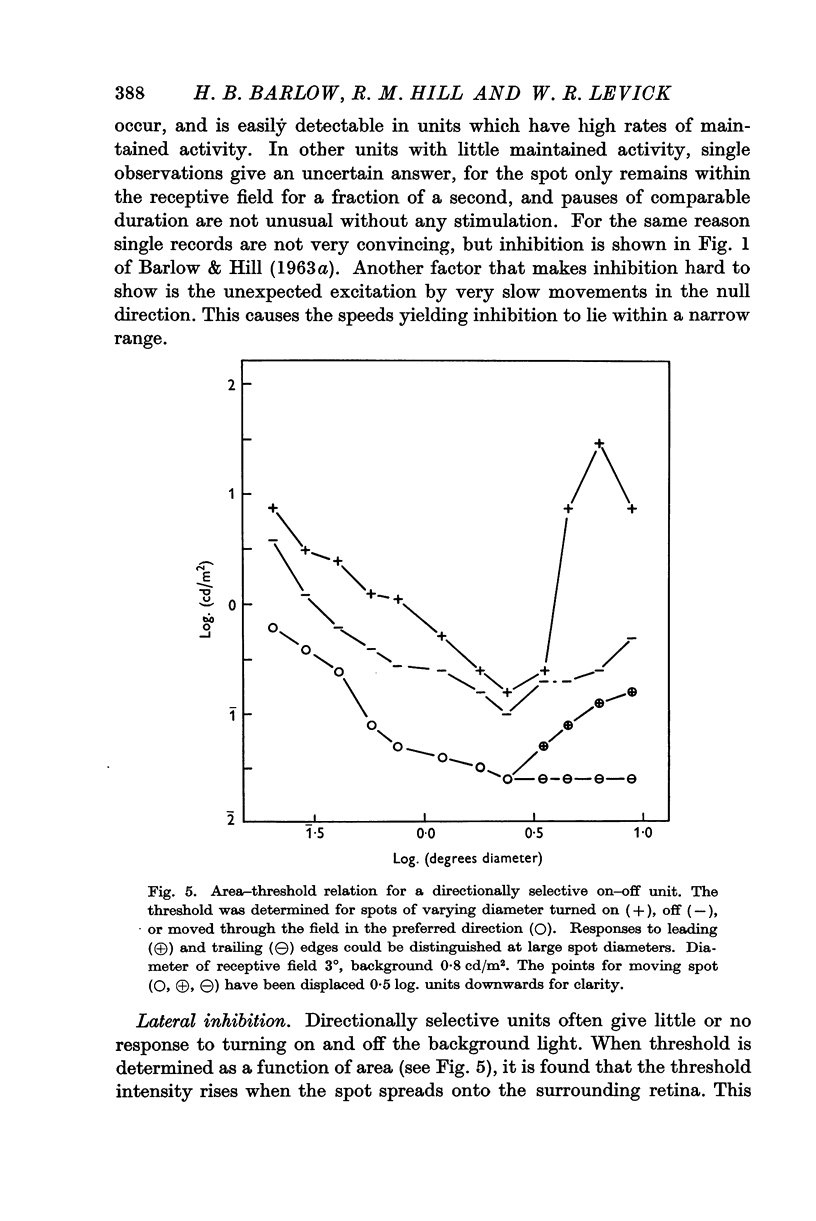
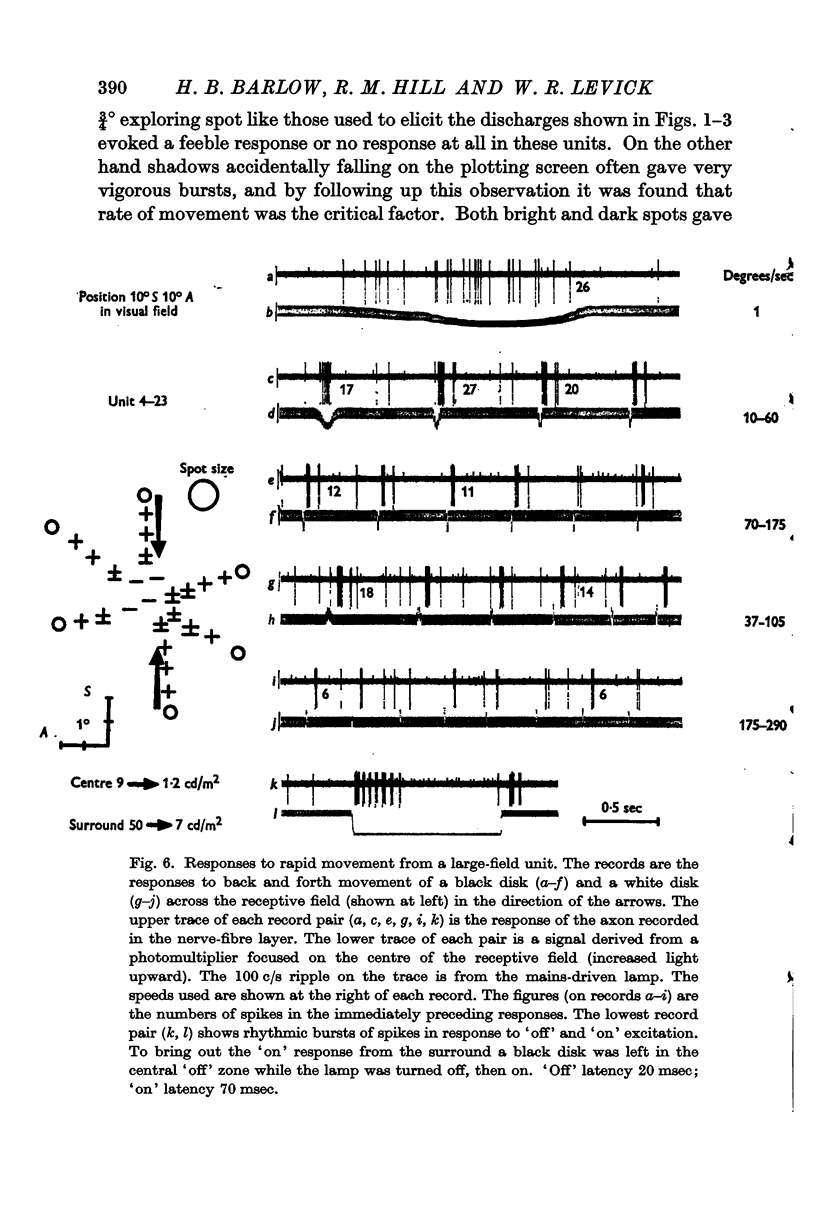
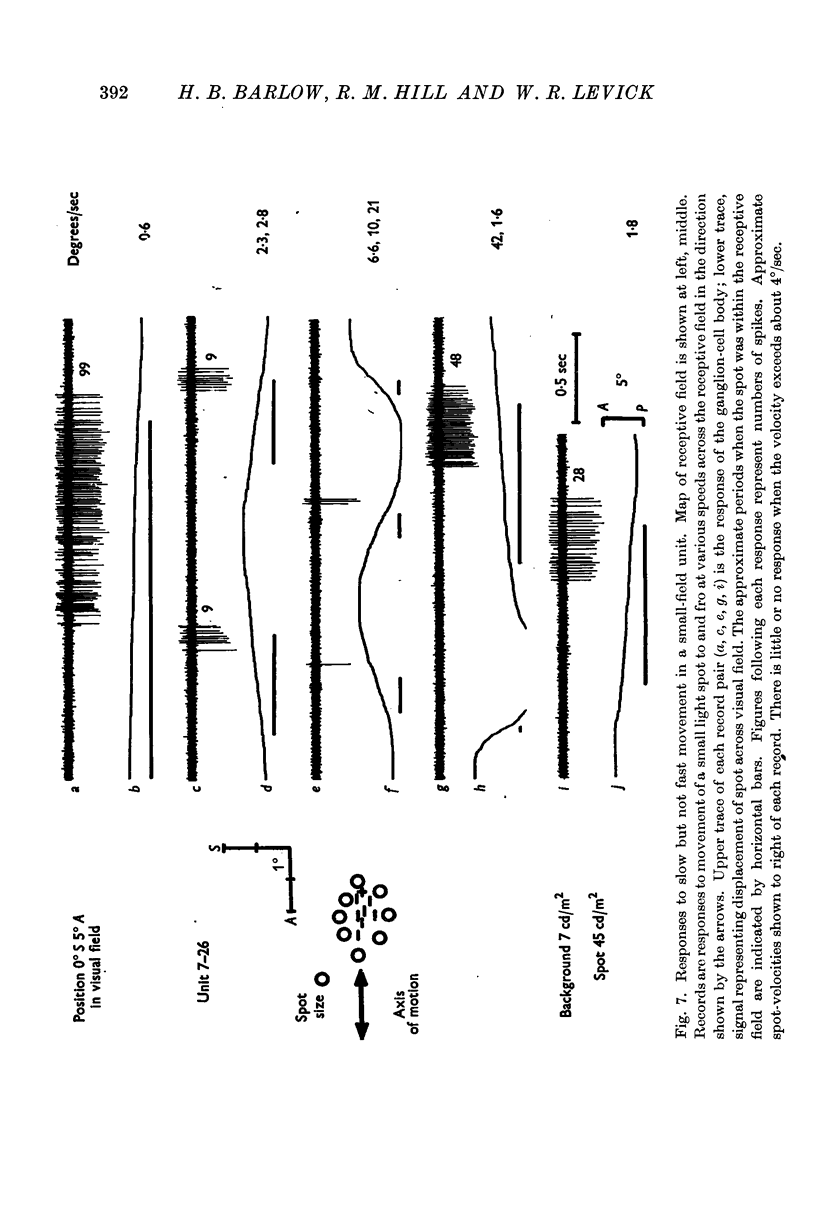
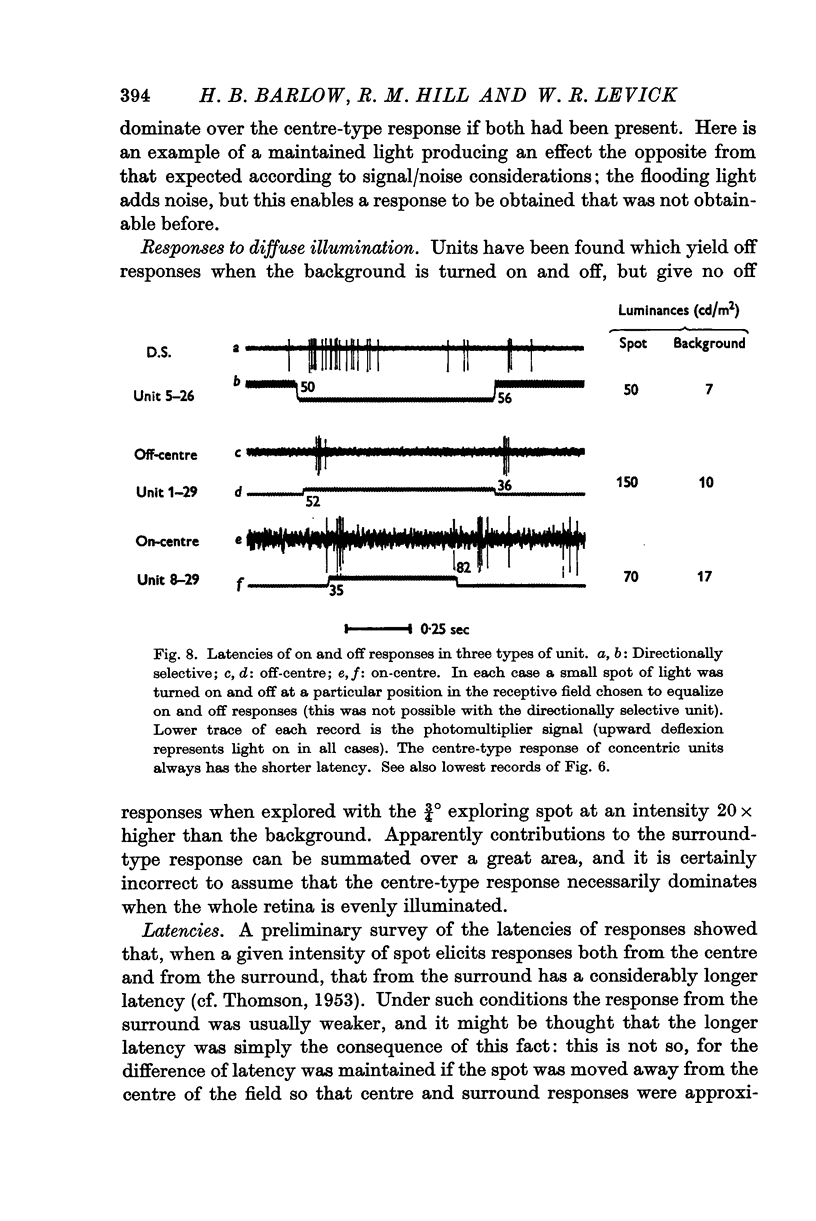
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARDEN G. B. Complex receptive fields and responses to moving objects in cells of the rabbit's lateral geniculate body. J Physiol. 1963 May;166:468–488. doi: 10.1113/jphysiol.1963.sp007117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARDEN G. B. Types of response and organization of simple receptive fields in cells of the rabbits lateral geniculate body. J Physiol. 1963 May;166:449–467. doi: 10.1113/jphysiol.1963.sp007116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. A method of determining the over-all quantum efficiency of visual discriminations. J Physiol. 1962 Jan;160:155–168. doi: 10.1113/jphysiol.1962.sp006838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., BRINDLEY G. S. INTER-OCULAR TRANSFER OF MOVEMENT AFTER-EFFECTS DURING PRESSURE BLINDING OF THE STIMULATED EYE. Nature. 1963 Dec 28;200:1347–1347. doi: 10.1038/2001347a0. