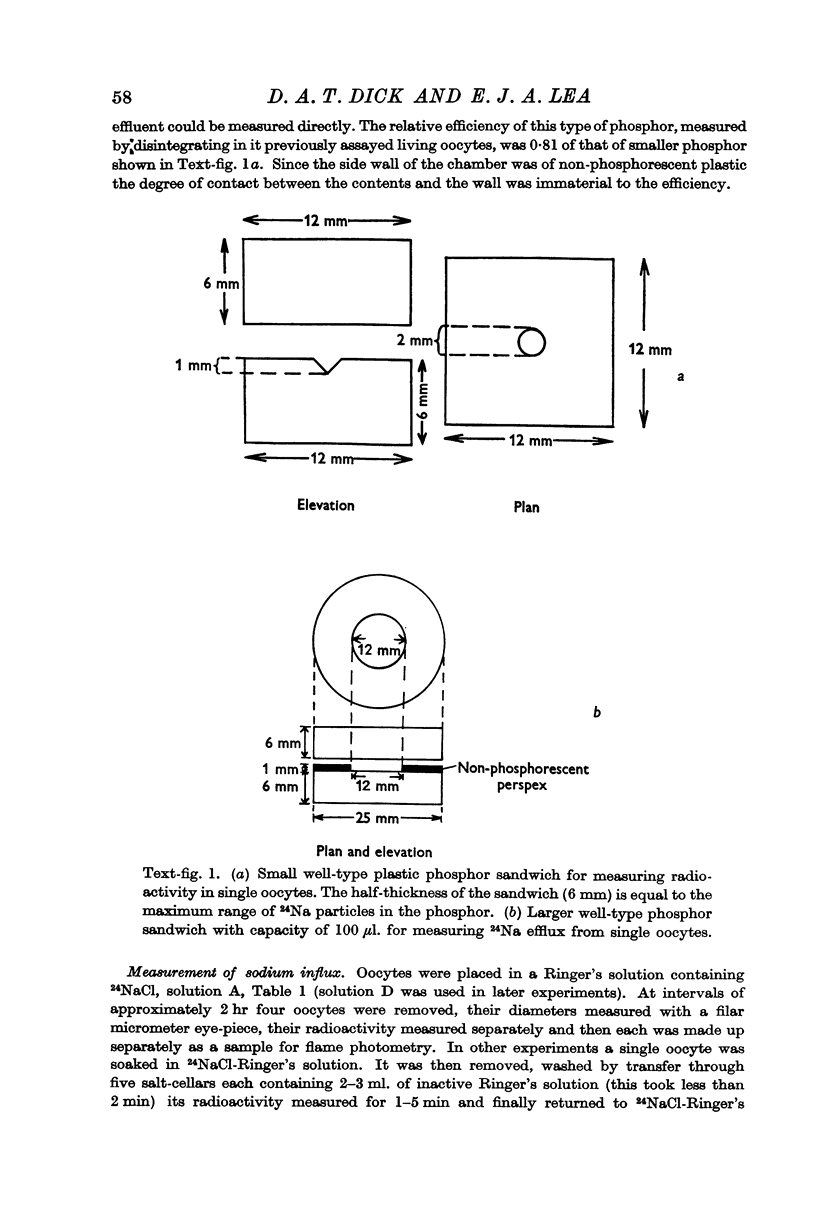
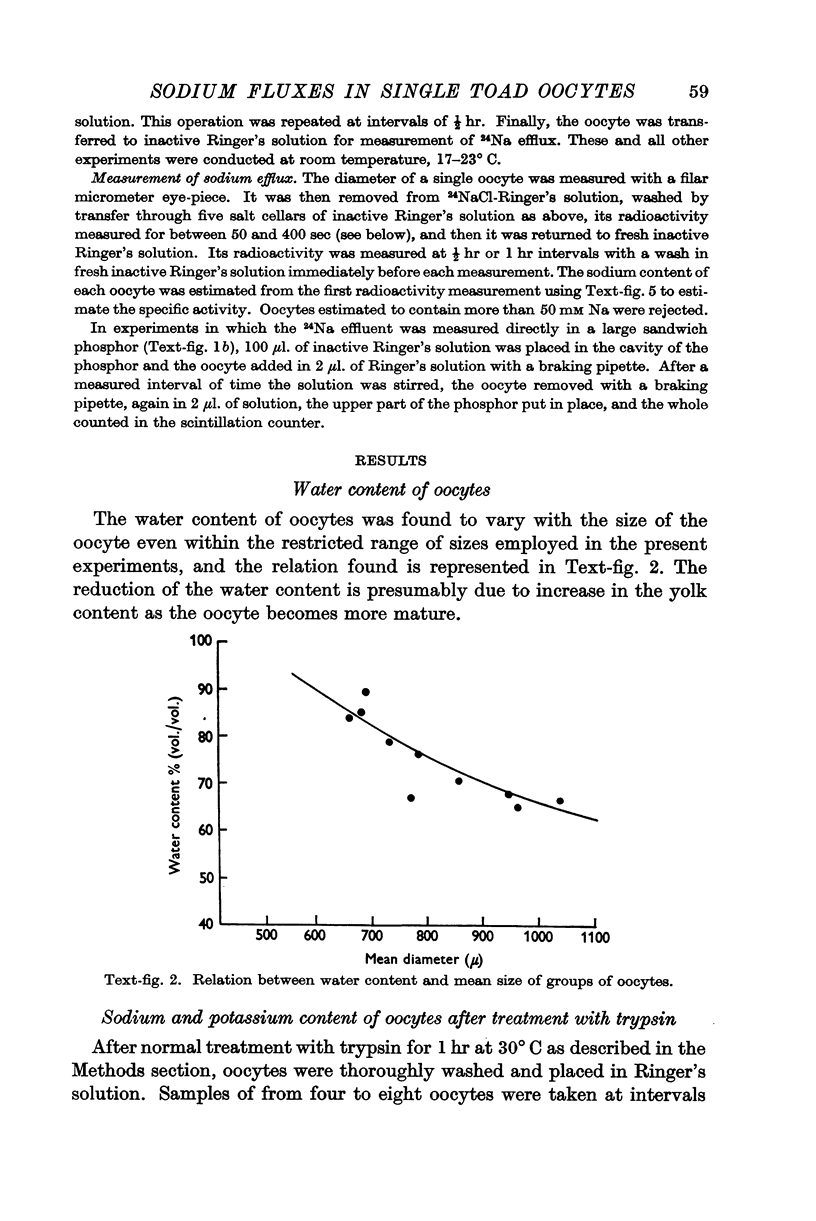
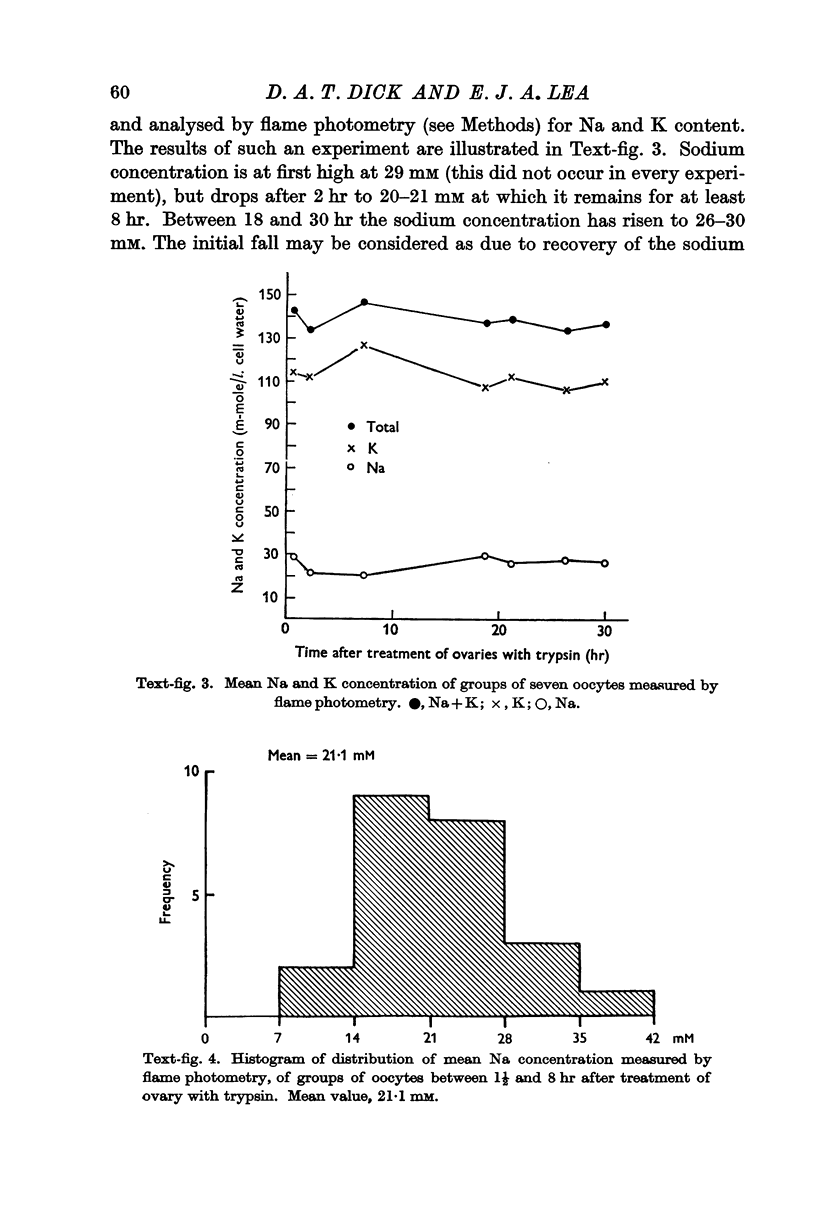
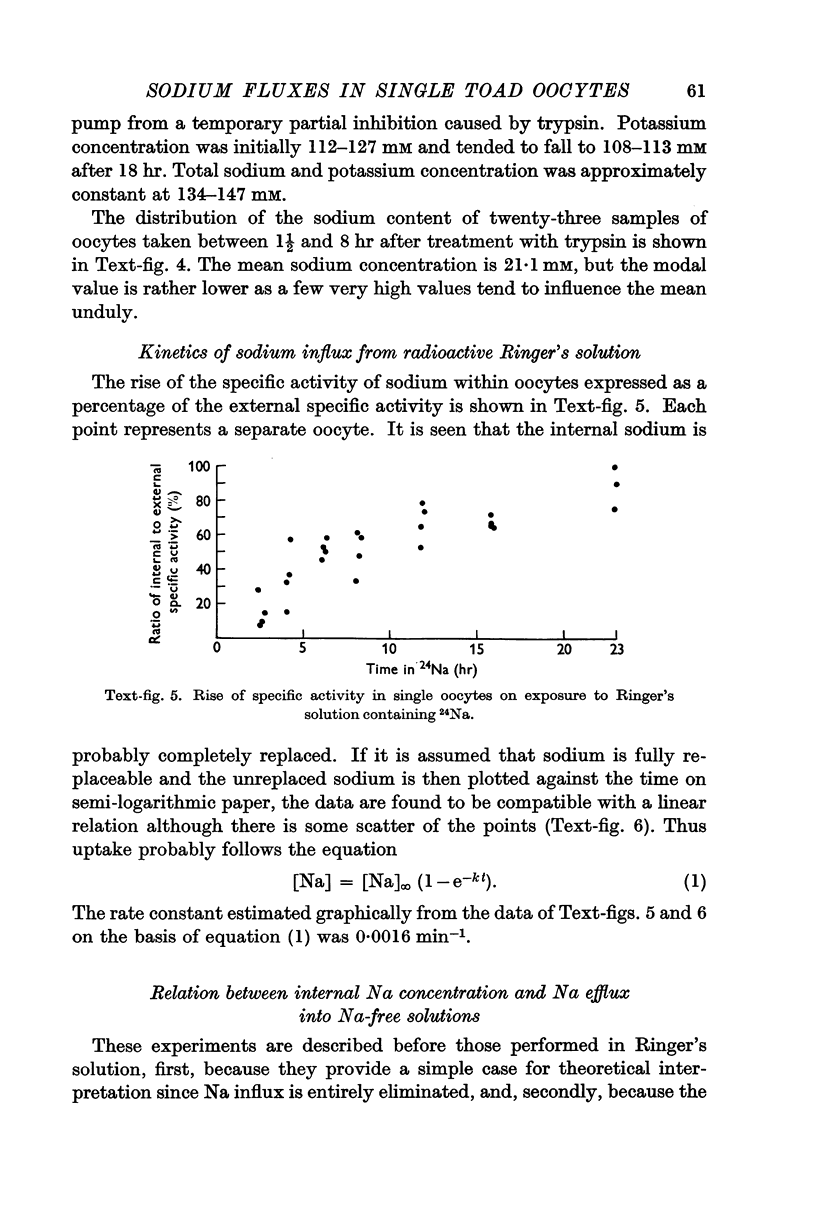
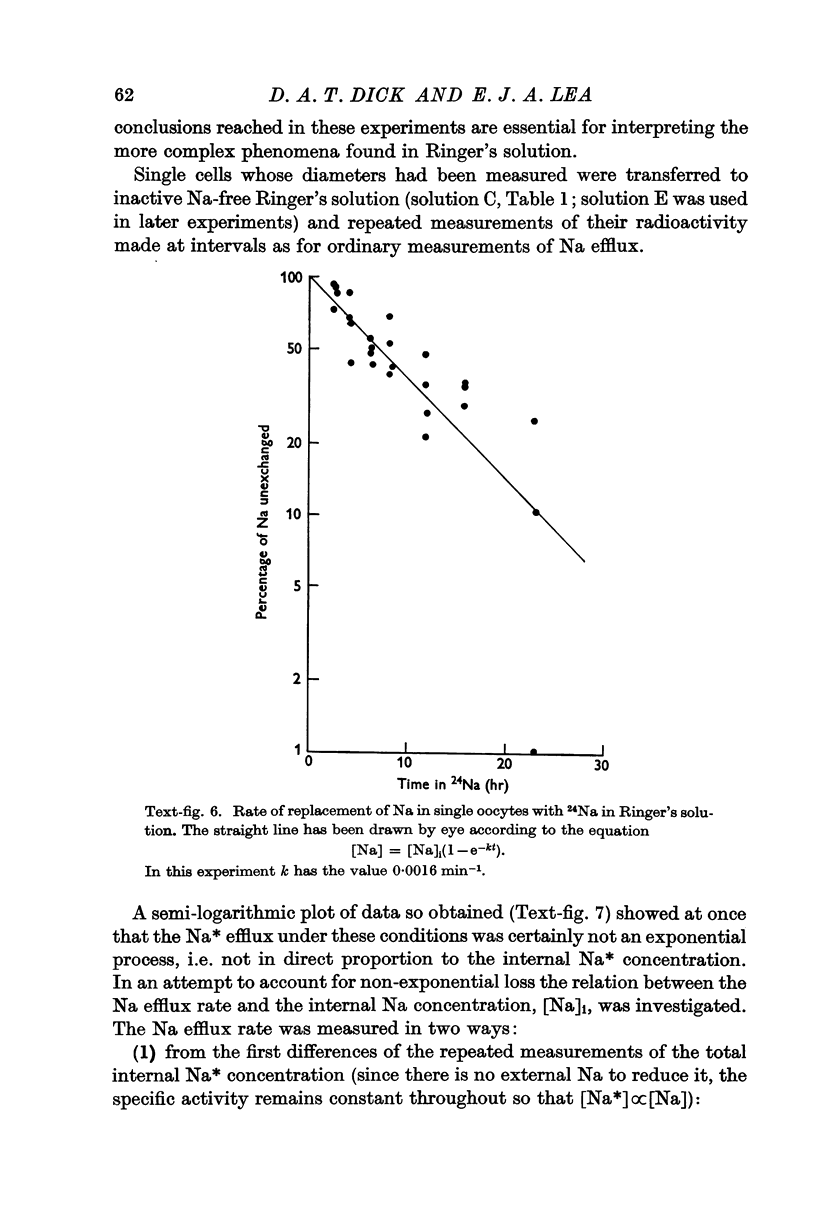
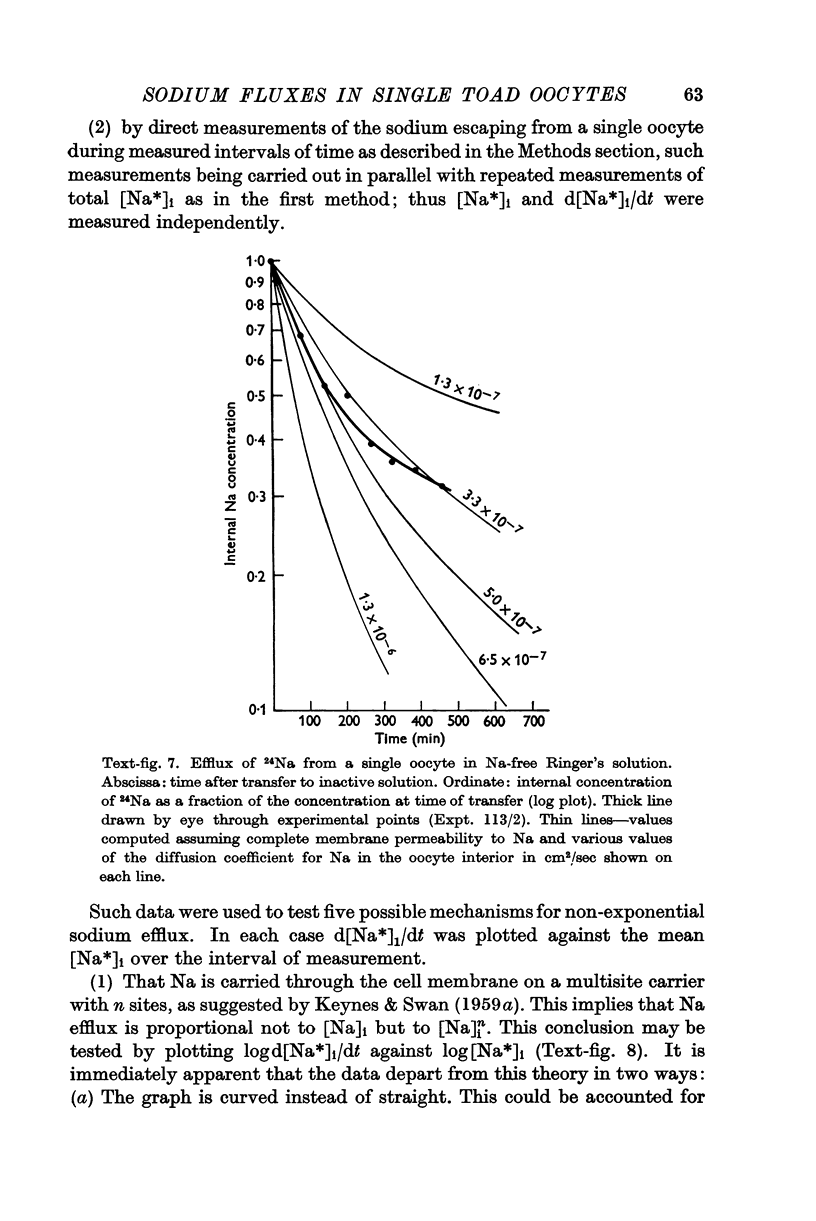
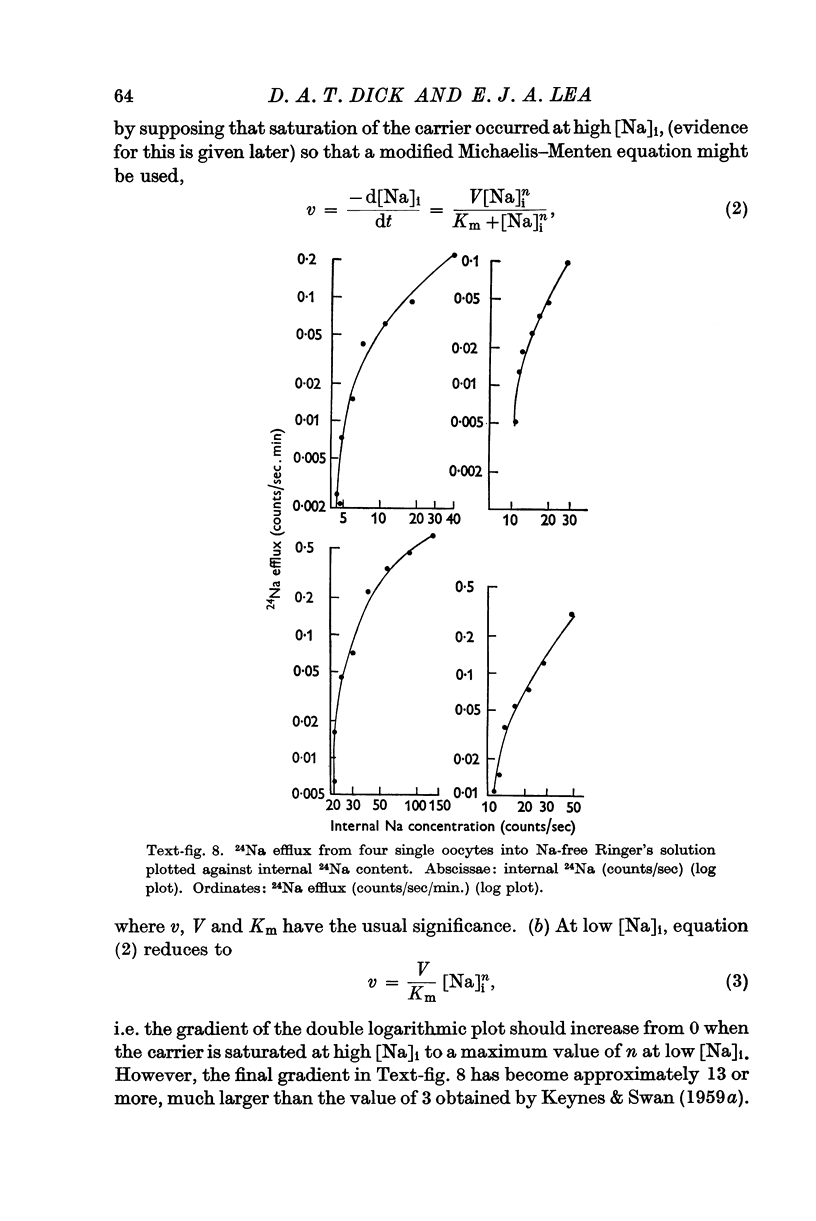
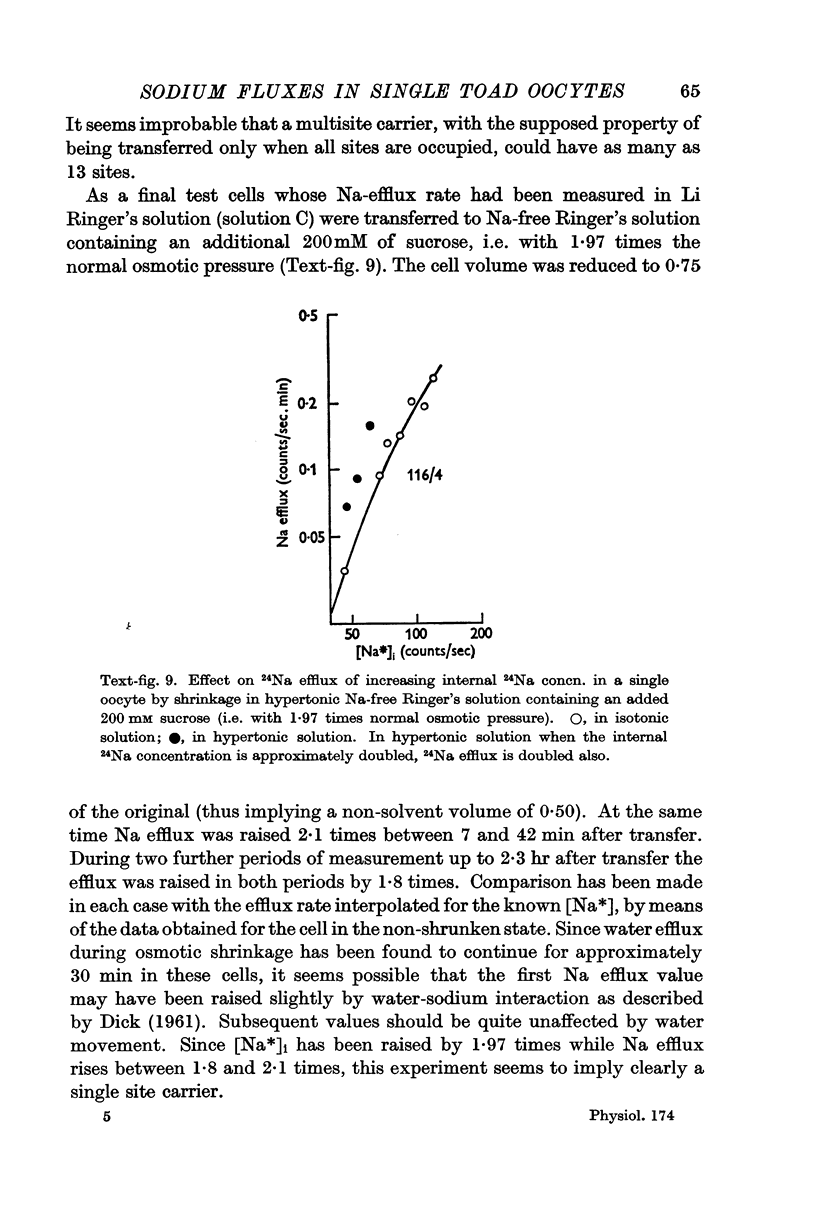
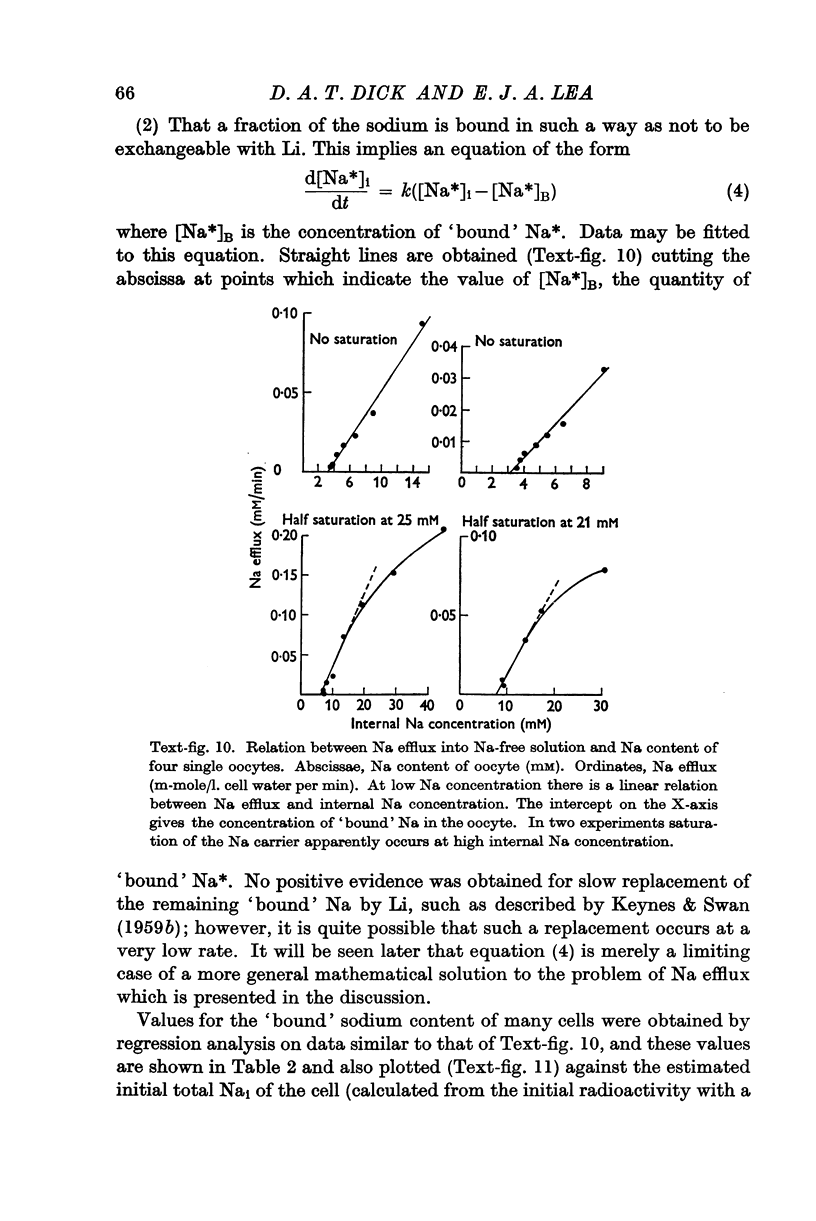
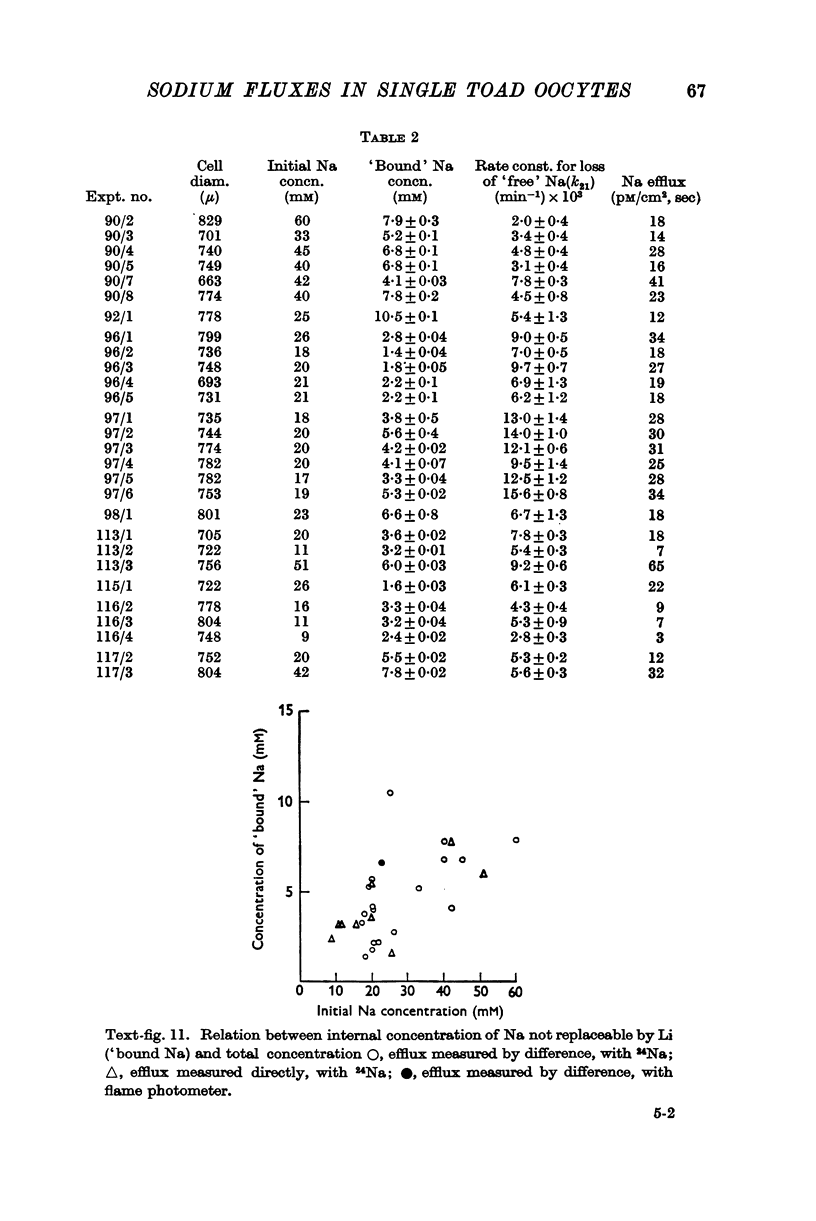
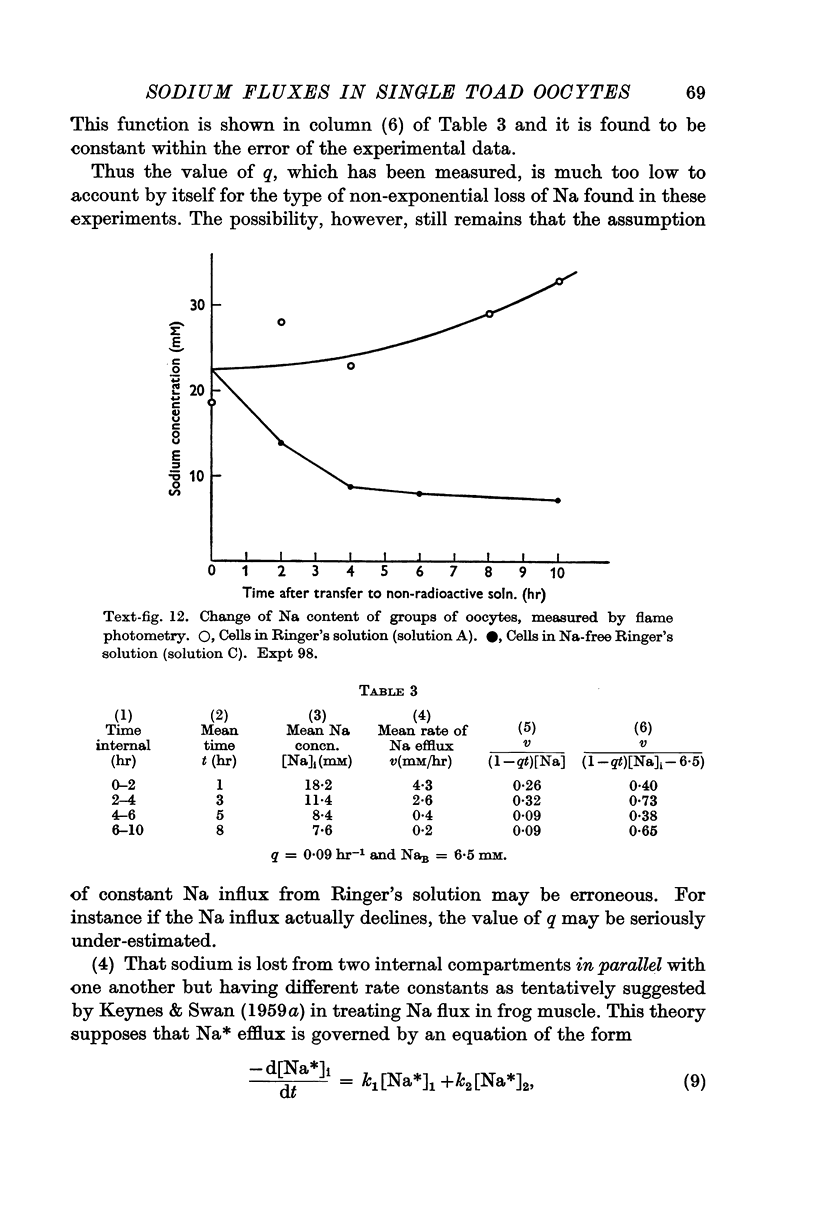
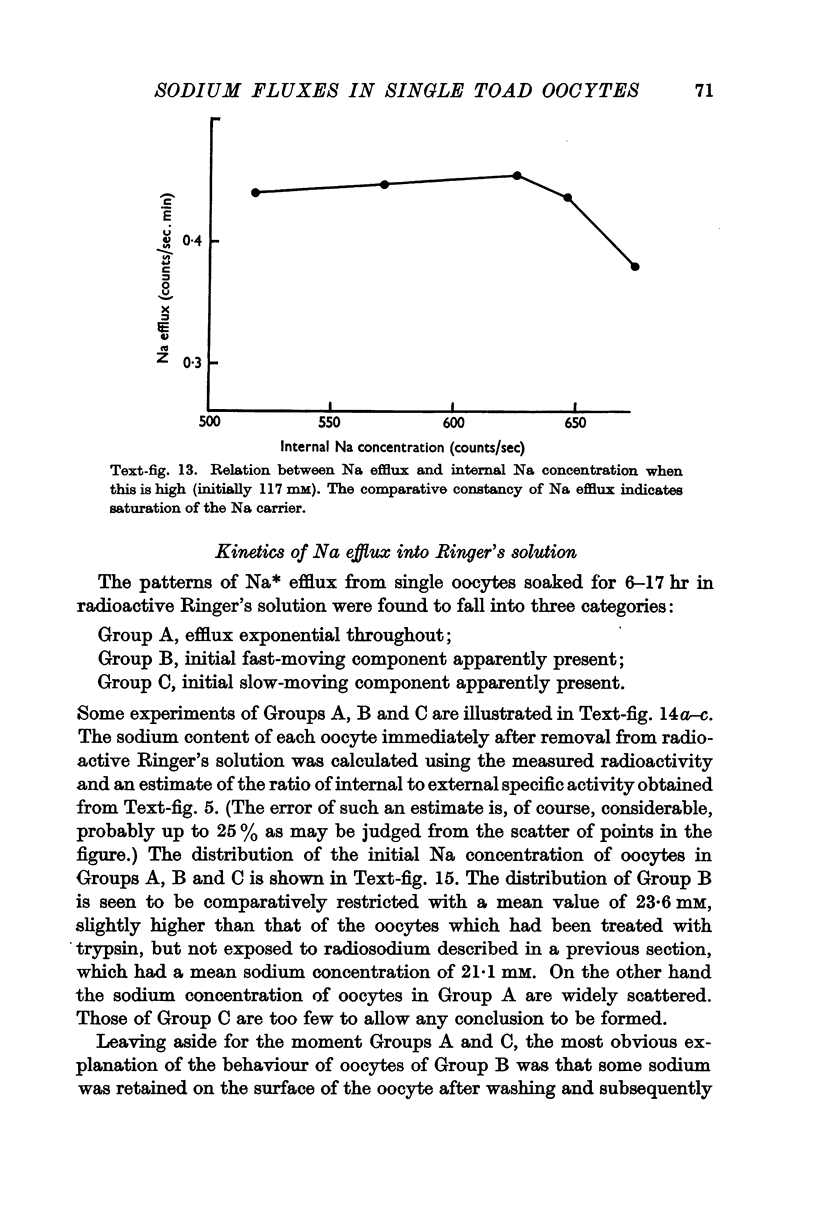
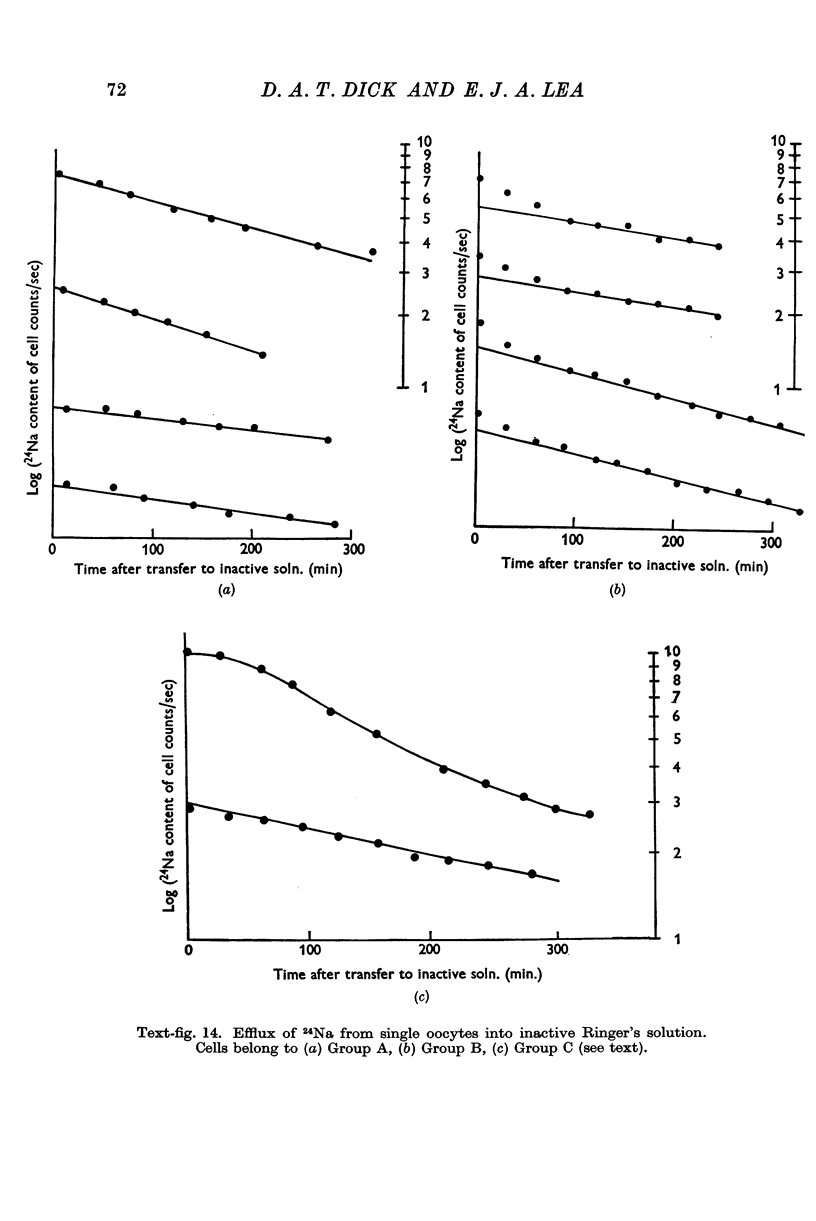
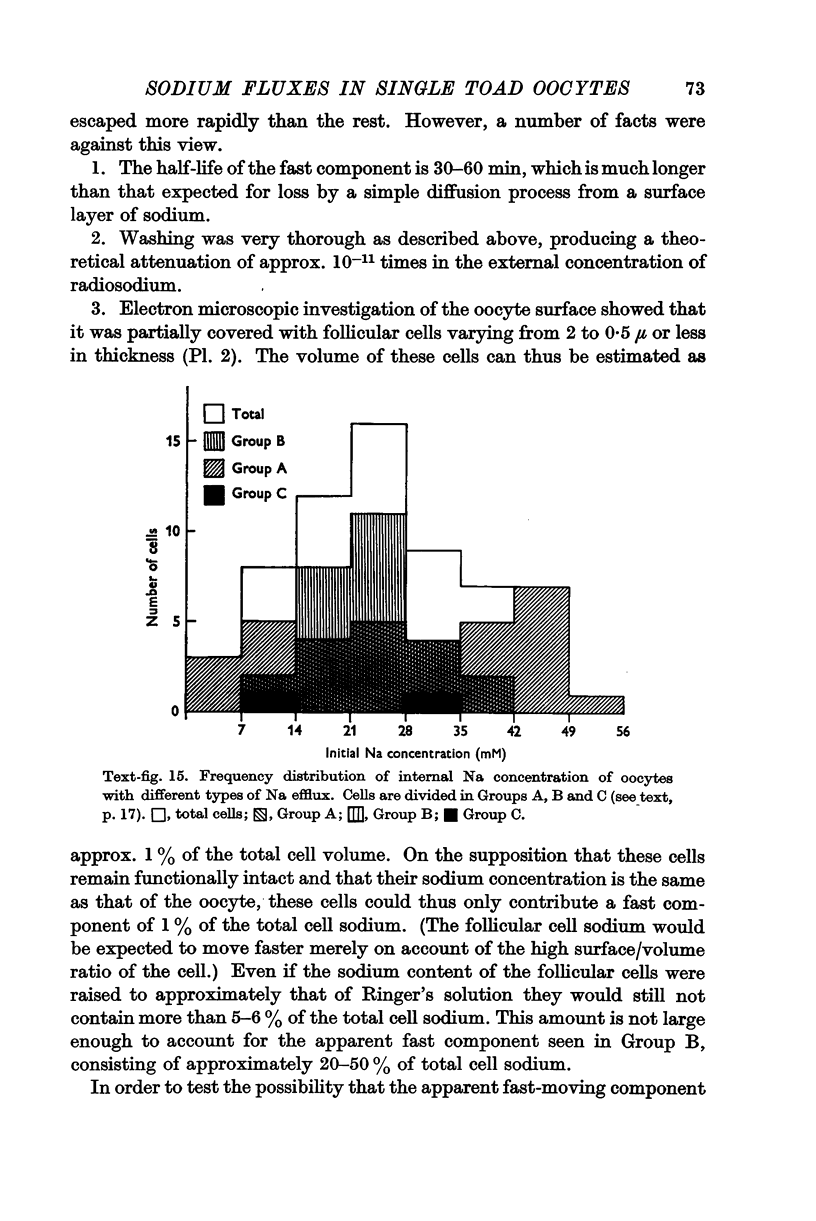
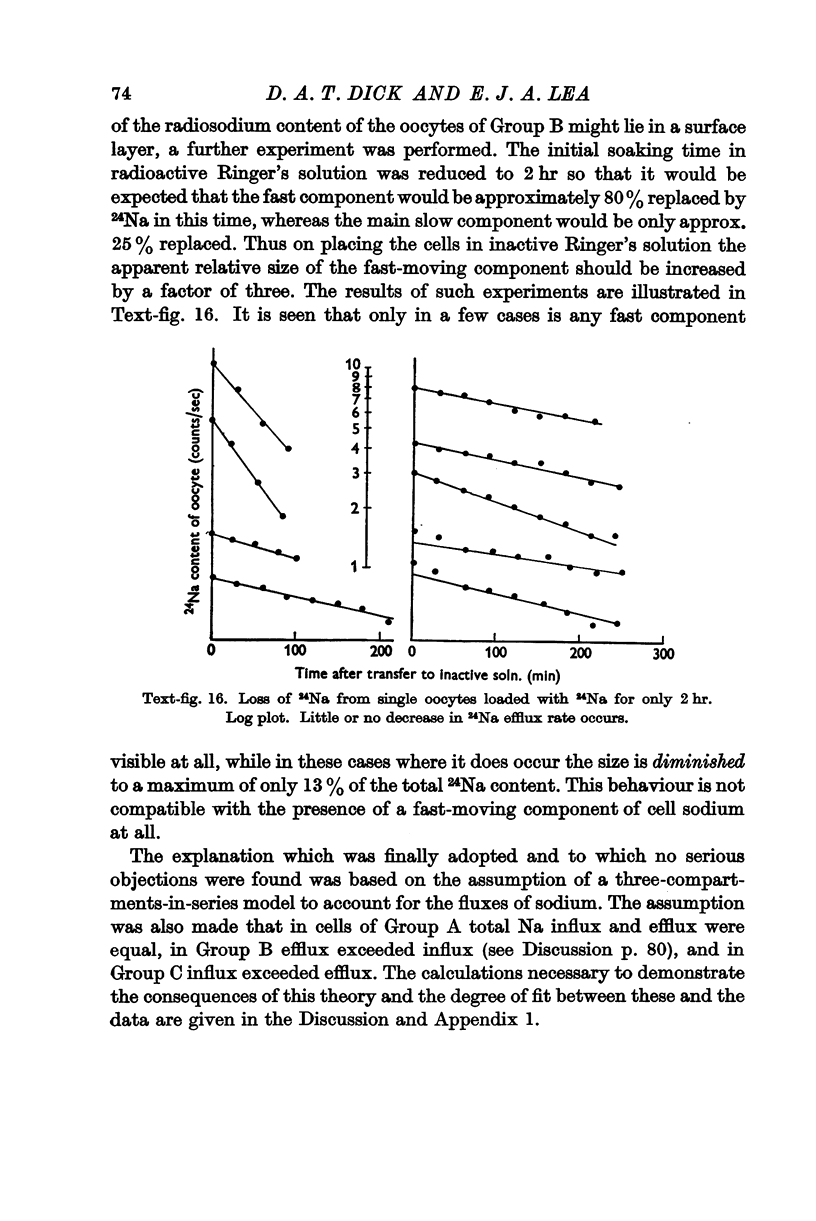
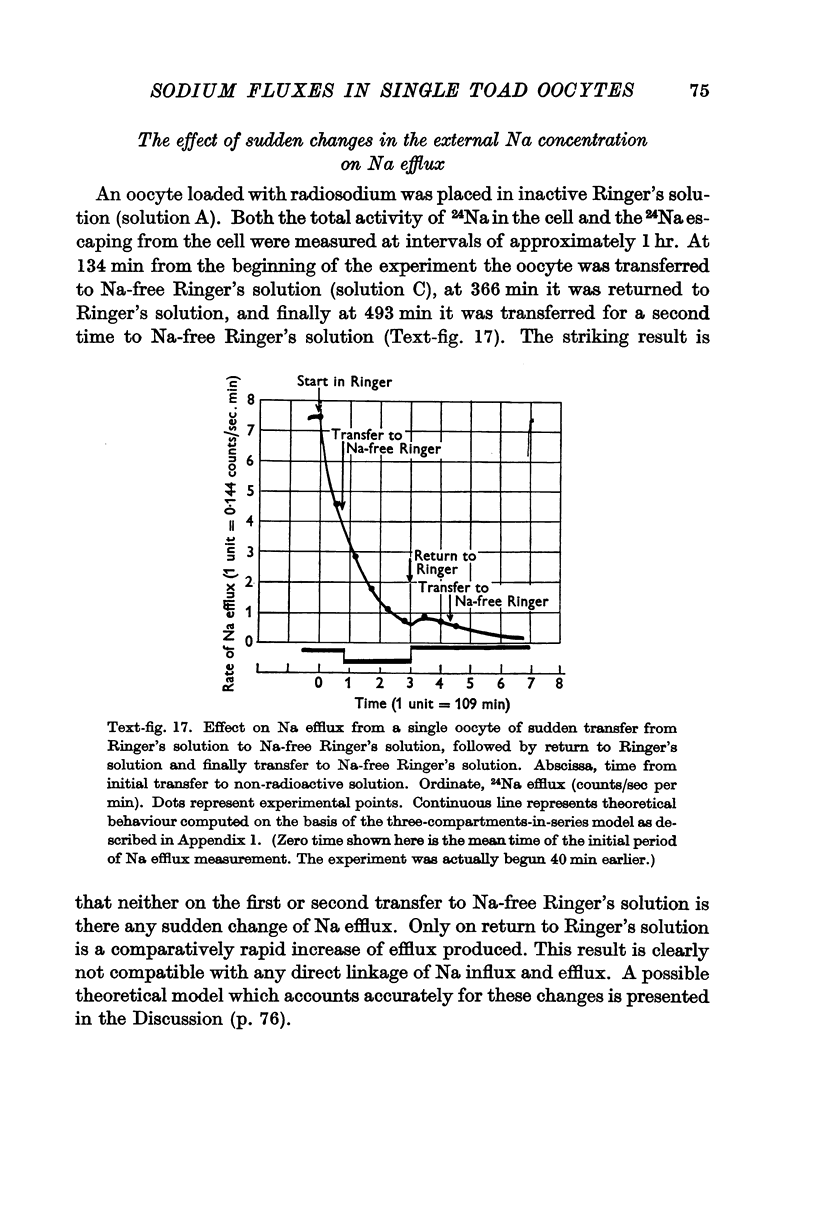
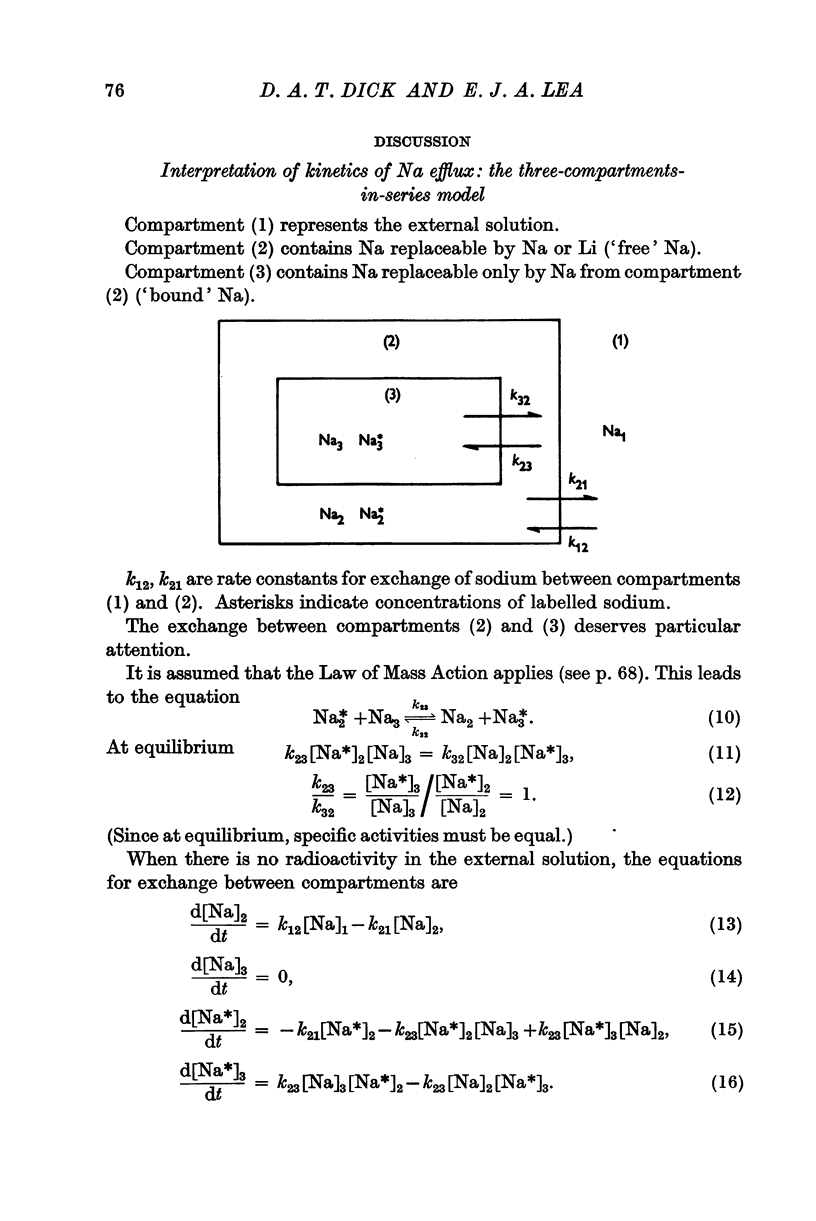
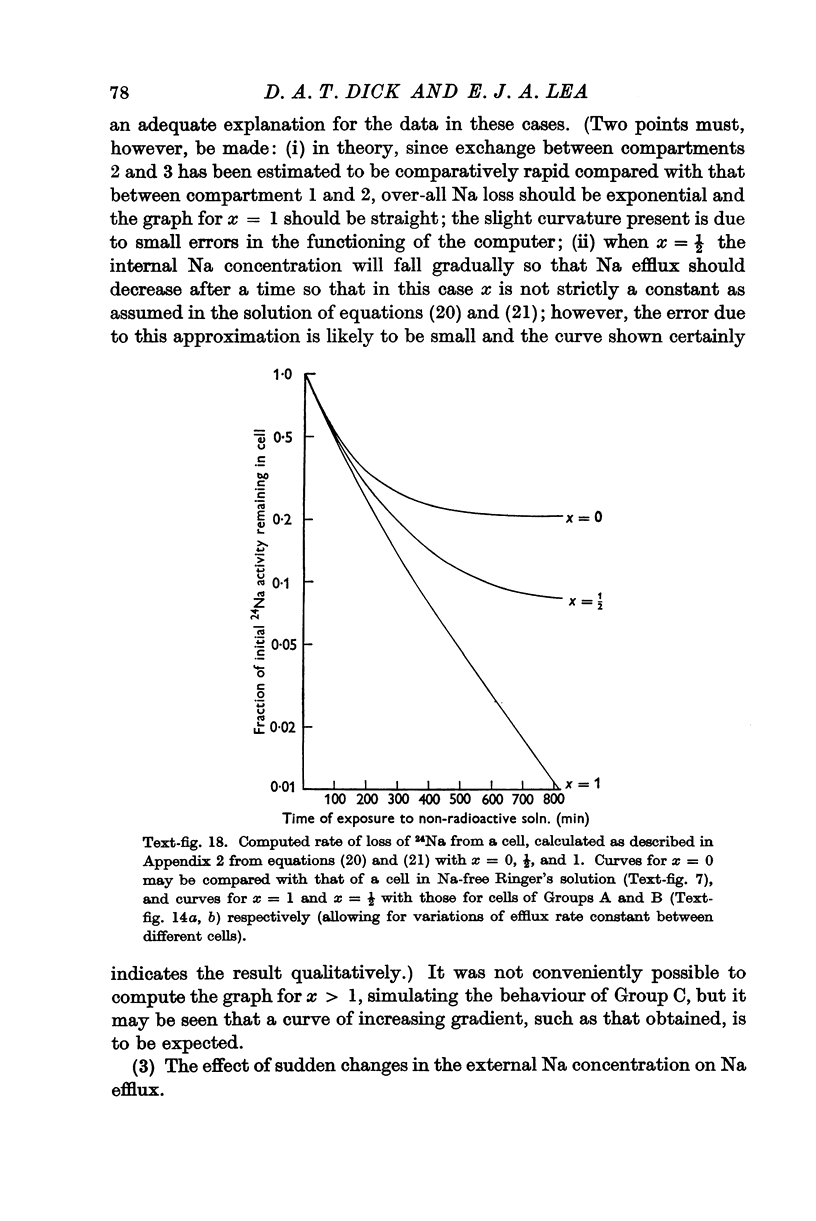
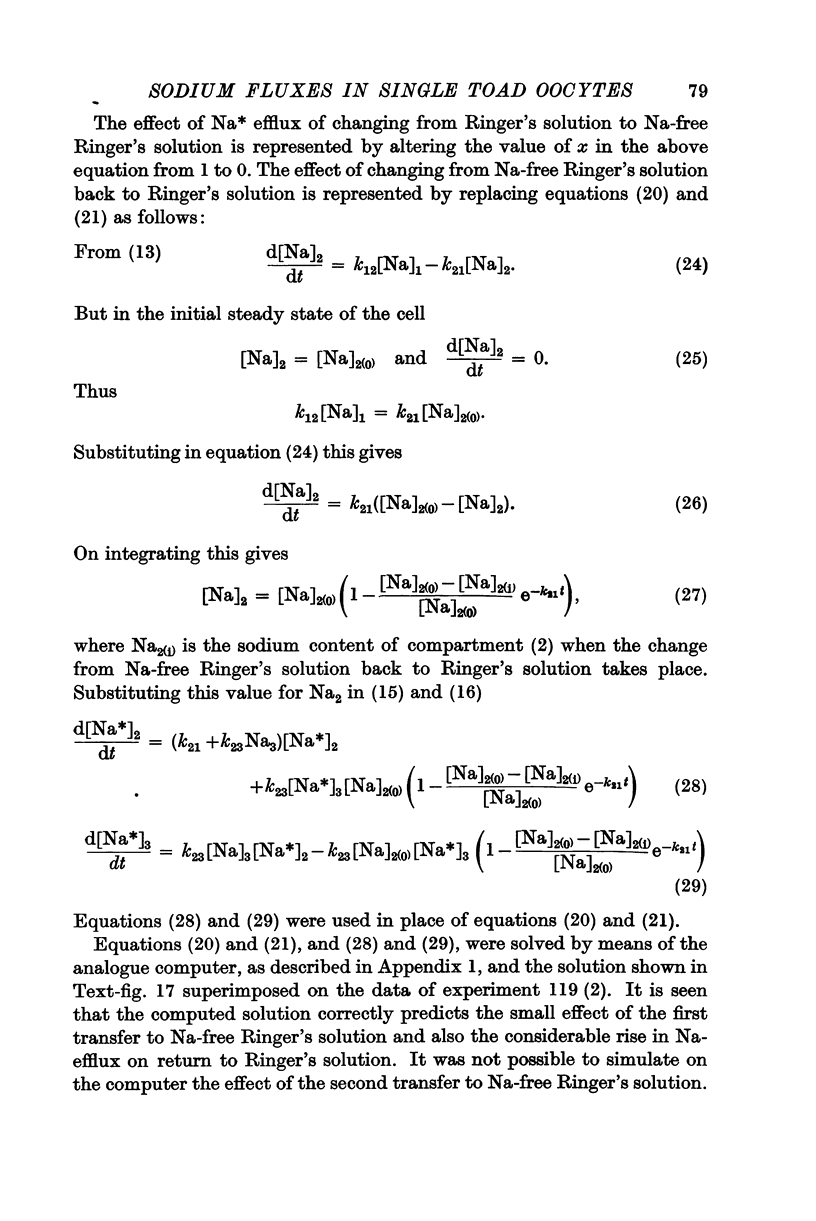
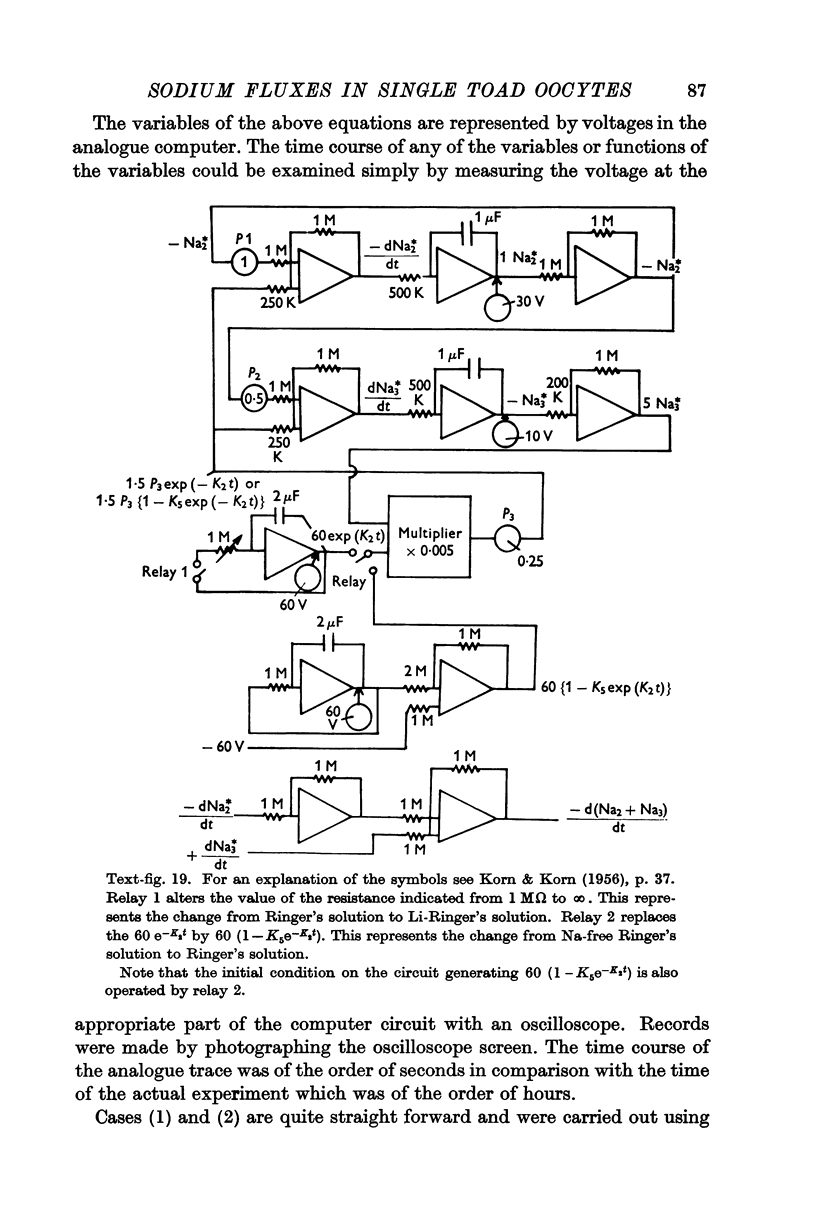
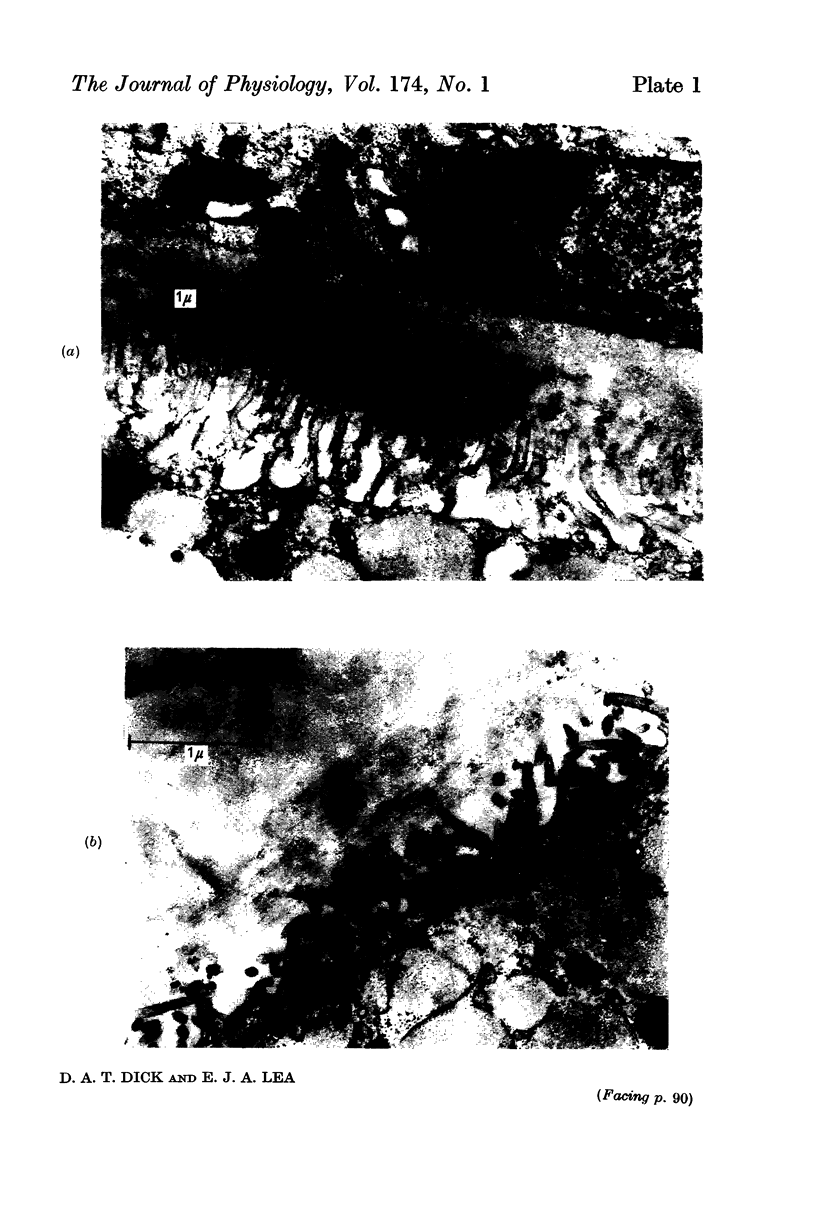
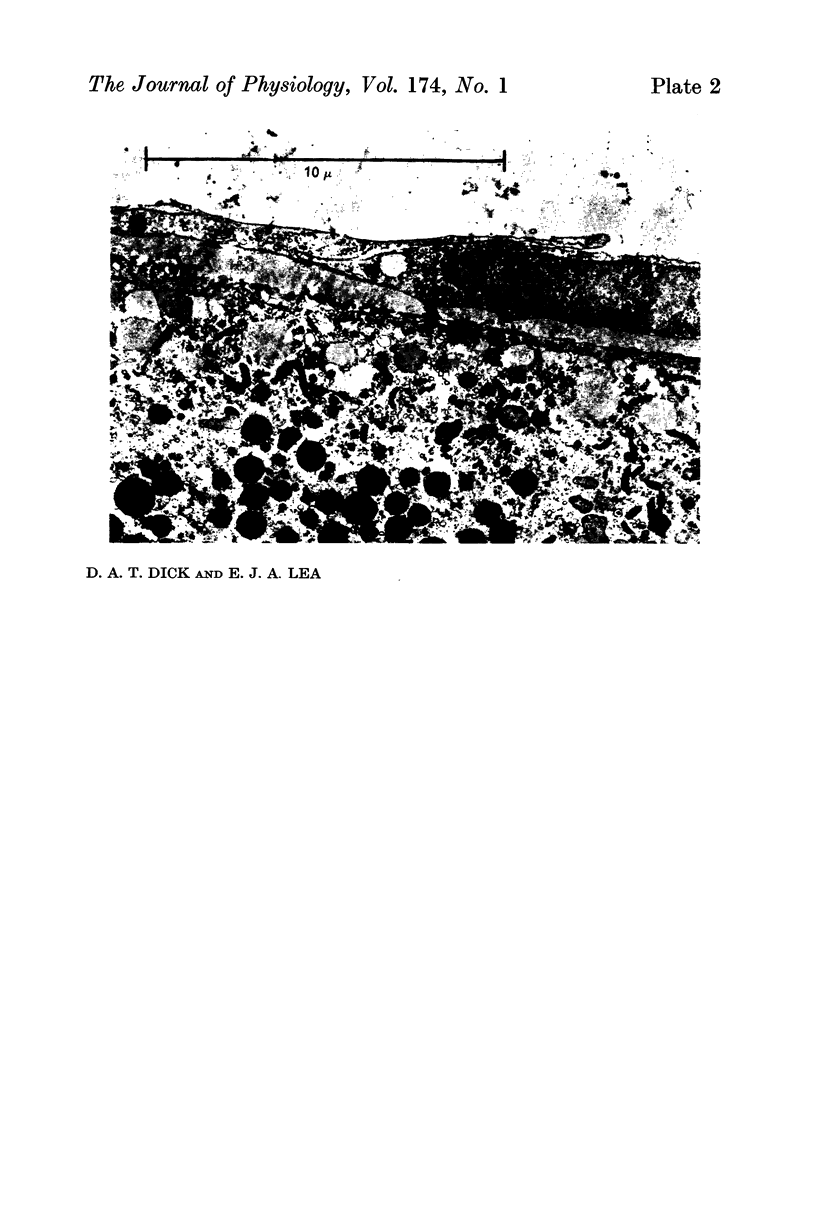
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMASSIAN V. E., DEVITO R. V. Unit activity in reticular formation and nearby structures. J Neurophysiol. 1954 Nov;17(6):575–603. doi: 10.1152/jn.1954.17.6.575. [DOI] [PubMed] [Google Scholar]
