Abstract
The consequences of a hepatitis A virus (HAV) infection on cell-based antiviral responses and the interactions between virus and host cells resulting in persistent infections are poorly understood. In this report, we show that HAV does inhibit double-stranded (dsRNA)-induced beta interferon (IFN-β) gene expression by influencing the IFN-β enhanceosome, as well as dsRNA-induced apoptosis, which suggests that both effects may be connected by shared viral and/or cellular factors. This ability of HAV, which preserves the sites of virus production for a longer time, may allow the virus to establish an infection and may be the presupposition for setting up persistent infections. Our results suggest that the inhibitory effect of HAV on the cellular defense mechanisms might not be sufficient to completely prevent the antiviral reactions, which may be induced by accumulating viral dsRNA, at a later stage of infection. However, HAV seems to counteract this situation by downregulation of viral replication and in the following production of viral dsRNA. This ability of noncytopathogenic HAV acts dominantly on cytopathogenic HAV in trans. The downregulation might ensure the moderate replication which seems necessary for inhibition of the antiviral mechanisms by HAV and therefore for the persistent state of the HAV infection.
In order to establish infections, picornaviruses are optimized to invade target cells, replicate large numbers of progeny virions, and spread these progeny to initiate new rounds of infection in neighboring cells. However, host organisms possess systemic, as well as cell-based, defense mechanisms to limit virus spread and to remove the virus at last. The innate cell-based responses to viral infections, which include the interferon (IFN) system and apoptosis of infected cells, are designed to act fast at the sites of infection. Induction of IFN synthesis in infected cells can curtail the spread of infection by inducing an antiviral state in neighboring cells, whereas induction of apoptosis can interrupt the infection cascade by entailing death of the infected cells without virus release. The inducer for IFN synthesis and in some cases also for apoptosis is double-stranded RNA (dsRNA) formed as a result of replication or convergent transcription of viral genomes (5, 6, 13, 18, 28, 34). The effectiveness of both antiviral responses has led to many viruses developing specific mechanisms that antagonize the production or actions of IFNs (25; for reviews, see references 12 and 26) and prevent apoptosis (for a review, see reference 23).
In particular viruses that establish persistent infections have to cope with the cellular antiviral responses. Although hepatitis A virus (HAV), which belongs to the picornavirus family, never causes chronic infection with viral persistence in the host organism in vivo, infection in cultured cells results in a persistent noncytopathic infection (7, 11, 31). At the cellular level this seems also to be true for the situation in vivo, in which massive hepatocyte destruction is mediated mainly by the action of cytotoxic T lymphocytes (32). These characteristics of HAV infection imply that, without involvement of the cellular immune system, HAV replication results in persistent infections and that therefore HAV has developed mechanisms to prevent or reduce cellular antiviral responses.
Indeed, various investigations demonstrated that HAV infection did not result in measurable human alpha IFN (IFN-α) levels in lymphocytes or IFN-β levels in fibroblasts (30). Furthermore, fibroblasts infected persistently with HAV did not interfere with the infection by other viruses (30, 31). This finding that HAV replication obviously does not induce IFN-α/β synthesis is supported by experiments that showed that even small amounts of exogenous IFN-α/β are able to eliminate persistent HAV infections in human fibroblasts (29, 31). In contrast to these experimental results, which give evidence that HAV cannot induce IFN-α/β expression, data were presented that demonstrate that IFN-γ is produced after HAV stimulation by HAV-specific HLA-dependent cytotoxic T lymphocytes in vitro and may play a role in the elimination of HAV infection in humans (19). The principal difference between the induction of these IFNs is that IFN-α/β is produced rapidly in direct response to viral replication by infected cells, whereas IFN-γ is synthesized in response to the recognition of infected cells by activated T lymphocytes later in the course of infection (12). Although it has been known for many years that HAV infection does not result in IFN-α/β synthesis in vitro, the mechanism by which HAV interferes with the expression of IFNs is not known, and therefore the role of IFN-α/β in the elimination of HAV during an acute infection is still uncertain.
The intention of our investigation was to find out at which level HAV interferes with IFN-β synthesis. We found that cells infected with HAV do not transcribe IFN RNA and do not synthesize IFN in response to the synthetic dsRNA poly(IC), which is an inducer of IFN synthesis. These cells also resist apoptosis caused by dsRNA molecules.
MATERIALS AND METHODS
Cells.
Fetal rhesus monkey kidney cells (FRhK-4), human embryonic lung fibroblasts (MRC-5), and human amnionic cells (WISH) were maintained as continuous cultures in Dulbecco modified Eagle medium (DMEM) supplemented with 1 or 3% (MRC-5) fetal calf serum (FCS). In order to split the cells at a ratio of 1:2 (MRC-5) and 1:5 (FRhK-4 and WISH), they were detached from the tissue culture plate with trypsin supplemented with 0.2 g of sodium-EDTA/liter and cultivated with DMEM-10% FCS as the growth medium.
FRhK-4/(−110IFN-β)-CAT cells were obtained by calcium phosphate transfection with FRhK-4 cells, which were cultivated on 6-cm dishes, and 5 μg of the (−110IFN-β)-CAT reporter plasmid, which was kindly provided by T. Maniatis (9). To establish stable cell lines containing the reporter plasmid the cells were cotransfected with 2 μg of pSV2neo (Clontech) and cultured with selection medium containing 600 μg of G418 per ml. To test induction of the chloramphenicol acetyltransferase (CAT) reporter gene, cell clones were transfected with 20 μg of poly(IC)/ml by using DEAE-dextran and CAT expression was analyzed by enzyme-linked immunosorbent assay (ELISA) 16 h after induction.
Viruses.
HAV/7 (noncytopathogenic) (3) and HAVcyt/HB1.1 (cytopathogenic) (1), which are variants of strain HM175, were propagated in FRhK-4 cells as described previously (1). HAV strain GBM (noncytopathogenic) (10) was propagated in MRC-5 cells at 37°C. Vesicular stomatitis virus (VSV) strain Indiana was passaged on FRhK-4 cells. HAV was prepared by triple freeze-thaw cycles, with VSV in one step, and then the cellular debris was removed.
The 50% tissue culture infective dose (TCID50) titers of HAV were determined by indirect immunofluorescence with the monoclonal mouse anti-HAV immunoglobulin G (IgG) antibody 7E7 (Mediagnost, Reutlingen, Germany), and the titer of VSV was determined by a plaque assay. The titers were calculated by the Kärber method (14).
In order to neutralize HAV, HAV-anti-HAV IgG immunocomplexes were prepared by incubating HAV (105 to 106 TCID50/ml) with 20 μg of anti-HAV IgG antibody 7E7 per ml for 2 h at room temperature in DMEM (8). Since these antibodies neutralize HAV upon infection of FRhK-4 cells, complex formation was assayed by infection inhibition on FRhK-4 cells, which was determined by titration and compared to that obtained with HAV alone in amounts equal to those in the samples with antibodies.
Detection of IFN activity and IFN mRNA.
IFN activity in supernatants of cells was measured by determination of the reduction of VSV growth in WISH cells (plaque reduction assay) (17). Cells cultivated in 96-well plates were incubated with serial dilutions of cell culture supernatants for 20 h at 37°C. After two washes, the cells were incubated with 80 PFU of VSV/well for 1 h at 37°C. After removal of the inoculum, DMEM supplemented with 1% methylcellulose and 2% FCS was added, and the cells were incubated for 2 days at 37°C, fixed with 4% formalin for 15 min at room temperature, and stained with crystal violet (0.4%) for 10 min at room temperature. IFN titers are expressed in terms of a reference standard provided by the NIH (Reference Reagent Repository, Gaithersburg, Md.). One unit of IFN is defined as the dilution that results in 50% plaque reduction. Incubation of WISH cells with IFN in the presence of HAV demonstrated that HAV in cell culture supernatants does not influence the plaque reduction assay.
