is associated with loss of islet structure and insulin production. In the present study, islets in pancreata of diabetic mice, regardless of their treatment, showed no staining for insulin (Fig. 7A), whereas islets from 4-week-old normal, healthy and untreated NOD mice were positive for insulin (Fig. 7D). Glucagon staining positively identified islets in diabetic mice (Fig. 7B). Islets in pancreata of aged (10-month-old) nondiabetic mice (protected either by CVB3/GA or CVB3/M) were immunoreactive to an anti-insulin antibody (Fig. 7E and F). Comparison of the patterns of inflammatory infiltrates in islets from hemotoxylin-stained sections from mice inoculated with CVB3/GA (Fig. 7H) or CVB3/M (Fig. 7I) with insulin-stained sections (Fig. 7E and F, respectively) revealed that inflamed regions of islets did not produce insulin, whereas uninvolved (noninfiltrated) regions continued to produce insulin (typical examples are indicated by arrows). Most islets remained functional at the level of insulin production in CVB3-protected mice, even despite extensive damage to acinar tissue due to the pancreatitis after initial inoculation of pathogenic strains such as CVB3/M.
FIG. 7.
Immunohistochemical analysis of pancreatic tissue for insulin production. Pancreas sections from mice inoculated either with CVB3/GA or CVB3/M and surviving through 10 months of age without T1D were compared to pancreas sections taken from normal (no virus) diabetic mice and young (4-week-old), normal healthy NOD mice for the expression of insulin by immunohistochemical staining. Typical sections were stained for insulin (A and D to F), glucagon (C), or with hemotoxylin to highlight islet inflammatory infiltrates (B and G to I). See the text for discussion. Sections A to C, D and G, E and H, and F and I are serial sections from the same pancreas. Arrowheads indicate islets. Regions staining for insulin in islets are brown (A, D to F); insulin staining occurs in noninflamed islet regions.
Apoptosis is a generalized protective mechanism against many viral infections and has been shown to occur as a consequence of CVB infection in mice and cell culture (45, 51, 70, 82). We therefore assayed pancreas sections from CVB3/M and CVB3/GA infected mice taken 2, 4, and 8 days (three mice per virus per time point) p.i. during acute viral replication for apoptosis by the TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) technique. Mice inoculated with CVB3/GA showed no pancreatitis (Fig. 8A) and, when stained for apoptotic nuclei, none were observed (Fig. 8B). Similarly, uninoculated control mice were also negative for TUNEL staining (data not shown). In contrast, pancreata from CVB3/M-inoculated mice showed evidence of active pancreatitis at 4 days p.i. in all mice (Fig. 8C). Pancreata of mice inoculated with CVB3/M were also positive for apoptotic nuclei in acinar tissue (Fig. 8D; Fig. E and F show examples at higher magnification). Apoptosis was first evident in CVB3/M-infected pancreata on day 4 p.i. and persisted through day 8. Apoptotic nuclei were not observed in islets in any section.
IgG antibody reactivity to CVB and pancreatic autoantigens in serum.
While TH2-like responses have been associated with lower T1D incidence in NOD mice, it is unclear what this may mean in terms of mechanism (93). TH1 and TH2 cells enhance isotype switching to IgG2a and IgG1, respectively; thus, greater ratios of insulin or HSP60-specific IgG1 or IgG2a should reflect greater TH2 responses and smaller ratios should reflect greater TH1 responses (78). We tested the hypothesis that the greater extent of protection afforded mice by CVB3/M may be due to the ability of this strain to induce a stronger TH2-like response than CVB3/GA against pancreatic autoantigens. Using sera from normal, as well as diabetic mice, and from mice that had been protected by either CVB3 strain, we screened for IgG2a (representative of a TH1 response) and IgG1 (TH2 response) immunoreactivity against two diabetic autoantigens, insulin and HSP60. No statistically significant variation was measured among the group studied for any immunoglobulin immunoreactivity (P ≥ 0.08; data not shown). Although for all groups there was on average an IgG1/IgG2a ratio of ca. 3 to 4, a finding consistent with a trend in all groups toward a TH2-like response, these ratios did not show statistically significant variation between groups for each capture antigen. We conclude from these studies that neither IgG1 nor IgG2a immunoreactivity against insulin or HSP60 is associated with the health outcome of these mice.
Western blot analysis with sera from diabetic and CVB3-protected mice.
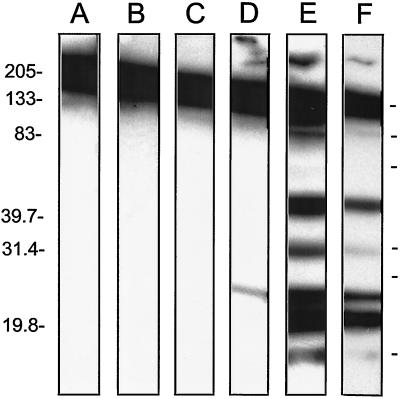
The extensive pancreatitis and pancreatic tissue damage induced by CVB3/M and other pathogenic CVB3 strains suggested the possibility that the NOD mouse immune system might mount an autoimmune reaction against pancreatic proteins. To test this hypothesis, we used sera from CVB3/M strain- and CVB3/GA-strain protected mice to probe Western blots of normal (4-week-old) NOD mouse pancreatic proteins. Sera were pooled from four to seven different mice (normal 4-week-old, diabetic mice between 15 and 25 weeks, and CVB3/M- or CVB3/GA-protected mice, aged 38 weeks), and identical strips of Western blotted normal NOD mouse pancreatic proteins were probed with each serum sample (Fig. 9). No signals were detected by either normal or diabetic NOD mouse sera (lanes B and C, respectively). Prominently detected across the tops of all lanes was mouse immunoglobulin. In contrast to the lack of signal detected by the diabetic or normal serum pools, several distinct bands ranging in size from 20 to 130 kDa were detected by the sera of CVB3/M-protected mice (see lane E; lane F is a shorter exposure of that seen in lane E). Unlike the pattern detected by the CVB3/M-protected mouse sera, sera from CVB3/GA-protected mice detected just a single band at ca. 28 kDa. None of the bands corresponded in size to GAD65, a diabetic autoantigen. The results suggest that the pancreatitis associated with CVB3/M infection resulted in a strong autoimmunity against numerous pancreatic proteins that was not observed as a consequence of CVB3/GA infection. Since both CVB3 strains replicated productively in the mouse pancreas (see Fig. 3 and 4), the strong inference from this experiment is that the differences we observed were not due simply to CVB3 replication but also due to the pancreatitis associated with pathogenic CVB3/M replication.