- BURNS B. D., ROBSON J. G. 'Weightless' micro-electrodes for recording extracellular unit action potentials from the central nervous system. Nature. 1960 Apr 16;186:246–247. doi: 10.1038/186246a0. [DOI] [PubMed] [Google Scholar]
- CARMELIET E. E. INFLUENCE OF LITHIUM IONS ON THE TRANSMEMBRANE POTENTIAL AND CATION CONTENT OF CARDIAC CELLS. J Gen Physiol. 1964 Jan;47:501–530. doi: 10.1085/jgp.47.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., PHILLIS J. W., WATKINS J. C. The chemical excitation of spinal neurones by certain acidic amino acids. J Physiol. 1960 Mar;150:656–682. doi: 10.1113/jphysiol.1960.sp006410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIETE-SPIFF K. The discharge pattern of muscle spindles of the rabbit on activation of intrafusal muscle fibres. J Physiol. 1961 Dec;159:282–296. doi: 10.1113/jphysiol.1961.sp006808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C. The central action of antidromic impulses in motor nerve fibres. Pflugers Arch. 1955;260(5):385–415. doi: 10.1007/BF00363548. [DOI] [PubMed] [Google Scholar]
- FATT P. Electric potentials occurring around a neurone during its antidromic activation. J Neurophysiol. 1957 Jan;20(1):27–60. doi: 10.1152/jn.1957.20.1.27. [DOI] [PubMed] [Google Scholar]