IFN mRNA was detected by reverse transcription-PCR (RT-PCR) by using total cellular RNA, which was prepared by acid guanidinium thiocyanate-phenol- chloroform extraction (2) and treated with DNase I (5 U/100 μl) for 30 min at 37°C. RT was performed for 45 min at 42°C in the presence of antisense primers and 2 U of reverse transcriptase in a total volume of 20 μl. Then, 5 μl of the RT product was used in the following PCR. The primers used were specific for IFN-α and IFN-β (27), respectively, and primers specific for β-actin were used as loading control in a separate reaction. After 2 min at 94°C, the reaction parameters used for 30 cycles were 1 min at 94°C, 2 min at 60°C, and 3 min at 72°C. The sequences of the primers used were IFN-α sense [5′-GGAAGCTT(CT)CTCCTG(CT)(CT)TGA(AT)GGACAGA-3′], IFN-α antisense [5′-GGGGATCCTCTGACAACCTCCCA(AGCT)GCACA-3′], IFN-β sense (5′-ACCAACAAGTGTCTCCTCCA-3′; nucleotides 361 to 380), IFN-β antisense (5′-GAGGTAACCTGTAAGTCTGT-3′; nucleotides 893 to 912), β-actin sense (5′-AGAAGAGCTACGAGCTGCCTGACG-3′; nucleotides 751 to 774), and β-actin antisense (3′-CGTCATACTCCTGCTTGCTGATCC-3′; nucleotides 1107 to 1131).
Induction of IFN synthesis and apoptosis by dsRNA.
IFN synthesis and apoptosis were induced by incubating cells cultivated in 6-cm dishes and washed once with DMEM with 2 ml of DMEM supplemented with 20 μg of polyinosinic-poly(C) [poly(IC)]/ml in the presence of 100 μg of DEAE-dextran/ml for 2 h at 37°C. After one wash with Hank's balanced salt solution, the cells were incubated for 12 to 16 h at 37°C, and samples were prepared to analyze IFN-β activity, IFN-β mRNA, and apoptosis, respectively.
Detection of apoptosis.
Apoptosis was analyzed by DNA fragmentation and quantified by flow cytometry (1). In order to investigate DNA fragmentation, 2 × 106 cells were washed twice with phosphate-buffered saline (PBS) and lysed in 400 μl of lysis buffer (10 mM Tris [pH 7.5], 0.5% Triton X-100) for 30 min on ice. Nuclei were removed, and the supernatants were extracted with phenol-chloroform. After precipitation, the samples were treated with RNase A (final concentration, 1 μg/ml) and analyzed electrophoretically.
For flow cytometric analysis, the cells were treated with trypsin, washed twice with PBS, and fixed with 70% ethanol in PBS at 4°C overnight. For nuclear staining, the cell were incubated with PBS supplemented with 50 μg of propidium iodide, 100 U of RNase A, and 0.1% glucose per ml for 30 min at room temperature. After transfer of the cells to 1 ml of isotonic buffer (Isoton II; Coulter), the DNA fluorescence profiles were recorded by using the EPICS XL flow cytometer (Coulter). Apoptotic nuclei were identified as a subdiploid peak with a DNA content of <2 N.
CAT reporter gene assay.
At 16 h postinduction with 20 μg of poly(IC) and 100 μg of DEAE-dextran per ml, FRhK-4/(−110IFN-β)-CAT-cell extracts were prepared by triple freeze-thaw cycles, and expression of the CAT reporter gene was analyzed by the CAT-ELISA (Roche) as described by the manufacturer, with normalization of the protein concentrations in the cell extracts.
Detection of HAV-specific dsRNA.
HAV dsRNA was detected by the hybrid detection assay (HDA) (24). In order to prepare dsRNA without contamination by single-stranded RNA, total cellular RNA, which was prepared by acid guanidinium thiocyanate-phenol-chloroform extraction, was treated with RNase A (final concentration, 35 μg/ml) in the presence of 0.3 M NaCl for 45 min at 15°C. After precipitation, the dsRNA extracts were hybridized with a biotinylated single-stranded DNA probe specific for HAV minus-strand RNA. The RNA-DNA hybrids were captured onto streptavidin-coated microwells, incubated with an anti-hybrid antibody conjugated to alkaline phosphatase (Digene Corporation, Gaithersburg, Md.), and detected with a chemiluminescent substrate by use of a luminometer.
RESULTS
HAV infection does not induce IFN-β synthesis.
Former investigations demonstrated that human fibroblasts infected with HAV do not secrete IFN-β (31). This implies that HAV replication in these cells does not result in IFN production.
In order to prove that the inability to respond to HAV infection with IFN synthesis is not an attribute of specific cell lines and/or HAV variants, the monkey kidney cell line FRhK-4, which is a standard cell line for HAV propagation, and the human lung fibroblast cells MRC-5 were infected at an multiplicity of infection (MOI) of 1 to 4 with HAV strain HM175 (HAV/7) and HAV strain GBM (HAV-GBM), respectively. IFN expression was analyzed by plaque reduction activity of the supernatants by using VSV as challenge virus as well as by IFN mRNA detection by RT-PCR at different times after infection. This allows the differentiation whether IFN synthesis is influenced at the level of transcription or posttranscriptionally. Infection of the cells was confirmed by indirect immunofluorescence.
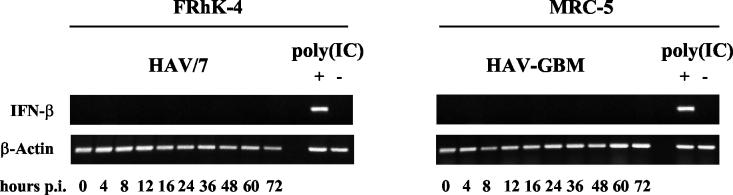
Up to 72 h postinfection (p.i.), at which time all cells were infected, no IFN activity could be detected at any time in the cell culture supernatants with both cell lines and virus strains used (data not shown). The same results were obtained with cells infected with cell lysates of noninfected cells as negative controls (data not shown). Also, no IFN mRNA was detectable in the cell extracts at the times indicated, as shown for FRhK-4 cells infected with HAV/7 (Fig. 1) and MRC-5 cells infected with HAV-GBM (Fig. 1). Transfection of noninfected cells with 20 μg of poly(IC) by DEAE-dextran resulted in IFN activity in the supernatants, as well as in IFN-β mRNA synthesis 12 to 16 h after induction (Fig. 1), but not in IFN-α mRNA synthesis (data not shown). This demonstrates that FRhK-4, as well as MRC-5, cells possess the ability to express IFN-β. Transfection with DEAE-dextran alone did not result in IFN expression, which proves that the cells IFN response was specific for dsRNA (Fig. 1), which is generally assumed to be the inducer under physiological conditions during viral infection (13).
FIG. 1.
RT-PCR analysis of IFN-β mRNA in cells infected with HAV. FRhK-4 cells were infected with HAV/7 at an MOI of 4 and MRC-5 cells were infected with HAV-GBM at an MOI of 1. Cells were transfected with 20 μg of poly(IC) [poly(IC)+] as positive controls and with DEAE-dextran without poly(IC) [poly(IC)−] as negative controls. Proof of β-actin mRNA by RT-PCR was included as control to ensure that equal quantities of RNA were analyzed. Shown are the data of an experiment representative for a series of three, each including two replicates.