FIG. 9.
Antibodies in sera from CVB3/M-protected NOD mice detect pancreatic proteins. Pancreatic proteins from normal NOD mice were electrophoresed in nonreducing SDS-containing 10 to 15% polyacrylamide gels and then Western blotted. Strips were cut and individually probed with HRP-labeled secondary antibody only (control) (lane A) or pooled sera from normal 4-week-old healthy (nondiabetic) NOD mice (lane B), normal mice that developed diabetes naturally (lane C), CVB3/GA-protected mice (lane D), or CVB3/M-protected mice (E and F). The exposure time for lanes A to E was 10 min; the exposure time for lane F was 3 min. Molecular mass markers are shown on the side in kilodaltons.
DISCUSSION
The mechanisms underlying the onset of T1D are not clearly understood. Although it has been well established that individual genetics can predispose one to T1D onset, human genetics are not the sole arbiter of whether T1D develops (8, 77). As a consequence, environmental agents (frequently infections by the CVB) have been suggested as precipitating factors. Despite a common supposition that CVB are candidate infectious agents that can trigger human T1D, there is no consensus regarding the etiologic role, if any, for the CVB in human T1D. A link between CVB infections and T1D onset has been proposed (see for example, references 18, 20, 21, 23, and 116), but results from other studies dispute that a direct link exists (see, for example, references 25, 32, 47, 52, and 91). Further, murine models of diabetes do not strongly support a common role for CVB infections as a cause of diabetes. While a few CVB strains have been reported to cause glucose abnormalities in some inbred strains (99), this is not a common CVB phenotype, and the effect is CVB strain specific (56). Although not disproven, support for a hypothetical role for CVB in the causation of diabetes is certainly much weaker than that available on the etiologic role of CVB in human myocarditis and pancreatitis. The CVB are readily demonstrable as causes of both human pancreatitis and myocarditis and induce these diseases in diverse mouse models (reviewed in references 60, 61, 68, and 85).
Given the lack of consensus for an etiologic role of CVB in human T1D, we were curious to determine the impact of CVB infection upon T1D incidence in the NOD mouse model of human T1D. We examined the impact of nine genetically and phenotypically diverse CVB strains upon T1D incidences in NOD mice. The results of these long-term studies were consistent and striking: all CVB strains significantly suppressed the incidence of diabetes between 2- to 10-fold relative to control, mock-infected mice. Similarly, inoculation of older (8-week-old, sexually mature) prediabetic mice resulted in emphatic protection from T1D. It has been proposed that CVB4/Edwards induced accelerated T1D onset in some NOD mice due to an expanded population of autoimmune T cells (94). However, if the state of a more advanced preexisting autoimmune process were the only key factor that affected accelerated disease onset, we should have observed accelerated T1D onset in all of the CVB-inoculated groups of older mice. This did not occur. The results presented in the present study of several diverse CVB strains suggest that the mechanism that underlies accelerated T1D development in NOD mice, when it occurs, must involve both the virus strain, as well as the age of the mouse (or, more precisely, the stage of autoimmune development). Notably, the T1D incidence never rose in any of the groups with accelerated T1D onset to that seen in the control mice: all groups of mice were protected as a function of CVB inoculation relative to mock-infected control mice. That induction of accelerated T1D onset appears to be related to specific (but not all) CVB strains suggests that it should be possible to dissect the viral genetics which control this process in much the same way that the CVB genetics which control the cardiovirulent phenotype in mice are being characterized (29). Precedent for the feasibility of establishing the genetics for this phenotype in CVB, the human virus group most often associated with the etiology of T1D onset, also comes from the work of Oldstone et al., who mapped the protective influence of the rodent virus LCMV, strain Pasteur, to the S genome segment (80).
The mechanism by which CVB infection in general lowers the risk of T1D onset in NOD mice or by which pathogenic CVB3 infection in particular provides a greater extent of protection from T1D remains, like the mechanism that underlies the onset of T1D in humans, unclear at this time. We have established that neither strain CVB3/GA nor strain CVB3/M replicate to detectable levels in pancreatic islets; other work has demonstrated this holds true for other CVB strains as well (S. Tracy and K. Drescher, unpublished data). Based on these and on results with CVB4/Edwards strain (75), the strong inference is that CVB does not replicate productively in normal NOD mouse pancreatic islets and, therefore, by extrapolation to humans, may not induce diabetes by destruction of the insulin producing beta cells. It is clear from results presented here that strain CVB3/M replicated to higher titers than did strain CVB3/GA and induced apoptosis during replication in the pancreas. We observed no significant differences among healthy versus diabetic mice as a function of the production of IgG2a or IgG1antibodies against two different diabetic autoantigens, insulin and HSP60. However, when we tested the hypothesis that the pancreatitis induced by a pathogenic strain such as CVB3/M might result in the generation of autoimmunity against other pancreatic antigens, we discovered by Western blot assay that CVB3/M induced a considerably more diverse autoimmune antibody response against pancreatic proteins than did the avirulent CVB3/GA. We have extended these results by using individual, rather than pooled, sera, and the results are consistent: antibodies in individual sera from T1D-protected mice that had been previously inoculated with CVB3/M, CVB3/AS, CVB3/20, or CVB3/ZU detected these bands (S. Carson and S. Tracy, data not shown). That we observed this reactivity only with sera from mice previously inoculated with pathogenic strains such as CVB3/M suggests that pancreatic autoimmunity in NOD mice is not detrimental to health per se but rather can be associated with a decreased risk for the development of T1D. The alacrity with which CVB replicate in pancreatic acinar tissue and the lack of any indication that CVB replicate productively in islets while (in the case of pathogenic strains) causing pancreatitis is consistent with a hypothesis that CVB3/M-induced antipancreatic immunity is an outcome largely due to lytic replication in the pancreatic exocrine tissue. It is not clear at present how or whether this CVB3/M-induced immunity accounts for the greater proportion of insulin-positive islets observed in CVB3/M-protected mice relative to CVB3/GA-protected mice, but the correlation of a vigorous antiviral and autoimmune response with protection suggests that this result must represent part of the mechanism by which pathogenic strains such as CVB3/M induce greater extents of protection from T1D than do avirulent strains. Efforts to identify pancreatic proteins that are detected by sera from CVB3/M-protected mice are under way.