- GAUTHIER C., MOLLICA A., MORUZZI G. Physiological evidence of localized cerebellar projections to bulbar reticular formation. J Neurophysiol. 1956 Sep;19(5):468–483. doi: 10.1152/jn.1956.19.5.468. [DOI] [PubMed] [Google Scholar]
- GERNANDT B. E., THULIN C. A. Effect of vestibular nerve section upon spinal influence of the bulbar reticular formation. Acta Physiol Scand. 1955;33(2-3):120–131. doi: 10.1111/j.1748-1716.1955.tb01198.x. [DOI] [PubMed] [Google Scholar]
- GERNANDT B. E., THULIN C. A. Reciprocal effects upon spinal motoneurons from stimulation of bulbar reticular formation. J Neurophysiol. 1955 Mar;18(2):113–129. doi: 10.1152/jn.1955.18.2.113. [DOI] [PubMed] [Google Scholar]
- GOODFORD P. J., HERMANSEN K. Sodium and potassium movements in the unstriated muscle of the guinea-pig taenia coli. J Physiol. 1961 Oct;158:426–448. doi: 10.1113/jphysiol.1961.sp006778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODFORD P. J. The sodium content of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1962 Oct;163:411–422. doi: 10.1113/jphysiol.1962.sp006986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS E. J., PRANKERD T. A. Diffusion and permeation of cations in human and dog erythrocytes. J Gen Physiol. 1957 Sep 20;41(1):197–218. doi: 10.1085/jgp.41.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS E. J. Permeation and diffusion of K ions in frog muscle. J Gen Physiol. 