These results confirm earlier suggestions that HAV infection does not result in IFN-β production in cultured cells (30) and show that the absent IFN-β response on HAV replication is based on the nonappearance of IFN mRNA transcription.
HAV inhibits dsRNA-induced expression of IFN-β.
Viruses vary considerably in their ability to induce IFN synthesis. This may reflect the fact that they are poor inducers of IFN or that they antagonize the induction of IFN transcription.
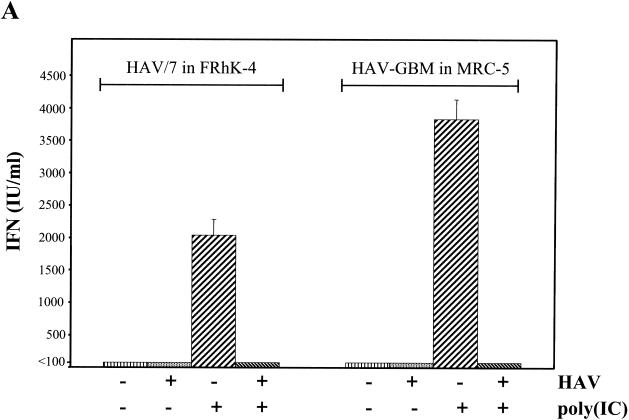
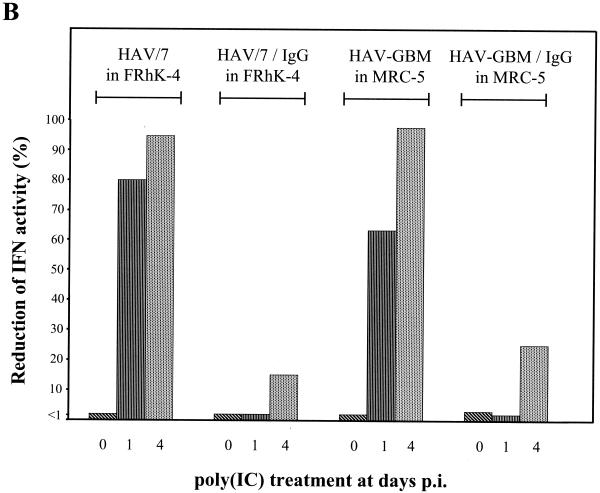
In order to examine whether HAV, which replicates exceptionally slowly in cultured cells, is a less potent IFN inducer or does inhibit IFN induction by dsRNA, poly(IC) was transfected into FRhK-4 cells infected with HAV/7 with an MOI of 0.1 and MRC-5 cells infected with HAV-GBM with an MOI of 0.1 at different times after inoculation. Transfection by the DEAE-dextran method does exclude the possibility that infection by HAV merely inhibits the uptake of poly(IC). Analysis by plaque reduction assay by using VSV showed that poly(IC) at 20 μg/ml in the presence of DEAE-dextran strongly induced the secretion of IFN into the supernatants of FRhK-4, as well as of MRC-5 cells, which was completely inhibited in the HAV-infected cells 4 days p.i. (Fig. 2A). Neither in HAV-infected nor in noninfected cells transfected without poly(IC) did IFN synthesis occur significantly (Fig. 2A). Compared to noninfected cells, induction of IFN synthesis immediately after HAV inoculation (day 0) and at 1 and 4 days p.i. by poly(IC) revealed that IFN activity in the supernatants is already strongly reduced by 80% in FRhK-4 cells and is reduced by 63.4% in MRC-5 cells by 1 day p.i. and is nearly absent by 4 days p.i. under these experimental conditions (Fig. 2B). This result strongly suggests that dsRNA-induced IFN synthesis is inhibited by the replicating HAV.
FIG. 2.
Influence of HAV infection on dsRNA-induced IFN-β secretion. FRhK-4 cells infected with HAV/7 and MRC-5 cells infected with HAV-GBM at an MOI of 0.1 were transfected with 20 μg of poly(IC)/ml at different times p.i. IFN-β was analyzed in the supernatants at 16 h after transfection by plaque reduction assay with VSV. (A) At 4 days p.i., IFN secretion was analyzed after induction by poly(IC) [+poly(IC)] or transfection without poly(IC) [−poly(IC)] in mock-infected cells (−HAV) and in cells infected with HAV (+HAV). IFN titers are expressed in terms of a reference standard provided by the NIH (IU). (B) Percentage by which IFN activity was reduced in HAV/7-infected FRhK-4 and HAV-GBM-infected MRC-5 cells after transfection with poly(IC) at days 0, 1, and 4 p.i. compared to the IFN activity obtained in mock-infected cells after transfection with poly(IC). As controls, cells were infected with HAV neutralized by anti-HAV IgG (HAV/IgG). The data represent means from two separate experiments including three replicates, and error bars indicate standard deviations.
This is supported by the result that under the same experimental settings, but with HAV neutralized by anti-HAV IgG, only a moderate reduction of IFN activity after poly(IC) induction was detected compared to noninfected cells (Fig. 2B), which presumably resulted from HAV not associated with IgG antibodies.
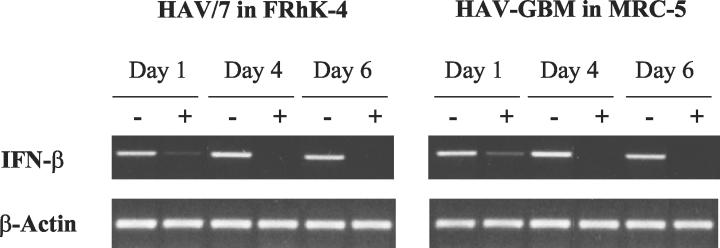
In order to investigate whether the inhibition of IFN secretion by HAV occurs at the level of IFN-β transcription, the expression of IFN-β mRNA was analyzed by RT-PCR. FRhK-4 and MRC-5 cells infected with HAV/7 (MOI of 3) and HAV-GBM (MOI of 1), respectively, were transfected with 20 μg of poly(IC)/ml by DEAE-dextran at days 1, 4, and 6 p.i., and cellular RNA was extracted 12 to 16 h after transfection. In comparison to noninfected cells, which were also treated with poly(IC) and in which strong signals for IFN-β mRNA were detected at the times analyzed, only weak signals indicating IFN-β mRNA were detectable at day 1 p.i. and no IFN-β mRNA at all was detectable 4 and 6 days after HAV infection (Fig. 3).
FIG. 3.
RT-PCR analysis of dsRNA-induced IFN-β mRNA in cells infected with HAV. FRhK-4 cells infected with HAV/7 at an MOI of 3 and MRC-5 cells infected with HAV-GBM at an MOI of 1 (+), as well as mock-infected cells (−) were transfected with 20 μg of poly(IC)/ml at days 1, 4, and 6 p.i., and IFN-β mRNA expression was analyzed 12 to 16 h after transfection. Proof of β-actin mRNA by RT-PCR was included as a control to ensure that equal quantities of RNA were analyzed. Shown are the data of an experiment representative for a series of three, each including two replicates.
These results demonstrate that HAV inhibits dsRNA-induced IFN-β gene expression and strongly imply that the inhibition of IFN-β synthesis occurs during the events leading to transcription of IFN-β mRNA.
HAV does not induce the IFN-β enhancer and inhibits the induction of IFN-β mRNA transcription by dsRNA.
In order to prove that HAV inhibits the transcription of IFN-β mRNA, FRhK-4 cells were stably transfected with CAT as a reporter gene linked to the intact IFN-β enhancer-promoter by calcium phosphate transfection of the (−110IFN-β)-CAT plasmid (9, 15).