Whether the pancreatic autoimmunity induced by pathogenic CVB3 strains is linked to the greater extents of protection provided by these viruses or whether it is a secondary event is an interesting problem. The hypothesis that greater extents of protection from T1D are due to specific antigenic epitopes in pathogenic CVB strain was largely ruled out by consistent results from two different experimental tests of the hypothesis. Inoculation of CVB3/M at 6 weeks of age after a previous CVB3/GA inoculation at 4 weeks protected fewer mice than did inoculation of just CVB3/M at 6 weeks of age. Preexisting anti-CVB3 immunity in these mice suppressed the extent of CVB3/M replication and prevented pancreatitis. It may be argued that an insufficient level of exposure to CVB3/M proteins occurred due to the anti-CVB3 immunity. However, we established in an earlier study that a chimeric CVB3 vector can induce an immune response to the non-CVB3 antigenic epitope even in the face of protective preexisting anti-CVB3 immunity (48), thereby demonstrating that anti-CVB immunity is not sterilizing but permits sufficient replication by the challenge strain to induce a new, CVB vector-associated immune response. The pancreas is a tissue in which early and high levels of CVB replication is seen and is likely to be a site in which CVB3 replication occurs even in the presence of standing immunity. Second, by using a series of recombinant CVB3 strains that differed only in the identity of the noncoding 5′ NTR of the genome (29) and which maintained all of the proteins encoded by the vector CVB3/20 strain, we did not observe extents of protection from T1D that were as high as that achieved by the vector strain itself, CVB3/20. Although these chimeric strains replicated to similar high titers in NOD mouse pancreatic tissue, they did not induce pancreatitis. Were antigenic epitopes of pathogenic CVB strains such as CVB3/M or CVB3/20 key to the better protection afforded by pathogenic strains or, indeed, merely a high virus load, we would have expected to observe similar extents of protection with these CVB3/20-based strains. This was not the case. At this time, therefore, the data show that CVB3-induced pancreatitis is directly linked to greater extents of protection from T1D onset, with the greatest protection provided by the most pancreovirulent CVB strains. These observations again suggest that understanding the CVB genetics underlying a pancreovirulent phenotype should help to highlight how such virus strains interact with the host to suppress T1D onset. If pancreatitis reveals specific host pancreatic antigens to the immune system that suppress the normal, genetically programmed autoimmune attack on NOD mouse pancreatic islets, it is intriguing to consider that expression of such key antigenic epitopes by an artificially attenuated pancreotropic CVB vector might serve to protect as many NOD mice, as does inoculation of pancreovirulent CVB strains without the induction of pancreatitis.
Results reported here do not support a hypothesis that states that the CVB are etiologic agents of T1D. None of the CVB strains tested in the present study resulted in as high an incidence of T1D as did the mock-infected control groups; instead, each CVB strain markedly reduced the incidence of T1D over 10 months of age. It is clear that our results cannot disprove the hypothesis that rare CVB strains (116) may induce T1D in humans. Nonetheless, it is worth noting that the T1D-associated strain in the present study (CVB4/Edwards) protected NOD mice from T1D onset. The strong inference from this work is that any CVB infection of younger NOD mice is protective, reducing the risk of T1D onset, rather than causative. Based upon these data derived from the relevant NOD mouse model of human T1D, although acknowledging the fact that it is not a perfect model of human T1D, we propose an alternative hypothesis: CVB infection(s) at a young age may generally lower the risk for the development of T1D even in individuals genetically predisposed to developing the disease. Although the mechanism underlying the CVB-induced anti-T1D protective effect in NOD mice has not yet been clarified, how CVB-induced protection from T1D onset occurs will likely involve several factors, among them the virus strain and its virulence phenotype, the NOD mouse host's immune response to the specific infection, and the age at which the first CVB infection occurs. Testing of the correlate that CVB exposure may also be protective in humans must rely on the NOD mouse model to provide virologic and immunologic correlates than can be evaluated inferentially in humans. For example, CVB commonly causes systemic disease in neonates and infants in which the infectious CVB strain can readily be isolated and specifically identified (26, 59, 62). It has been suggested as well that infections within the first year of life provide greater protection from T1D onset than when infections occur later in life (13, 38, 57). Both retro- and prospective studies of this specific subset of patients could be used to test whether verified CVB infections of children are associated with more or fewer cases of T1D onset as the children age. Understanding that the NOD mouse remains only a model of human T1D, we nonetheless suggest that the consistent and marked suppression of T1D in the inbred, genetically T1D-prone NOD mice by all CVB strains used in the present study is an encouraging finding, one that suggests that it may be possible one day to induce CVB vaccine-mediated suppression of T1D in the genetically outbred human population.
Acknowledgments
We thank Charles Gauntt for many useful discussions and a critical reading of the text. We also thank B. Barnes and K. Delaney for excellent technical assistance.