1957 Sep 20;41(1):169–195. doi: 10.1085/jgp.41.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNANDEZ-PEON R., HAGBARTH K. E. Interaction between afferent and cortically induced reticular responses. J Neurophysiol. 1955 Jan;18(1):44–55. doi: 10.1152/jn.1955.18.1.44. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Movements of Na and K in single muscle fibres. J Physiol. 1959 Mar 3;145(2):405–432. doi: 10.1113/jphysiol.1959.sp006150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMES R. L., WOLSTENCROFT J. H. Accessory sources of blood supply to the brain of the cat. J Physiol. 1959 Oct;148:93–107. doi: 10.1113/jphysiol.1959.sp006275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMP N. E. Electron microscopy of growing oocytes of Rana pipiens. J Biophys Biochem Cytol. 1956 May 25;2(3):281–292. doi: 10.1083/jcb.2.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The effect of external sodium concentration on the sodium fluxes in frog skeletal muscle. J Physiol. 1959 Oct;147:591–625. doi: 10.1113/jphysiol.1959.sp006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The permeability of frog muscle fibres to lithium ions. J Physiol. 1959 Oct;147:626–638. doi: 10.1113/jphysiol.1959.sp006265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAPORTE Y., LUNDBERG A. Functional organization of the dorsal spino-cerebellar tract in the cat. III. Single fibre recording in Flechsig's fasciculus on adequate stimulation of primary afferent neurons. Acta Physiol Scand. 1956 Mar 24;36(1-2):204–218. doi: 10.1111/j.1748-1716.1956.tb01318.x. [DOI] [PubMed] [Google Scholar]
- LAPORTE Y., LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spino-cerebellar tract in the cat. I. Recording of mass discharge in dissected Flechsig's fasciculus. Acta Physiol Scand. 1956 Mar 24;36(1-2):175–187. doi: 10.1111/j.1748-1716.1956.tb01316.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spinocerebellar tract in the cat. IV. Synaptic connections of afferents from Golgi tendon organs and muscle spindles. Acta Physiol Scand. 1956 Dec 29;38(1):53–75. doi: 10.1111/j.1748-1716.1957.tb00173.x. [DOI] [PubMed] [Google Scholar]
- MAGNI F., WILLIS W. D. IDENTIFICATION OF RETICULAR FORMATION NEURONS BY INTRACELLULAR RECORDING. Arch Ital Biol. 1963 Oct 5;101:681–702. [PubMed] [Google Scholar]