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., HILL R. M. EVIDENCE FOR A PHYSIOLOGICAL EXPLANATION OF THE WATERFALL PHENOMENON AND FIGURAL AFTER-EFFECTS. Nature. 1963 Dec 28;200:1345–1347. doi: 10.1038/2001345a0. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B., HILL R. M. Selective sensitivity to direction of movement in ganglion cells of the rabbit retina. Science. 1963 Feb 1;139(3553):412–414. doi: 10.1126/science.139.3553.412. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B. Summation and inhibition in the frog's retina. J Physiol. 1953 Jan;119(1):69–88. doi: 10.1113/jphysiol.1953.sp004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., KOZAK W., LEVICK W. R., VAKKUR G. J. The determination of the projection of the visual field on to the lateral geniculate nucleus in the cat. J Physiol. 1962 Oct;163:503–539. doi: 10.1113/jphysiol.1962.sp006991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOURAS P. Spreading depression of activity in amphibian retina. Am J Physiol. 1958 Oct;195(1):28–32. doi: 10.1152/ajplegacy.1958.195.1.28. [DOI] [PubMed] [Google Scholar]
- HAMASAKI D., MARG E. Microelectrode study of accessory optic tract in the rabbit. Am J Physiol. 1962 Mar;202:480–486. doi: 10.1152/ajplegacy.1962.202.3.480. [DOI] [PubMed] [Google Scholar]
- HILL R. M., MARG E. Single-cell responses of the nucleus of the transpeduncular tract in rabbit to monochromatic light on the retina. J Neurophysiol. 1963 Mar;26:249–257. doi: 10.1152/jn.1963.26.2.249. [DOI] [PubMed] [Google Scholar]
- HILL R. M. Unit responses of the rabbit lateral geniculate nucleus to monochromatic light on the retina. Science. 1962 Jan 12;135(3498):98–99. doi: 10.1126/science.135.3498.98. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H. Single unit activity in lateral geniculate body and optic tract of unrestrained cats. J Physiol. 1960 Jan;150:91–104. doi: 10.1113/jphysiol.1960.sp006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of optic nerve fibres in the spider monkey. J Physiol. 1960 Dec;154:572–580. doi: 10.1113/jphysiol.1960.sp006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of single neurones in the cat's striate cortex. J Physiol. 