This work was supported in part by grants from the Juvenile Diabetes Research Foundation, the National Institutes of Health, and the American Cancer Society.
REFERENCES
- 1.Akhtar, N., J. Ni, D. Stromberg, G. L. Rosenthal, N. E. Bowles, and J. A. Towbin. 1999. Tracheal aspirate as a substrate for polymerase chain reaction detection of viral genome in childhood pneumonia and myocarditis. Circulation 99:2011-2018. [DOI] [PubMed] [Google Scholar]
- 2.Arnesjo, B., T. Eden, I. Ihse, E. Nordenfelt, and B. Ursing. 1976. Enterovirus infections in acute pancreatitis: a possible etiological connection. Scand. J. Gastroenterol. 11:645-649. [PubMed] [Google Scholar]
- 3.Atkinson, M. A., M. Bowman, L. Campbell, B. Darrow, D. L. Kaufman, and N. K. McLaren. 1994. Cellular immunity to a determinant common to glutamate decarboxylase and coxsackie virus in insulin-dependent diabetes. J. Clin. Investig. 94:2125-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson, M. A., and E. H. Leiter. 1999. The NOD mouse model of type 1 diabetes: as good as it gets? Nat. Med. 5:601-604. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson, M. A., and N. Maclaren. 1994. The pathogenesis of insulin-dependent diabetes mellitus. N. Engl. J. Med. 331:1428-1436. [DOI] [PubMed] [Google Scholar]
- 6.Bach, J.-F. 1994. Insulin-dependent diabetes mellitus as an autoimmune disease. Endocrine Rev. 15:516-535. [DOI] [PubMed] [Google Scholar]
- 7.Baekkeskov, S., and B. Hansen. 1990. Human diabetes: genetic, environmental, and autoimmune etiology. Curr. Top. Microbiol. Immunol. 164:143-168. [DOI] [PubMed] [Google Scholar]
- 8.Barnett, A. H., C. Eff, R. Leslie, and D. Pyke. 1981. Diabetes in identical twins: a study of 200 pairs. Diabetologia 20:87-93. [DOI] [PubMed] [Google Scholar]
- 9.Beck, M., N. Chapman, B. McManus, J. Mullican, and S. Tracy. 1990. Secondary enterovirus infection in the murine model of myocarditis: pathologic and immunologic aspects. Am. J. Pathol. 136:669-681. [PMC free article] [PubMed] [Google Scholar]
- 10.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 11.Bergelson, J. M., A. Krithivas, L. Celi, G. Droguett, M. S. Horwitz, T. Wickham, R. L. Crowell, and R. W. Finberg. 1998. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J. Virol. 72:415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertrera, S., A. Alexander, N. Giannoukakis, P. Robbins, and M. Trucco. 1999. Immunology of type 1 diabetes. Pediatr. Endocrinol. 28:841-864. [DOI] [PubMed] [Google Scholar]
- 13.Blom, L., L. Nystrom, and G. Dahlquist. 1991. The Swedish childhood diabetes study: vaccinations and infections as risk determinants for diabetes in childhood. Diabetologia 34:176-181. [DOI] [PubMed] [Google Scholar]
- 14.Caggana, M., P. Chan, and A. L. Ramsingh. 1993. Identification of a single amino acid residue in the capsid protein VP1 of coxsackievirus B4 that determines the virulent phenotype. J. Virol. 67:4797-4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carson, S., N. Chapman, and S. Tracy. 1997. Purification of the putative coxsackievirus B receptor from HeLa cells. Biochem. Biophys. Res. Commun. 233:325-328. [DOI] [PubMed] [Google Scholar]
- 16.Carson, S., J. T. Hobbs, S. Tracy, and N. Chapman. 1999. Expression of the coxsackievirus and adenovirus receptor in cultured human umbilical vein endothelial cells: regulation in response to cell density. J. Virol. 73:7077-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castano, L., and G. S. Eisenbarth. 1990. Type 1 diabetes: a chronic autoimmune disease of human, mouse and rat. Annu. Rev. Immunol. 8:647-679. [DOI] [PubMed] [Google Scholar]
- 18.Champsaur, H., E. Dussaix, D. Samolyk, M. Fabre, C. Bach, and R. Assan. 1980. Diabetes and coxsackievirus B5 infection. Lancet i:251. [DOI] [PubMed] [Google Scholar]
- 19.Chapman, N., Z. Tu, S. Tracy, and C. Gauntt. 1994. An infectious cDNA copy of the genome of a noncardiovirulent coxsackievirus B3 strain: its complete sequence analysis and comparison to the genomes of cardiovirulent coxsackieviruses. Arch. Virol. 135:115-130. [DOI] [PubMed] [Google Scholar]
- 20.Chowdhury, T. A., C. Mijovic, and A. H. Barnett. 1999. The aetiology of type I diabetes. Baillieres Best Pract. Res. Clin. Endocrinol. Metab. 13:181-195. [DOI] [PubMed] [Google Scholar]
- 21.Clements, G. B., D. Galbraith, and K. Taylor. 1995. Coxsackie B virus infection and onset of childhood diabetes. Lancet 346:221-223. [DOI] [PubMed] [Google Scholar]
- 22.Craighead, J. E. 1975. The role of viruses in the pathogenesis of pancreatic disease and diabetes mellitus. Prog. Med. Virol. 19:161-214. [PubMed] [Google Scholar]
- 23.D'Alessio, D. 1992. A case-control study of group B coxsackievirus immunoglobulin M antibody prevalence and HLA-DR antigens in newly diagnosed cases of insulin-dependent diabetes mellitus. Am. J. Epidemiol. 135:1331-1338. [DOI] [PubMed] [Google Scholar]
- 24.Dalldorf, G. 1955. The coxsackie viruses. Annu. Rev. Microbiol. 9:277-296. [DOI] [PubMed] [Google Scholar]
- 25.Dippe, S. E., P. Bennet, M. Miller, J. Maynard, and K. Berquist. 1975. Lack of causal association between coxsackie B4 virus and diabetes. Lancet i:1314-1317. [DOI] [PubMed] [Google Scholar]