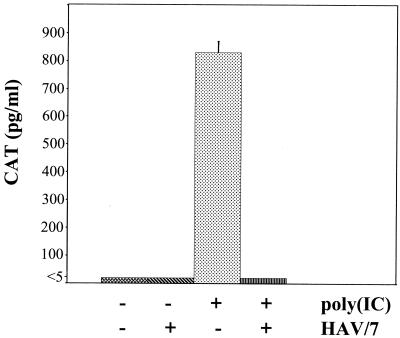
After transfection of these cells with 20 μg of poly(IC)/ml by DEAE-dextran, proteins were extracted 16 h after transfection and 100 μg of protein were analyzed for expression of the CAT reporter gene by the CAT-ELISA. In these experiments high levels of CAT were detected (Fig. 4), which shows that the IFN-β enhancer was induced strongly by dsRNA. Transfection without poly(IC), which was used as a control, did not result in CAT expression (Fig. 4).
FIG. 4.
Influence of HAV infection on the assembly of the IFN-β enhanceosome. FRhK-4 cells were stably transfected with the (−110IFN-β)-CAT reporter plasmid. CAT expression was analyzed by ELISA 16 h after transfection with 20 μg of poly(IC)/ml in cells mock-infected [+poly(IC); −HAV] or infected with HAV/7 at an MOI of 4 [+poly(IC); +HAV] 1 day prior transfection. As negative controls, cells were mock infected and mock transfected [−poly(IC); −HAV]. The influence of HAV/7 on CAT expression was examined by infection of the mock-transfected cells with HAV/7 at an MOI of 4 [−poly(IC); +HAV]. The data are means obtained from two separate experiments including two replicates, and error bars indicate standard deviations.
In order to examine whether HAV replication does not induce the IFN-β enhancer, FRhK-4/(−110IFN-β)-CAT cells were infected with HAV/7 with an MOI of 4 and analyzed for CAT expression 2 days p.i. As shown in Fig. 4, no CAT was detectable, which demonstrates that HAV does not induce the IFN-β enhancer and therefore IFN-β mRNA transcription.
Moreover, induction of CAT expression by poly(IC) in FRhK-4/(−110IFN-β)-CAT cells was completely inhibited in cells, which were infected with HAV/7 at an MOI of 4 1 day prior induction (Fig. 4). Thus, HAV does inhibit activation of the IFN-β enhancer in response to dsRNA.
HAV inhibits dsRNA-induced apoptosis.
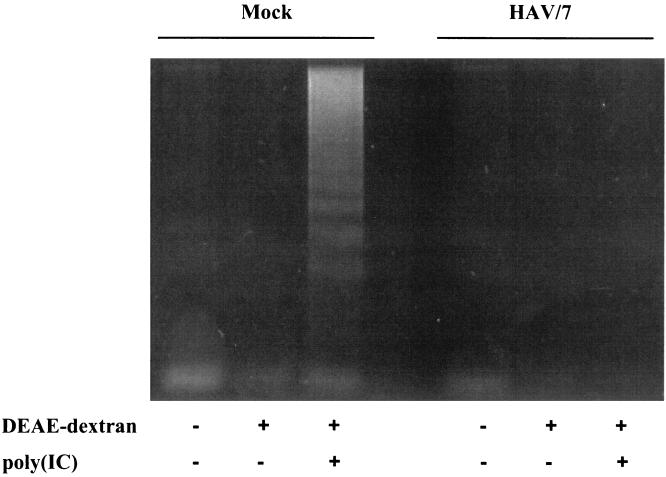
Transfection of 20 μg of poly(IC)/ml with DEAE-dextran into FRhK-4 cells resulted not only in induction of IFN-β production but also in the induction of apoptotic cell death, which was analyzed by DNA fragmentation 12 h after transfection (Fig. 5). However, in FRhK-4 cells, which were infected persistently with HAV/7, which does not induce DNA fragmentation on its own (1), no DNA fragmentation occurred after treatment with poly(IC) (Fig. 5). Therefore, it was supposed that HAV/7 inhibits dsRNA-induced apoptosis.
FIG. 5.
Analysis of apoptosis-specific DNA fragmentation in mock-infected and persistently HAV/7-infected FRhK-4 cells after transfection with poly(IC). Cytoplasmic DNA extracts were prepared 12 h after transfection with 20 μg of poly(IC)/ml [poly(IC)+]. After treatment with RNase A, the samples were analyzed on a 1.6% agarose gel. As controls, the cells were mock transfected [poly(IC)−; DEAE-dextran+] and not transfected [poly(IC)−; DEAE-dextran−]. Shown are the data of an experiment representative for a series of three, each including two replicates.
In order to obtain more quantitative data with regard to the inhibitory effect of HAV on dsRNA-induced apoptosis, we analyzed by flow cytometry the DNA contents of FRhK-4 cells that either were not infected or were infected with HAV/7 after treatment with poly(IC). For this purpose, cells were infected with an MOI of 0.1. Immediately after inoculation with HAV (day 0) and at days 1 and 4 p.i., cells were transfected with 20 μg of poly(IC)/ml by DEAE-dextran and after an incubation time of 12 h, cells were prepared for fluorescence-activated cell sorting (FACS) analysis. Apoptotic nuclei were identified as a subdiploid peak (DNA content, <2 N). To demonstrate the specificity of the viral effect, HAV/7 neutralized by anti-HAV IgG and cell lysates of noninfected cells supplemented with or without anti-HAV IgG in amounts equal to those used for neutralization of HAV, respectively, were used as controls. The results obtained are shown in Table 1. For cells infected with HAV/anti-HAV IgG complexes, the percentage of apoptotic cells was identical to that inoculated with the cell lysate and the lysates supplemented with IgG, with values between 7 and 12.5 after treatment with poly(IC) at days 0, 1, and 4 p.i. However, after infection with HAV/7, the proportion of apoptotic cells after poly(IC) treatment decreased from 12.5% at day 0 p.i., which correponds to the value obtained with noninfected cells, to 3.4% at day 1 p.i. and 2.1% at day 4 p.i., which almost corresponds to the mean value of 1.3% apoptotic cells for FRhK-4 cells transfected with DEAE-dextran without poly(IC).
TABLE 1.
Fraction of HAV/7-infected FRhK-4 cells with a DNA content of <2 N after transfection with poly(IC) in comparison with the controls and HAV/7-anti-HAV IgG-infected cellsa
| Day(s) p.i.c | % Apoptotic cells
|
||||
|---|---|---|---|---|---|
| Controlsb
|
HAV/7 | HAV/7- anti-HAV IgG complexes | |||
| −HAV/7 and −poly(IC) | −HAV/7 and −anti-HAV IgG | −HAV/7 and +anti-HAV IgG | |||
| 0 | 1.2 | 10.3 | 11.7 | 12.5 | 8.2 |
| 1 | 1.4 | 8.6 | 6.9 | 3.4 | 6.7 |
| 4 | 1.3 | 10.5 | 12.0 | 2.1 | 9.2 |
Cells were inoculated for 2 h at 34°C with HAV/7-anti-HAV IgG complexes or HAV/7 in amounts equivalent to those in the complex inoculum (MOI 0.1), transfected with poly(IC) by DEAE-dextran at different times p.i. and after an incubation time of 12 h prepared for FACS analysis. Shown are the data of an experiment representative for a series of three.
As controls, cell lysates of noninfected cells supplemented with (+) or without (−) anti-HAV IgG in amounts equal to those used for neutralization of HAV were used as the inoculum. In addition, mock-infected cells were transfected with DEAE-dextran without (−) poly(IC).
Day p.i. at which poly(IC) treatment (20 μg/ml) was begun.