- MAIZELS M., REMINGTON M. Cation exchanges of human erythrocytes. J Physiol. 1959 Mar 12;145(3):641–657. doi: 10.1113/jphysiol.1959.sp006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLLICA A., MORUZZI G., NAQUET R. Décharges réticulaires induites par la polarisation du cervelet: leurs rapports avec le tonus postural et la réaction d'éveil. Electroencephalogr Clin Neurophysiol. 1953 Nov;5(4):571–584. doi: 10.1016/0013-4694(53)90034-0. [DOI] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B., DAVIES P. W., BERMAN A. L. Response properties of neurons of cat's somatic sensory cortex to peripheral stimuli. J Neurophysiol. 1957 Jul;20(4):374–407. doi: 10.1152/jn.1957.20.4.374. [DOI] [PubMed] [Google Scholar]
- MULLINS L. J., ADELMAN W. J., Jr, SJODIN R. A. Sodium and potassium ion effluxes from squid axons under voltage clamp conditions. Biophys J. 1962 May;2:257–274. doi: 10.1016/s0006-3495(62)86854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLINS L. J., FRUMENTO A. S. The concentration dependence of sodium efflux from muscle. J Gen Physiol. 1963 Mar;46:629–654. doi: 10.1085/jgp.46.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSCARSSON O. Functional organization of the ventral spino-cerebellar tract in the cat. I. Electrophysiological identification of the tract. Acta Physiol Scand. 1956 Dec 31;38(2):145–165. doi: 10.1111/j.1748-1716.1957.tb01379.x. [DOI] [PubMed] [Google Scholar]