- 26.Disney, M. E., E. M. Howard, and B. S. B. Wood. 1953. Myocarditis in children. BMJ I:1351-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drescher, K. M., P. Murray, X. Lin, J. Carlino, and M. Rodriguez. 2000. TGF-β2 reduces demyelination, virus antigen expression, and macrophage recruitment in a viral model of multiple sclerosis. J. Immunol. 164:3207-3213. [DOI] [PubMed] [Google Scholar]
- 28.Drescher, K. M., and J. A. Whittum-Hudson. 1996. Herpes simplex virus type 1 alters transcript levels of tumor necrosis factor-α and interleukin-6 in retinal glial cells. Investig. Opthalmol. Visual Sci. 37:2302-2312. [PubMed] [Google Scholar]
- 29.Dunn, J. J., N. M. Chapman, S. Tracy, and J. R. Romero. 2000. Natural genetics of cardiovirulence in coxsackievirus B3 clinical isolates: localization to the 5′ nontranslated region. J. Virol. 74:4787-4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenbarth, G. 1986. Type 1 diabetes mellitus: a chronic autoimmune disease. N. Engl. J. Med. 314:1360-1368. [DOI] [PubMed] [Google Scholar]
- 31.Flodstrom, M., A. Maday, B. Balakrishna, M. Cleary, A. Yoshimura, and N. Sarvetnick. 2002. Target cell defense prevents the development of diabetes after viral replication. Nat. Immunol. 3:373-382. [DOI] [PubMed] [Google Scholar]
- 32.Foulis, A. K., M. Farquharson, S. Cameron, M. McGill, H. Schoenke, and R. Kandolf. 1990. A search for the presence of enteroviral capsid protein VP1 in pancreases of patients with type 1 diabetes and pancreases and hearts of infants who died of coxsackieviral myocarditis. Diabetology 33:290-298. [DOI] [PubMed] [Google Scholar]
- 33.Fuchtenbusch, M., A. Irnstetter, G. Jager, and A.-G. Ziegler. 2001. No evidence for an association of coxsackievirus infections during pregnancy and early childhood with development of islet autoantibodies in offspring of mothers or fathers with type 1 diabetes. J. Autoimmun. 17:333-340. [DOI] [PubMed] [Google Scholar]
- 34.Gamble, D. R. 1976. A possible virus etiology for juvenile diabetes, p. 95-105. In W. Kreutzfeld, J. Kobberling, and J. Neel (ed.), The genetics of diabetes mellitus. Springer Verlag, Berlin, Germany.
- 35.Gamble, D. R., M. Kinsley, M. Fitzgerald, R. Bolton, and K. W. Taylor. 1969. Viral antibodies in diabetes mellitus. BMJ 3:627-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gauntt, C., M. Trousdale, D. LaBadie, R. E. Paque, and T. Nealon. 1979. Properties of coxsackievirus B3 variants which are amyocarditic or myocarditic for mice. J. Med. Virol. 3:207-220. [DOI] [PubMed] [Google Scholar]
- 37.Gianani, R., and N. Sarvetnick. 1996. Viruses, cytokines, antigens, and autoimmunity. Proc. Natl. Acad. Sci. USA 93:2257-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gibbon, C., T. Smith, P. Egger, P. Betts, and D. Phillips. 1997. Early infection and subsequent insulin-dependent diabetes. Arch. Dis. Child 77:384-385. [DOI] [PubMed] [Google Scholar]
- 39.Gomez, R., E. Lascano, and M. Berria. 1991. Murine acinar pancreatitis preceding necrotizing myocarditis after coxsackievirus B3 inoculation. J. Med. Virol. 35:71-75. [DOI] [PubMed] [Google Scholar]
- 40.Gomez, R. M., X. Cui, C. G. Castagnino, and M. I. Berria. 1993. Differential behavior in pancreas and heart of two coxsackievirus B3 variants. Intervirology 36:153-160. [DOI] [PubMed] [Google Scholar]
- 41.Gooby-Toedt, D., J. Byrd, and D. Omori. 1996. Coxsackievirus-associated pancreatitis mimicking metastatic carcinoma. S. Med. J. 89:441-443. [DOI] [PubMed] [Google Scholar]
- 42.Grist, N. R. 1972. Viruses and myocarditis. Postgrad. Med. J. 48:750-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halim, S., and A. I. Ramsingh. 2000. A point mutation in VP1 of coxsackievirus B4 alters antigenicity. Virology 269:86-94. [DOI] [PubMed] [Google Scholar]
- 44.Harkonen, T., H. Lankinen, B. Davydova, T. Hovi, and M. Roivainen. 2002. Enterovirus infection can induce immune responses that cross-react with beta cell autoantigen tyrosine phosphatase IA-2/IAR. J. Med. Virol. 66:340-350. [DOI] [PubMed] [Google Scholar]
- 45.Henke, A., M. Nestler, S. Strunze, H. Saluz, P. Hortschansky, B. Menzel, U. Martin, R. Zell, and A. Stelzner. 2001. The apoptotic capability of coxsackievirus B3 is influenced by the efficient interaction between the capsid protein VP2 and the proapoptotic host protein Siva. Virology 289:15-22. [DOI] [PubMed] [Google Scholar]
- 46.Hermitte, L., B. Vialettes, P. Naquet, C. Atlan, M. J. Payan, and P. Vague. 1990. Paradoxical lessening of autoimmune processes in non-obese diabetic mice after infection with the diabetogenic variant of encephalomyocarditis virus. Eur. J. Immunol. 20:1297-1303. [DOI] [PubMed] [Google Scholar]
- 47.Hierholzer, J. C., and W. Farris. 1974. Follow-up of children infected in a coxsackievirus B3 and B4 outbreak: no evidence of diabetes mellitus. J. Infect. Dis. 129:741-746. [DOI] [PubMed] [Google Scholar]
- 48.Hofling, K., S. Tracy, N. Chapman, and S. L. Leser. 2000. Expression of the antigenic adenovirus type 2 hexon protein L1 loop region in a group B coxsackievirus. J. Virol. 74:4570-4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horwitz, M., C. Fine, A. Ilic, and N. Sarvetnick. 2001. Requirements for viral-mediated autoimmune diabetes: beta cell damage and immune infiltration. J. Autoimmun. 16:211-217. [DOI] [PubMed] [Google Scholar]