These results demonstrate that HAV/7 inhibits apoptotic cell death induced by poly(IC) in FRhK-4 cells. In contrast to this, HAV is not able to prevent apoptosis induced by actinomycin D and cycloheximide, which are inhibitors of macromolecular synthesis, on which we reported previously (1).
A cytopathogenic variant of HAV also does not induce IFN-β mRNA transcription but is deficient in inhibiting dsRNA-induced IFN-β mRNA transcription and apoptosis.
Cytopathogenic HAV variants replicate much faster and generate significantly more viral products than noncytopathogenic HAV. This is reflected by exceptionally high viral titers and results in apoptotic cell death, which was demonstrated for HAVcyt/HB1.1-infected FRhK-4 cells (1). Therefore, it was of interest to investigate the effects of HAVcyt replication on dsRNA-induced IFN-β mRNA transcription and apoptosis in FRhK-4 cells.
In FRhK-4 cells, which were infected with HAVcyt/HB1.1 with an MOI of 4, no IFN-β mRNA synthesis was detectable by RT-PCR in the course of 72 h after infection (data not shown). Also, no CAT expression did occur in FRhK-4/(−110IFN-β)-CAT cells 2 days after infection with the cytopathogenic virus (data not shown). These findings indicate that HAVcyt/HB1.1 does not induce IFN-β mRNA transcription, which is in accordance with the results obtained with the noncytopathogenic HAV/7 virus.
In order to investigate the influence of HAVcyt/HB1.1 on dsRNA-induced synthesis of IFN-β, FRhK-4 cells infected with an MOI of 4 with HAV/7 and HAVcyt/HB1.1, respectively, were treated with poly(IC), and at 2 days p.i., IFN-β mRNA was analyzed by RT-PCR and IFN activity in cell supernatants was tested by the plaque reduction assay. Under these conditions, at which virtually every cell is infected, no IFN was detectable in cells infected with HAV/7, whereas in cells which were infected with HAVcyt/HB1.1, IFN as well as IFN-β mRNA could be detected in low amounts (data not shown). A mixed population of HAVcyt/HB1.1 and HAV/7 can be excluded, as HAVcyt/HB1.1 was clonally selected for three times.
Thereupon, in order to obtain more quantitative data, we examined the consequence of HAVcyt/HB1.1 replication on induction of the IFN-β enhancer by using FRhK-4/(−110IFN-β)-CAT cells. The cells were infected with HAVcyt/HB1.1 with MOIs of 10, 4, 0.4, and 0.04. Induction with 20 μg of poly(IC)/ml by DEAE-dextran was performed at 24 h p.i., and CAT expression was analyzed 16 h after induction. Cells infected with HAV/7 with MOIs of 4, 0.4, and 0.04 were treated identically in parallel. As controls, cells were mock infected with medium and FRhK-4 cell lysates, respectively. The results shown in Table 2 demonstrated that HAVcyt/HB1.1 also inhibits activation of the IFN-β enhancer in response to dsRNA but to a notably lesser extent than HAV/7, which replicates significantly more slowly than HAVcyt/HB1.1. Experiments with preparations of empty HAV capsids (22), as well as with inactivated vaccine virus in amounts equivalent and higher than in the infectious virus preparation, revealed that replication is necessary for HAV to inhibit the effect of poly(IC), since no inhibitory effect on dsRNA-induced CAT expression was observed (data not shown). It is therefore remarkable that the attenuated inhibition on dsRNA-induced CAT expression by HAVcyt occurred in the presence of a vast viral replication.
TABLE 2.
Influence of the HAVcyt/HB1.1 infection on dsRNA-induced CAT expression in FRhK-4/(−110IFN-β)-CAT cells in comparison with mock- and HAV/7-infected cellsa
| Infection (MOI) | Mean CAT concn (pg/ml)b | % Reduction of CAT compared to medium | HAV TCID50/mlb |
|---|---|---|---|
| Medium | 844 (83) | 0 | 0 |
| Cell lysate | 830 (40) | 2 | 0 |
| HAV/7 (0.04) | 535 (36) | 37 | NDc |
| HAV/7 (0.4) | 138 (18) | 84 | 2.2 × 103 (0.6) |
| HAV/7 (4) | 5 (2) | 99 | 3.3 × 105 (0) |
| HAVcyt/HB1.1 (0.04) | 968 (50) | 0 | ND |
| HAVcyt/HB1.1 (0.4) | 614 (40) | 27 | 1.3 × 106 (0.6) |
| HAVcyt/HB1.1 (4) | 142 (12) | 83 | 1.5 × 107 (0.3) |
| HAVcyt/HB1.1 (10) | 68 (9) | 92 | ND |
Mock- and HAV-infected cells were transfected with 20 μg of poly(IC)/ml by DEAE-dextran 24 h p.i. and 16 h after induction, CAT expression was analyzed, and the TCID50 titer was determined. The data are means obtained from two separate experiments, including two replicates.
The standard deviation is shown in parentheses.
ND, not determined.
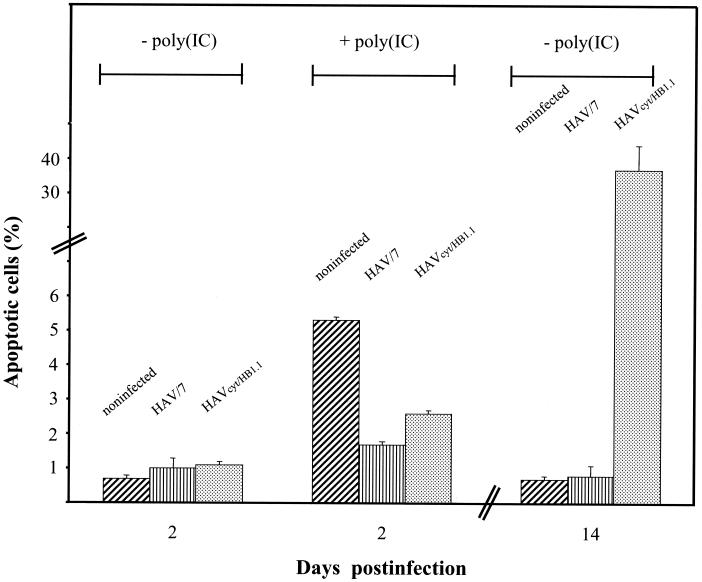
We also examined whether HAVcyt/HB1.1 affects dsRNA-induced apoptosis. FRhK-4 cells were infected with HAVcyt/HB1.1 with an MOI of 4, and apoptosis was induced by transfection with 20 μg of poly(IC)/ml at 24 h p.i. Apoptotic nuclei were determined by flow cytometry 12 h after induction. In order to compare the cytopathogenic variant with the noncytopathogenic variant, HAV/7-infected cells were treated in the same way. In noninfected cells, which were used as controls, poly(IC) induction resulted in 5.3% apoptotic cells (Fig. 6). This proportion was reduced to 2.6% in cells infected with HAVcyt/HB1.1. At this time p.i., no apoptotic cell death caused by HAVcyt/HB1.1 was detectable. An even stronger reduction to a value of 1.7% was obtained with HAV/7. Although HAVcyt/HB1.1 was able to reduce dsRNA-induced apoptosis, the proportion of apoptotic cells increased with advancing incubation periods, reaching more than 20% at day 14 p.i. without poly(IC) induction (Fig. 6).
FIG. 6.