- POMPEIANO O., SWETT J. E. ACTIONS OF GRADED CUTANEOUS AND MUSCULAR AFFERENT VOLLEYS ON BRAIN STEM UNITS IN THE DECEREBRATE, CEREBELLECTOMIZED CAT. Arch Ital Biol. 1963 Oct 5;101:552–583. [PubMed] [Google Scholar]
- POMPEIANO O., SWETT J. E. CEREBELLAR POTENTIALS AND RESPONSES OF RETICULAR UNITS EVOKED BY MUSCULAR AFFERENT VOLLEYS IN THE DECEREBRATE CAT. Arch Ital Biol. 1963 Oct 5;101:584–613. [PubMed] [Google Scholar]
- SCHEIBEL M., SCHEIBEL A., MOLLICA A., MORUZZI G. Convergence and interaction of afferent impulses on single units of reticular formation. J Neurophysiol. 1955 Jul;18(4):309–331. doi: 10.1152/jn.1955.18.4.309. [DOI] [PubMed] [Google Scholar]
- SHIMAMURA M., LIVINGSTON R. B. Longitudinal conduction systems serving spinal and brain-stem coordination. J Neurophysiol. 1963 Mar;26:258–272. doi: 10.1152/jn.1963.26.2.258. [DOI] [PubMed] [Google Scholar]
- SPRAGUE J. M., CHAMBERS W. W. Control of posture by reticular formation and cerebellum in the intract, anesthetized and unanesthetized and in the decerebrated cat. Am J Physiol. 1954 Jan;176(1):52–64. doi: 10.1152/ajplegacy.1953.176.1.52. [DOI] [PubMed] [Google Scholar]