- 50.Horwitz, M. S., L. M. Bradley, J. Harbertson, T. Krahl, J. Lee, and N. Sarvetnick. 1998. Diabetes induced by coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat. Med. 4:781-785. [DOI] [PubMed] [Google Scholar]
- 51.Huber, S. A. 2000. T cells expressing the gamma-delta T-cell receptor induce apoptosis in cardiac myocytes. Cardiovasc. Res. 45:579-587. [DOI] [PubMed] [Google Scholar]
- 52.Huff, J. C., J. C. Hierholzer, and W. Farris. 1974. An “outbreak” of juvenile diabetes mellitus: consideration of a viral etiology. Am. J. Epidemiol. 100:277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hyoty, H., M. Hiltunen, and M. Lonnrot. 1998. Enterovirus infections and insulin-dependent diabetes mellitus: evidence for causality. Clin. Diagn. Virol. 9:77-84. [DOI] [PubMed] [Google Scholar]
- 54.Imrie, C. W., J. Ferguson, and R. Sommerville. 1977. Coxsackie and mumpsvirus infection in a prospective study of acute pancreatitis. Gut 18:53-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jenson, A., and H. Rosenberg. 1984. Multiple viruses in diabetes mellitus. Prog. Med. Virol. 29:197-217. [PubMed] [Google Scholar]
- 56.Jordan, W., V. Bolton, and N. J. Schmidt. 1985. Diabetogenic potential of coxsackie B viruses in nature. Arch. Virol. 86:213-221. [DOI] [PubMed] [Google Scholar]
- 57.Juhela, S., H. Hyoty, M. Roivainen, T. Harkonen, A. Putto-Laurila, O. Simell, and J. Ilonen. 2000. T-cell responses to enterovirus antigens in children with type 1 diabetes. Diabetes 49:1308-1313. [DOI] [PubMed] [Google Scholar]
- 58.Kennedy, J. D., I. C. Talbot, and M. S. Tanner. 1986. Severe pancreatitis and fatty liver progressing to cirrhosis associated with coxsackie B4 infection in a three year old with α-1-antitrypsin deficiency. Acta Paediatr. Scand. 75:336-339. [DOI] [PubMed] [Google Scholar]
- 59.Kibrick, S., and K. Benirschke. 1958. Severe generalized disease (encephalohepatomyocarditis) occurring in the newbord period and due to infection with coxsackie virus, group B. Pediatrics 22:857-874. [PubMed] [Google Scholar]
- 60.Kim, K.-S., G. Hufnagel, N. Chapman, and S. Tracy. 2001. The group B coxsackieviruses and myocarditis. Rev. Med. Virol. 11:355-368. [DOI] [PubMed] [Google Scholar]
- 61.Kim, K.-S., K. Hofling, S. D. Carson, N. M. Chapman, and S. Tracy. 2002. The primary viruses of myocarditis, p. 23-33. In L. T. Cooper and K. Knowlton (ed.), Myocarditis. Mayo Academic Press, Chicago, Ill.
- 62.Kleinert, S., R. Weintraub, J. Wilkinson, and C. Chow. 1997. Myocarditis in children with dilated cardiomyopathy: incidence and outcome after dual therapy immunosuppression. J. Heart Lung Transplant. 16:1248-1254. [PubMed] [Google Scholar]
- 63.Knip, M., and H. K. Akerblom. 1999. Environmental factors in the pathogenesis of type 1 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 107(Suppl. 3):S93-S100. [DOI] [PubMed] [Google Scholar]
- 64.Kukreja, A., and N. Maclaren. 1999. Auotimmunity and diabetes. J. Clin. Endocrinol. Metab. 84:4371-4378. [DOI] [PubMed] [Google Scholar]
- 65.Laemmli, U. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 66.Lal, S., D. Fowler, C. Losasso, and G. Berg. 1988. Coxsackie virus-induced acute pancreatitis in a long-term dialysis patient. Am. J. Kidney Dis. 11:434-436. [DOI] [PubMed] [Google Scholar]
- 67.Lee, C., E. Maull, N. Chapman, S. Tracy, G. Wood, and C. Gauntt. 1997. Generation of an infectious cDNA of a highly cardiovirulent coxsackievirus B3(CVB3m) and comparison to other infectious CVB3 cDNAs. Virus Res. 50:225-235. [DOI] [PubMed] [Google Scholar]
- 68.Leslie, K., R. Blay, C. Haisch, A. Lodge, A. Weller, and S. A. Huber. 1989. Clinical and experimental aspects of viral myocarditis. Clin. Microbiol. Rev. 2:191-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lo, S., R. Tun, M. Hawa, and R. Leslie. 1991. Studies of diabetic twins. Diabetes Metab. Rev. 7:223-228. [DOI] [PubMed] [Google Scholar]
- 70.Luo, H., B. Yanagawa, J. Zhang, Z. Luo, M. Zhang, M. Esfandiarei, C. Carthy, J. E. Wilson, D. Yang, and B. McManus. 2002. Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J. Virol. 76:3365-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maclaren, N., D. Schatz, A. Drash, and G. Grave. 1989. Initial pathogenic events in T1D: conference summary. Diabetes 38:534-537. [DOI] [PubMed] [Google Scholar]
- 72.Makino, S., K. Kunimoto, Y. Muraoka, Y. Mizushima, K. Katagiri, and Y. Tochino. 1980. Breeding of a non-obese, diabetic strain of mice. Exp. Anim. 29:1-13. [DOI] [PubMed] [Google Scholar]
- 73.Martin, A. B., S. Webber, F. J. Fricker, R. Jaffe, G. Demmler, D. Kearney, Y. H. Zhang, J. Bodurtha, B. Gelb, J. Ni, J. Bricker, and J. Towbin. 1994. Acute myocarditis: rapid diagnosis by PCR in children. Circulation 90:330-339. [DOI] [PubMed] [Google Scholar]
- 74.Martino, T. A., P. Liu, M. Petric, and M. J. Sole. 1995. Enteroviral myocarditis and dilated cardiomyopathy: a review of clinical and experimental studies, p. 291-352. In H. A. Rotbart (ed.), Human enterovirus infections. ASM Press, Washington, D.C.