Influence of HAVcyt/HB1.1 on dsRNA-induced apoptosis. HAVcyt/HB1.1-infected FRhK-4 cells, as well as HAV/7-infected FRhK-4 cells (MOI 4) and mock-infected FRhK-4 cells, were transfected with 20 μg of poly(IC)/ml at 24 h p.i. [+poly(IC)]. The percentage of cells with a DNA content of <2N, which is indicative of apoptosis, was determined by flow cytometry 12 h after treatment with poly(IC). As controls, the cells were mock transfected [−poly(IC)]. Additionally shown is the analysis of mock-transfected cells 14 days p.i. as an example for the effect of the HAVcyt/HB1.1 variant with regard to the induction of apoptosis at a later stage of infection. The data are means obtained from two separate experiments, including two replicates, and error bars indicate the standard deviations.
These results show that HAVcyt/HB1.1 inhibits apoptosis, which is induced by dsRNA, but not as effectively as the noncytopathogenic HAV/7.
Appearance and relative amounts of viral dsRNA during infection with noncytopathogenic and cytopathogenic HAV variants.
As the competence to inhibit cellular antiviral mechanisms, which are induced by dsRNA, is different in the case of cytopathogenic and noncytopathogenic HAV, we recorded the relative amounts of viral dsRNA during infection with both variants in the course of 72 h. FRhK-4 cells were infected with HAVcyt/HB1.1 and HAV/7, respectively, with an MOI of 1. At various times cellular RNA was extracted and treated with RNase A under conditions specific for the degradation of single-stranded RNA, and the remaining dsRNA was analyzed for HAV-specific dsRNA by HDA. We detected HAV dsRNA for the first time 40 h after inoculation with HAVcyt/HB1.1 with increasing amounts till 48 h p.i., which declined till 72 h p.i. (Table 3). In contrast, HAV/7 dsRNA could be detected for the first time 72 h p.i. These results, together with the finding that HAVcyt/HB1.1 is deficient in its inhibitory effect on dsRNA-induced apoptosis, might imply that HAVcyt dsRNA is involved in induction of apoptotic cell death after infection with cytopathogenic HAV variants. This is supported by the observation that the appearance and amount of dsRNA correlate with morphological changes of the cells infected with HAVcyt/HB1.1 that are indicative of apoptosis, whereas with HAV/7 even after the appearance of small quantities of viral dsRNA, no cytopathic effect (CPE) could be detected (Table 3). However, IFN-β synthesis is induced neither by HAVcyt nor by noncytopathogenic HAV.
TABLE 3.
Proof of HAV dsRNA in FRhK-4 cells infected with HAV/7 and HAVcyt/HB1.1 with an MOI of 1 by HDAa
| Time p.i. (h) | HAV/7
|
HAVcyt/HB1.1
|
||
|---|---|---|---|---|
| dsRNAb | CPEc | dsRNAb | CPEc | |
| 0 | − | − | − | − |
| 8 | − | − | − | − |
| 16 | − | − | − | − |
| 24 | − | − | − | − |
| 40 | − | − | + | + |
| 48 | − | − | +++ | + |
| 72 | + | − | ++ | ++ |
Additionally, the morphological findings (CPE) indicative for apoptosis are included. Shown are the data of two separate experiments, including two replicates.
−, not detected; +, 50 to 500 luminescence counts per second (LCPS)/105; ++, 500 to 1,000 LCPS/105; +++, >1000 LCPS/105.
That is, the presence of scattered regions with small, shrunken, and rounded cells. −, no CPE; +, small regions; ++, large regions.
Noncytopathogenic HAV interferes with HAVcyt.
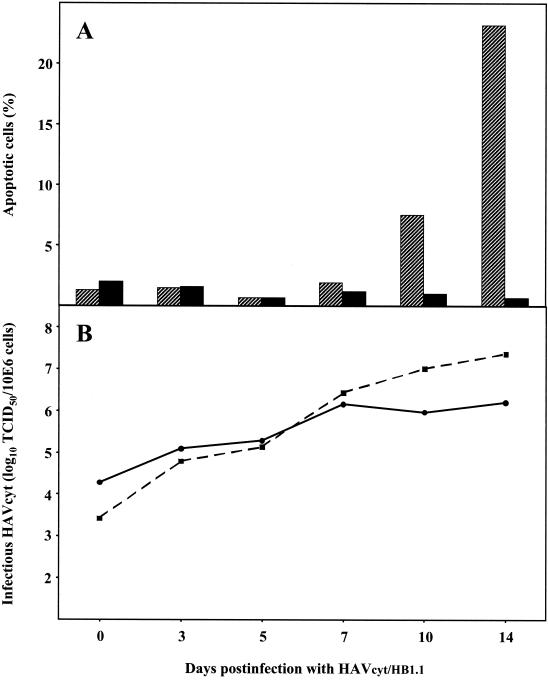
In further experiments we examined whether noncytopathogenic HAV influences the apoptotic reaction induced by HAVcyt. We infected FRhK-4 cells, which were persistently infected with HAV/7 with a titer of 106 TCID50/106 cells, with HAVcyt/HB1.1 at an MOI of 0.07. Analysis of the fraction of apoptotic cells by flow cytometry demonstrated that no apoptosis was induced in the course of 14 days p.i., whereas in cells noninfected with HAV/7 apoptosis occurred between days 7 and 10 after infection with HAVcyt/HB1.1, reaching more than 20% apoptotic cells by day 14 p.i. (Fig. 7A).
FIG. 7.
Influence of persistent HAV/7 infection on infection with HAVcytHB1.1. FRhK-4 cells persistently infected with HAV/7, as well as noninfected FRhK-4 cells as controls, were infected with HAVcyt/HB1.1 with an MOI of 0.07. (A) The percentage of cells with a DNA content of <2 N, which is indicative for apoptosis, was determined by flow cytometry in the course of 14 days p.i. with HAVcyt/HB1.1 in FRhK-4 cells (▨) or FRhK-4 cells persistently infected with HAV/7 (▪). (B) Replication kinetics of HAVcyt/HB1.1 in FRhK-4 cells (▪) or FRhK-4 cells persistently infected with HAV/7 (•) by using the same samples as those used for the analysis of apoptosis-specific DNA-fragmentation. The TCID50 titers were determined in FRhK-4 cells 2 weeks after inoculation by the CPE induced by HAVcyt/HB1.1. Shown are the data of an experiment representative for a series of four.
The records of the replication kinetics of HAVcyt/HB1.1 revealed that, under these conditions, HAVcyt/HB1.1 replicated in FRhK-4 cells, which were persistently infected with HAV/7, considerably more slowly than in cells noninfected with HAV/7 (Fig. 7B). From day 5 p.i. onward the TCID50 titers of HAVcyt/HB1.1 were reduced significantly in the presence of HAV/7, reaching 1.6 × 106 TCID50/106 cells compared to 2.3 × 107 TCID50/106 cells in cells noninfected with HAV/7 (Fig. 7B). These titers were assessed by the morphological characteristics of apoptotic cells (1). This is possible because simultaneous inoculation of FRhK-4 cells with HAVcyt/HB1.1 and HAV/7 does not influence the replication of HAVcyt/HB1.1, as well as the apoptotic reaction induced by this variant (data not shown). It could also be demonstrated that the titers determined for HAVcyt/HB1.1 by CPE and by immunofluorescence are identical (data not shown).
These results show that, at a later stage of infection, HAV/7 is equipped with functions to reduce the replication of HAVcyt/HB1.1 and most likely its own also. Reduction of replication means less HAV dsRNA molecules and thus possibly prevention of the induction of apoptosis in the infected cells. The mechanisms involved in the control of the viral replication are not known.
The total TCID50 titer in cells infected with both HAV variants was 1.1 × 107/106 cells 14 days p.i. As the indication for an apoptotic reaction after infection with HAVcyt/HB1.1 correlates with a TCID50 titer of above 107/106 cells (data not shown), it is also possible that at this time HAV/7 directly inhibited the induction of apoptosis, as described for induction of apoptosis by poly(IC).