- TASAKI I. Demonstration of two stable states of the nerve membrane in potassium-rich media. J Physiol. 1959 Oct;148:306–331. doi: 10.1113/jphysiol.1959.sp006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THULIN C. A. Motor effects from stimulation of the vestibular nuclei and the reticular formation. Acta Physiol Scand Suppl. 1953;28(103):1–61. [PubMed] [Google Scholar]
- TORVIK A., BRODAL A. The origin of reticulospinal fibers in the cat; an experimental study. Anat Rec. 1957 May;128(1):113–137. doi: 10.1002/ar.1091280110. [DOI] [PubMed] [Google Scholar]
- VERHAART W. J. The fiber structure of cord in the cat. Acta Anat (Basel) 1953;18(2):88–100. doi: 10.1159/000140830. [DOI] [PubMed] [Google Scholar]
- VON BAUMGARTEN R., MOLLICA A. Der Einfluss sensibler Reizung auf die Entladungsfrequenz kleinhirnabhängiger Reticulariszellen. Pflugers Arch. 1954;259(1):79–96. doi: 10.1007/BF00363577. [DOI] [PubMed] [Google Scholar]
- VON BAUMGARTEN R., MOLLICA A., MORUZZI G. Modulierung der Entladungsfrequenz einzelner Zellen der Substantia reticularis durch corticofugale und cerebelläre Impulse. Pflugers Arch. 1954;259(1):56–78. doi: 10.1007/BF00363576. [DOI] [PubMed] [Google Scholar]
- WITTEK M. La vitellogénèse chez les amphibiens. Arch Biol (Liege) 1952;58(2):133–198. [PubMed] [Google Scholar]