- 75.Mena, I., C. Fischer, J. Gebhard, C. Perry, S. Harkins, and J. L. Whitton. 2000. Coxsackievirus infection of the pancreas: evaluation of receptor expression, pathogenesis, and immunopathology. Virology 271:276-288. [DOI] [PubMed] [Google Scholar]
- 76.Menser, M. A., J. Forrest, and R. Bransby. 1978. Rubella infection and diabetes mellitus. Lancet i:57-60. [DOI] [PubMed] [Google Scholar]
- 77.Metcalfe, K., G. Hitman, R. Rowe, M. Hawa, X. Huang, T. Stewart, and R. Leslie. 2001. Concordance for type 1 diabetes in identical twins is affected by insulin genotype. Diabetes Care 24:838-842. [DOI] [PubMed] [Google Scholar]
- 78.Mosmann, T., and R. Coffman. 1989. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv. Immunol. 46:111-132. [DOI] [PubMed] [Google Scholar]
- 79.Oldstone, M. B. A. 1988. Prevention of type 1 diabetes in NOD mice by virus infection. Science 239:500-502. [DOI] [PubMed] [Google Scholar]
- 80.Oldstone, M. B. A., R. Ahmed, and M. Salvato. 1990. Viruses as therapeutic agents. II. Viral reassortants map prevention of insulin-dependent diabetes mellitus to the small RNA of lymphocytic choriomeningitis virus. J. Exp. Med. 171:2091-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ou, D., L. Mitchell, D. Metzger, S. Gillam, and A. Tingle. 2000. Cross reactive rubella virus and glutamic acid decarboxylase (65 and 67) protein determinants recognized by T cells of patients with type I diabetes mellitus. Diabetologia 43:750-762. [DOI] [PubMed] [Google Scholar]
- 82.Peng, T., T. Sadusky, Y. Li, G. Coulton, H. Zhang, and Archard.L. 2001. Altered expression of Bag-1 in coxsackievirus B3-infected mouse heart. Cardiovasc. Res. 50:46-55. [DOI] [PubMed] [Google Scholar]
- 83.Ramsingh, A., A. Hixson, B. Duceman, and J. Slack. 1990. Evidence suggesting that virulence maps to the P1 region of the coxsackievirus B4 genome. J. Virol. 64:3078-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramsingh, A., J. Salck, M. Silkworth, and A. Hixson. 1989. Severity of disease induced by a pancreotropic coxsackie B4 virus correlates with the H2Kq locus of the major histocompatibility complex. Virus Res. 14:347-358. [DOI] [PubMed] [Google Scholar]
- 85.Ramsingh, A. I. 1997. Coxsackievirus and pancreatitis. Front. Biosci. 2:53-62. [DOI] [PubMed] [Google Scholar]
- 86.Ramsingh, A. I., W. T. Lee, D. N. Collins, and L. E. Armstrong. 1999. T cells contribute to disease severity during coxsackievirus B4 infection. J. Virol. 73:3080-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ross, M. E., K. Hayashi, and A. L. Notkins. 1974. Virus-induced pancreatic disease: alterations in concentration of glucose and amylase in blood. J. Infect. Dis. 129:669-676. [DOI] [PubMed] [Google Scholar]
- 88.Rotter, J., C. Vadheim, and D. Rimoin. 1990. Genetics of diabetes mellitus, p. 378-413. In H. Rifkin and D. Porte (ed.), Diabetes mellitus: theory and practice. Elsevier, Amsterdam, The Netherlands.
- 89.Rueckert, R. R. 1996. Picornaviridae: the viruses and their replication, p. 609-654. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Raven Press, New York, N.Y.