DISCUSSION
We investigated the consequences of HAV infections on the induction of IFN-α/β synthesis and apoptosis. These two reactions, which can be induced by dsRNA, are components of the cell-based antiviral mechanisms, which obviously are not effective against HAV infections in cultured cells, as HAV normally establishes persistent infections in cell culture (31). In former experiments no IFN-β could be detected after infection of human fibroblasts with HAV-GBM, and no interference with other viruses occurred (30, 31). This suggests that no endogenous IFN-β was produced and is supported by the fact that already small amounts of exogenous IFN-β could erase the infection (29, 31). Therefore, HAV seems unable to inhibit IFN-β-mediated gene expression and antiviral activities but seems able to influence IFN-β synthesis.
Using different strains of HAV and cell lines originating from various species and able to respond to induction with the production of IFN-β, we show that HAV infections in general do not result in transcription of IFN-β mRNA. Taking advantage of the CAT gene as reporter, we could prove that the transcriptional induction of IFN-β by dsRNA is inhibited by the replicating HAV by influencing the formation of the transcription complex, the enhanceosome (20).
Furthermore, we demonstrate that HAV also inhibits dsRNA-induced apoptosis. This may support the suggestion that shared transcription factors might be involved in dsRNA-induced IFN synthesis and apoptosis.
HAVcyt/HB1.1, which is an exceptionally fast-replicating, apoptosis-inducing cytopathogenic FRhK-4 cell culture variant, also does not initiate IFN-β transcription, although this virus impairs less dsRNA-triggered IFN-β transcription than noncytopathogenic HAV. Despite the fact that HAVcyt/HB1.1 additionally produces more dsRNA than noncytopathogenic HAV, the attenuated repression on transcriptional activation of IFN-β seems sufficient to prevent IFN-β synthesis.
This might be different for the induction of apoptosis, because HAVcyt/HB1.1 is also deficient in inhibiting dsRNA-induced apoptosis. The mechanism by which HAVcyt/HB1.1 causes apoptosis is not known. Previously, we reported that HAVcyt/HB1.1 might interfere with the cellular control of apoptosis-promoting and apoptosis-preventing functions by competing with the cellular synthesis of macromolecules, because HAV is not equipped with antiapoptotic functions in the case of restrained macromolecular synthesis (1). Based on the finding that the inhibitory effect on dsRNA-induced apoptosis is restricted in HAVcyt/HB1.1 infected cells, the higher amount of viral dsRNA molecules might contribute to the induction of the apoptotic cascade.
Besides this speculation, the attenuation of HAVcyt/HB1.1 in its ability to interfere with both dsRNA-induced IFN-β synthesis and apoptosis supports the suggestion that the two reactions are connected by common factors. The reduced ability of HAVcyt/HB1.1 to interfere with these cellular antiviral responses might be caused by mutational changes, which occurred during adaptation of the virus to better growth in FRhK-4 cells, and might refer to viral proteins, which interact with the signaling pathways leading to IFN-β synthesis and apoptosis. Recently, it was demonstrated that the IFN regulatory factor 3 (IRF3), together with the transcriptional coactivators CREB-binding protein (CBP) and p300, is involved in the induction of apoptosis caused by infection with Newcastle disease virus or stimulation with dsRNA independent from IFN (33). Since these transcription factors are also involved in the transcriptional activation of IFN-β (20) and since FRhK-4 cells are deficient in an IFN response, so that apoptosis induced by dsRNA or HAVcyt is independent of the action of IFN (unpublished results), it may be that HAV might interact with IRF3 and/or CBP/p300 to inhibit dsRNA-induced IFN-β synthesis and apoptosis. Since the attenuated competence to inhibit cellular antiviral responses seems to correlate with the degree of the adaptation of HAV to growth in cell culture, it can be assumed that wild-type HAV is equipped with a significant ability to inhibit these antiviral actions and that this attribute may play an important role for the pathogenesis during infections in vivo. The results support older suggestions that IFN-α/β is not involved in the elimination of HAV under natural conditions (30).
The ability of HAV to modulate dsRNA-induced IFN-β expression and apoptosis may assist or even allow the virus to establish an infection in vivo and in cell culture. After entry into host cells, HAV replicates exceptionally slowly. However, this may not ensure that activation of cellular dsRNA-induced antiviral responses such as IFN-β production is prevented. Therefore, the ability to repress dsRNA-induced transcriptional activation of IFN-β may guarantee that no antiviral activities by IFN-stimulated proteins are induced in the already infected cell as well as in surrounding noninfected cells. This would allow HAV to produce sufficient progeny virus to spread to neighboring cells and to establish new rounds of infections there. In order to prevent apoptotic cell death which might be caused by accumulating viral dsRNA, HAV is able to repress dsRNA-induced apoptosis by a mechanism, which is possibly based on the same mechanism as that responsible for the inhibition of IFN-β expression. Therefore, the usage of these anti-antiviral properties of HAV, by which already existing sites of virus production can be preserved and new sites of virus production can be initiated, may be the basic strategy to allow this slowly growing virus to establish infections and may be the presupposition for the persistent character of HAV infections.
The inhibition of dsRNA-induced cellular defense principles allows HAV to evade detection by the infected cells and may save time to establish infection. However, the anti-antiviral abilities of HAV may not be sufficient to completely inhibit the cellular defense principles at a later stage of infection when the cellular viral load is high and to allow HAV to establish a persistent infection. At least this seems to be true for the induction of apoptosis. As demonstrated with cytopathogenic variants, apoptosis can be triggered depending on the cellular viral load and possibly on the velocity of the viral replication by a mechanism which may be dependent on dsRNA. Previous studies suggested that the replication of noncytopathogenic HAV is downregulated at a later stage of infection (4, 21), but elements in HAV RNA and factors which are involved in the regulation of viral RNA replication could not be positively defined until now (16, 21). Our results also indicate that noncytopathogenic HAV is equipped with functions that result in a reduction of the viral replication at later times in the replication cycle and which ensure that viral dsRNA does not accumulate to amounts required to overcome the inhibitory functions of HAV on dsRNA-induced cellular defense mechanisms. This would result in the moderate replication allowing HAV to completely inhibit the cellular antiviral mechanisms and thus guarantee the persistent state of the infection. In addition, HAV seems to be able to make both functions, the inhibition of the dsRNA-induced cellular defense mechanisms and the downregulation of viral replication, dominantly available to HAVcyt in trans.
In summary, the results presented here show three new qualities of HAV, by which the virus interacts with host cells to establish persistent infections. (i) HAV inhibits dsRNA-induced IFN expression. (ii) HAV inhibits dsRNA-induced apoptosis. (iii) HAV is able to downregulate its own replication in cis, as well as that of cytopathogenic variants in trans. Further investigations with specific elements of the promoter-enhancer for IFN-β are required to dissect the mechanism by which HAV interferes with the transcription of IFN-β mRNA. It also remains to be shown by which mechanisms HAV interferes with apoptosis and by which further mechanisms HAV is able to establish persistent infections.
Acknowledgments
We thank T. Maniatis, Harvard University, Cambridge, Mass., for providing the plasmid (−110IFN-β)-CAT; Y. Kusov and V. Gauss-Müller, Medical University of Luebeck, Luebeck, Germany, for providing the empty HAV capsids; the NIAID Reference Reagent Repository, Gaithersburg, Md., for providing IFN reference standards; and Mediagnost, Reutlingen, Germany, for providing the monoclonal anti-HAV antibody 7E7.
This work was supported by the Tönjes-Vagt-Stiftung, Bremen, Germany.
Footnotes
This work is dedicated to H.-J. Gerth on the occasion of his 75th birthday.