1959 Oct;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- LIPETZ L. E. A mechanism of light adaptation. Science. 1961 Mar 3;133(3453):639–640. doi: 10.1126/science.133.3453.639. [DOI] [PubMed] [Google Scholar]
- MATURANA H. R., FRENK S. DIRECTIONAL MOVEMENT AND HORIZONTAL EDGE DETECTORS IN THE PIGEON RETINA. Science. 1963 Nov 15;142(3594):977–979. doi: 10.1126/science.142.3594.977. [DOI] [PubMed] [Google Scholar]
- MATURANA H. R., LETTVIN J. Y., MCCULLOCH W. S., PITTS W. H. Anatomy and physiology of vision in the frog (Rana pipiens). J Gen Physiol. 1960 Jul;43(6):129–175. doi: 10.1085/jgp.43.6.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVER P. H. TWO SPECTRAL SENSITIVITY CURVES OF XENOPUS LAEVIS OBTAINED BY USING THE MELANOPHORE RESPONSE TO LIGHT ON WHITE AND BLACK BACKGROUNDS. J Physiol. 1963 Nov;169:1–9. doi: 10.1113/jphysiol.1963.sp007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMSON L. C. The localization of function in the rabbit retina. J Physiol. 1953 Feb 27;119(2-3):191–209. doi: 10.1113/jphysiol.1953.sp004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]