- 90.Sadeharju, K., M. Lonnrot, T. Kimpimaki, K. Savola, S. Erkkila, T. Kalliokoski, P. Savolainen, P. Koskela, J. Ilonen, O. Simell, M. Knip, and H. Hyoty. 2001. Enterovirus antibody levels during the first two years of life in prediabetic autoantibody-positive children. Diabetologia 44:818-823. [DOI] [PubMed] [Google Scholar]
- 91.Schmidt, W. A. K., L. Brade, H. Muntefering, and M. Klein. 1978. Course of coxsackie B antibodies during juvenile diabetes. Med. Microbiol. Immunol. 164:219-298. [DOI] [PubMed] [Google Scholar]
- 92.Serreze, D. V., H. Chapman, D. Varnum, M. Hanson, P. Reifsnyder, S. Richard, S. Fleming, E. H. Leiter, and L. Shultz. 1996. B lymphocytes are critical antigen-presenting cells for the initiation of T-cell-mediated autoimmune diabetes in nonobese diabetic mice: analysis of a new “speed congenic” stock of NOD Ig mu null mice. J. Exp. Med. 184:2049-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Serreze, D. V., N. Chapman, C. Post, E. Johnson, W. Suarez-Pinon, and A. Rabinovitch. 2001. Th1 to Th2 cytokine shifts in nonobese diabetic mice: sometimes an outcome, rather than the cause, of diabetes resistance elicited by immunostimulation. J. Immunol. 166:1352-1359. [DOI] [PubMed] [Google Scholar]
- 94.Serreze, D. V., E. W. Ottendorfer, T. M. Ellis, C. J. Gauntt, and M. A. Atkinson. 2000. Acceleration of type 1 diabetes by a coxsackievirus infection requires a preexisting critical mass of autoreactive T cells in pancreatic islets. Diabetes 49:708-711. [DOI] [PubMed] [Google Scholar]
- 95.Sperling, M. A. 1997. Aspects of the etiology, prediction, and prevention of insulin-dependent diabetes mellitus in childhood. Pediatr. Clin. N. Am. 44:269-284. [DOI] [PubMed] [Google Scholar]
- 96.Takei, I., Y. Asaba, T. Kasatani, T. Maruyama, K. Watanabe, T. Yanagawa, T. Saruta, and T. Ishii. 1992. Suppression of development of diabetes in NOD mice by lactate dehydrogenase virus infection. J. Autoimmun. 5:665-673. [DOI] [PubMed] [Google Scholar]
- 97.Tian, J., D. Zekzer, L. R. Hanssen, Y. Lu, A. Olcutt, and D. L. Kaufman. 2001. Lipopolysaccharide-activated B cells downregulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J. Immunol. 167:1081-1089. [DOI] [PubMed] [Google Scholar]
- 98.Tomko, R., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Toniolo, A., T. Onodera, G. Jordan, J. Yoon, and A. L. Notkins. 1982. Virus-induced diabetes mellitus: glucose abnormalities produced in mice by the six members of the coxsackie B virus group. Diabetes 31:496-499. [DOI] [PubMed] [Google Scholar]
- 100.Tracy, S., N. Chapman, B. McManus, M. Pallansch, M. Beck, and J. Carstens. 1990. A molecular and serologic evaluation of enteroviral involvement in human myocarditis. J. Mol. Cell. Cardiol. 22:403-414. [DOI] [PubMed] [Google Scholar]
- 101.Tracy, S., N. Chapman, and H. Liu. 1985. Molecular cloning and partial characterization of the coxsackievirus B3 genome. Arch. Virol. 85:157-163. [DOI] [PubMed] [Google Scholar]
- 102.Tracy, S., N. Chapman, and B. Mahy. 1997. The coxsackie B viruses. Curr. Top. Microbiol. Immunol. 223:1-303.9294922 [Google Scholar]
- 103.Tracy, S., N. Chapman, and Z. Tu. 1992. Coxsackievirus B3 from an infectious cDNA copy of the genome is cardiovirulent in mice. Arch. Virol. 122:399-409. [DOI] [PubMed] [Google Scholar]
- 104.Tracy, S., and C. Gauntt. 1988. Phenotypic and genotypic differences among naturally occurring coxsackie B3 virus variants. Eur. Heart J. 8(Suppl. J):445-448. [Google Scholar]
- 105.Tracy, S., K. Hofling, S. Pirruccello, P. H. Lane, S. M. Reyna, and C. Gauntt. 2000. Group B coxsackievirus myocarditis and pancreatitis in mice: connection between viral virulence phenotypes. J. Med. Virol. 62:70-81. [DOI] [PubMed] [Google Scholar]
- 106.Tu, Z., N. Chapman, G. Hufnagel, S. Tracy, J. R. Romero, W. H. Barry, K. Currey, and B. Shapiro. 1995. The cardiovirulent phenotype of coxsackievirus B3 is determined at a single site in the genomic 5′ nontranslated region. J. Virol. 69:4607-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ursing, B. 1973. Acute pancreatitis in coxsackie B infection. BMJ 3:524-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van Creveld, S., and H. De Jager. 1956. Myocarditis in newborns, caused by coxsackie virus. Ann. Pediatr. 187:100-112. [PubMed] [Google Scholar]
- 109.Verlinde, J., H. Van Tongeren, and A. Kret. 1956. Myocarditis in newborns due to group B coxsackievirus: virus studies. Acta Pediatr. 187:113-118. [Google Scholar]
- 110.von Herrath, M. G. 2000. Obstacles to identifying viruses that cause autoimmune disease. J. Neuroimmunol. 107:154-160. [DOI] [PubMed] [Google Scholar]
- 111.von Herrath, M. G., D. Homan, J. Gairin, and M. B. A. Oldstone. 1996. Pathogenesis and treatment of virus-induced autoimmune diabetes: novel insights gained from the RIP-LCMV transgenic mouse model. Biochem. Soc. Trans. 25:630-635. [DOI] [PubMed] [Google Scholar]
- 112.Webb, S., R. Loria, G. Madge, and S. Kibrick. 1976. Susceptibility of mice to group B coxsackievirus is influenced by the diabetic gene. J. Exp. Med. 143:1239-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wilberz, S., H. Partke, F. Dagnaes-Hansen, and L. Herberg. 1991. Persistent MHV (mouse hepatitis virus) infection reduces the incidence of diabetes mellitus in non-obese diabetic mice. Diabetologia 34:2-5. [DOI] [PubMed] [Google Scholar]
- 114.Wolf, E., K. Spenser, and A. Cudworth. 1983. The genetic susceptibility to type 1 diabetes. Analysis of the HLA-DR association. Diabetologia 24:224-230. [DOI] [PubMed] [Google Scholar]
- 115.Wong, F. S., and C. A. Janeway, Jr. 1999. Insulin-dependent diabetes mellitis and its animal models. Curr. Opin. Immunol. 11:643-647. [DOI] [PubMed] [Google Scholar]
- 116.Yoon, J., M. Austin, T. Onodera, and A. Notkins. 1979. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N. Engl. J. Med. 300:1173-1179. [DOI] [PubMed] [Google Scholar]