REFERENCES
- 1.Brack, K., W. Frings, A. Dotzauer, and A. Vallbracht. 1998. A cytopathogenic, apoptosis-inducing variant of hepatitis A virus. J. Virol. 72:3370-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 3.Cohen, J. I., B. Rosenblum, J. T. Ticehurst, R. J. Daemer, S. M. Feinstone, and R. H. Purcell. 1987. Complete nucleotide sequence of an attenuated hepatitis A virus: comparison with wild-type virus. Proc. Natl. Acad. Sci. USA 84:2497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Chastonay, J., and G. Siegl. 1987. Replicative events in hepatitis A virus-infected MRC-5 cells. Virology 157:268-275. [DOI] [PubMed] [Google Scholar]
- 5.Der, S. D., Y.-L. Yang, C. Weissmann, and B. R. G. Williams. 1994. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc. Natl. Acad. Sci. USA 94:3279-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz-Guerra, M., C. Rivas, and M. Esteban. 1997. Activation of the IFN-inducible enzyme RNase L causes apoptosis of animal cells. Virology 236:354-363. [DOI] [PubMed] [Google Scholar]
- 7.Dotzauer, A., S. M. Feinstone, and G. Kaplan. 1994. Susceptibility of nonprimate cell lines to hepatitis A virus infection. J. Virol. 68:6064-6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dotzauer, A., U. Gebhardt, K. Bieback, U. Göttke, A. Kracke, J. Mages, S. M. Lemon, and A. Vallbracht. 2000. Hepatitis A virus-specific immunoglobulin A mediates infection of hepatocytes with hepatitis A virus via the asialoglycoprotein receptor. J. Virol. 74:10950-10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du, W., D. Thanos, and T. Maniatis. 1993. Mechanisms of transcriptional synergism between distinct virus-inducible enhancer elements. Cell 74:887-898. [DOI] [PubMed] [Google Scholar]
- 10.Flehmig, B., H. Frank, G. G. Frösner, and H.-J. Gerth. 1977. Hepatitis A-virus particles in stools of patients from a natural hepatitis outbreak in Germany. Med. Microbiol. Immunol. 163:209-214. [DOI] [PubMed] [Google Scholar]
- 11.Gauss-Müller, V., and F. Deinhardt. 1984. Effect of hepatitis A virus infection on cell metabolism in vitro. Proc. Soc. Exp. Biol. Med. 175:10-15. [DOI] [PubMed] [Google Scholar]
- 12.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral responses and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs, B. L., and J. O. Langland. 1996. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology 219:339-349. [DOI] [PubMed] [Google Scholar]
- 14.Kärber, G. 1931. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch. Exp. Pathol. Pharmakol. 162:480-483. [Google Scholar]
- 15.Kim, T., T. Y. Kim, W. G. Lee, J. Yim, and T. K. Kim. 2000. Signaling pathways to the assembly of an interferon-β enhanceosome. J. Biol. Chem. 275:16910-16917. [DOI] [PubMed] [Google Scholar]
- 16.Kusov, Y., M. Weitz, G. Dollenmeier, V. Gauss-Müller, and G. Siegl. 1996. RNA-protein interactions at the 3′ end of the hepatitis A virus RNA. J. Virol. 70:1890-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langford, M. P., D. A. Weigent, G. J. Stanton, and S. Baron. 1981. Virus plaque-reduction assay for interferon: microplaque and regular macroplaque reduction. Methods Enzymol. 78:339-346. [DOI] [PubMed] [Google Scholar]
- 18.Lee, S. B., and M. Esteban. 1994. The interferon-induced double-stranded RNA-activated protein kinase induces apoptosis. Virology 199:491-496. [DOI] [PubMed] [Google Scholar]
- 19.Maier, K., P. Gabriel, E. Koscielniak, Y.-D. Stierhof, K. H. Wiedmann, B. Flehmig, and A. Vallbracht. 1988. Human gamma interferon production by cytotoxic T lymphocytes sensitized during hepatitis A virus infection. J. Virol. 62:3756-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maniatis, T., J. V. Falvo, T. H. Kim, T. K. Kim, C. H. Lin, B. S. Parekh, and M. G. Wathelet. 1998. Structure and function of the interferon-β enhanceosome. Cold Spring Harbor Symp. Quant. Biol. 63:609-620. [DOI] [PubMed] [Google Scholar]
- 21.Nüesch, J. P. F., M. Weitz, and G. Siegl. 1993. Proteins specifically binding to the 3′ untranslated region of hepatitis A virus RNA in persistently infected cells. Arch. Virol. 128:65-79. [DOI] [PubMed] [Google Scholar]
- 22.Probst, C., M. Jecht, and V. Gauss-Müller. 1998. Processing of proteinase precursors and their effect on hepatitis A virus particle formation. J. Virol. 72:8013-8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roulston, A., R. C. Marcellus, and P. E. Branton. 1999. Viruses and apoptosis. Annu. Rev. Microbiol. 53:577-628. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz, G., and A. Dotzauer. 1998. Proof of hepatitis A virus negative-sense RNA by RNA/DNA-hybrid detection: a method for specific detection of both viral negative- and positive-strand RNA species. Nucleic Acids Res. 26:5230-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schweizer, M., and E. Peterhans. 2001. Noncytopathic bovine viral diarrhea virus inhibits double-stranded RNA-induced apoptosis and interferon synthesis. J. Virol. 75:4692-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sen, G. C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255-281. [DOI] [PubMed] [Google Scholar]
- 27.Takizawa, T., R. Fukuda, T. Miyawaki, K. Ohashi, and Y. Nakaanishi. 1995. Activation of the apoptotic Fas antigen-encoding gene upon influenza virus infection involving spontaneously produced beta-interferon. Virology 209:288-296. [DOI] [PubMed] [Google Scholar]
- 28.Takizawa, T., K. Ohashi, and Y. Nakanishi. 1996. Possible involvement of double-stranded RNA-activated protein kinase in cell death by influenza virus infection. J. Virol. 70:8128-8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallbracht, A., and B. Flehmig. 1985. Elimination of a persistent hepatitis A virus infection in cell cultures by interferon, p. 339-345. In H. Kirchner and H. Schellekens (ed.), The biology of the interferon system 1984. Elsevier Science Publishers B.V., Amsterdam, The netherlands.
- 30.Vallbracht, A., P. Gabriel, J. Zahn, and B. Flehmig. 1985. Hepatitis A virus infection and the interferon system. J. Infect. Dis. 152:211-213. [DOI] [PubMed] [Google Scholar]
- 31.Vallbracht, A., L. Hofman, K. G. Wurster, and B. Flehmig. 1984. Persistent infection of human fibroblasts by hepatitis A virus. J. Gen. Virol. 65:609-615. [DOI] [PubMed] [Google Scholar]
- 32.Vallbracht, A., K. Maier, Y.-D. Stierhof, K. H. Wiedmann, B. Flehmig, and B. Fleischer. 1989. Liver-derived cytotoxic T cells in hepatitis A virus infection. J. Infect. Dis. 160:209-217. [DOI] [PubMed] [Google Scholar]
- 33.Weaver, B. K., O. Ando, K. Prasanna Kumar, and N. C. Reich. 2001. Apoptosis is promoted by the dsRNA-activated factor (DRAF1) during viral infection independent of the action of interferon or p53. FASEB J. 15:501-514. [DOI] [PubMed] [Google Scholar]
- 34.Zhou, A., J. Paranjape, T. L. Brown, H. Nie, S. Naik, B. Dong, A. Chang, B. Trapp, R. Fairchild, C. Colmenares, and R. H. Silverman. 1997. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. EMBO J. 16:6355-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]