Abstract
Hepatitis delta virus (HDV) requires host RNA editing at the viral RNA amber/W site. Of the two host genes responsible for RNA editing via deamination of adenosines in double-stranded RNAs, short inhibitory RNA-mediated knockdown of host ADAR1 expression but not that of ADAR2 led to decreased HDV amber/W editing and virus production. Despite substantial sequence and structural variation among the amber/W sites of the three HDV genotypes, ADAR1a was primarily responsible for editing all three. We conclude that ADAR1 is primarily responsible for editing HDV RNA at the amber/W site during HDV infection.
Hepatitis delta virus (HDV) infection causes severe acute and chronic hepatitis in those infected with its helper, hepatitis B virus (32). A central event in the HDV replication cycle is an RNA editing event that allows the virus to produce two forms of the sole viral protein, hepatitis delta antigen (HDAg), that have different and opposed functions in the HDV replication cycle (reviewed in reference 14). Editing involves the specific deamination of the amber/W site adenosine to inosine and changes the stop codon of HDAg-S to a tryptophan codon for HDAg-L (4, 7, 26, 30).
In mammals, the ADAR1 and ADAR2 genes encode proteins that edit specific adenosines in double-stranded RNA segments (reviewed in references 15, 20, and 33), and ADAR1 and ADAR2 proteins can specifically edit the amber/W site in HDV RNA (18, 33, 36) as well as adenosines in several cellular pre-mRNA substrates (15, 20, 34). The product of a third related gene, ADAR3, has no apparent deaminase activity on other ADAR1 or ADAR2 substrates (9, 27) and is unlikely to edit HDV RNA. ADAR1 is expressed in many tissues, while the highest level of ADAR2 expression is found in the brain (21, 28). The relative levels of ADAR1 and ADAR2 RNA expression have been analyzed by Northern blotting for some tissues (9, 22) but not for the liver. Using Northern blot hybridization and reverse transcription-PCR (RT-PCR), we analyzed ADAR1 and ADAR2 expression both in cultured Huh-7 human hepatoma cells and in HDV-infected liver tissue and found that the expression level of ADAR1 is 10- to 20-fold higher than that of ADAR2. These data are consistent with the general pattern of ADAR1 and ADAR2 expression (9, 21, 27) and could suggest that ADAR1 is principally responsible for HDV amber/W editing in infected hepatocytes. However, these enzymes can exhibit differential activities on some substrates (28, 33, 36). Although previous studies (18, 33, 36) showed that both ADAR1 and ADAR2 can edit HDV RNA when overexpressed in Huh-7 cells, their relative activities on the HDV amber/W site were not investigated: amber/W editing activities were analyzed only at very high, possibly saturating, levels of ADAR expression.
We sought to determine the extent to which ADAR1 and ADAR2 and their splice variants are responsible for HDV RNA editing in vivo by using short inhibitory RNAs (siRNAs) (2, 10) to specifically knock down expression of ADAR1 or ADAR2 in cultured Huh-7 cells. siRNAs (Table 1) were designed as double-stranded RNAs with 19 or 20 bp and 2-nucleotide 3′ overhangs, as described previously (2, 11). GenBank searches (1) indicated that only the targeted genes matched the siRNA sequences perfectly; the closest nontargeted genes were mismatched with the siRNAs in at least two positions and would not likely be targeted for siRNA-mediated knockdown of expression (12). siRNAs were obtained as annealed duplexes from Dharmacon Research Inc. (Lafayette, Colo.) (11) and transfected into cultured Huh-7 cells as reported previously (2).
TABLE 1.
Sequence of siRNA duplexes used to knock down ADAR expression
| siRNA | Sequence | Region targeteda | mRNA targeted |
|---|---|---|---|
| siAD1 | 5′ CGCAGAGUUCCUCACCUGUATT 3′ | +271 to +293 | All ADAR1 mRNAs |
| 3′ TTGCGUCUCAAGGAGUGGACAU 5′ | |||
| siAD1a | 5′ GAGAGGAGGAGCAUAGUUCTT 3′ | +1563 to +1583 | ADAR1a |
| 3′ TTCUCUCCUCCUCGUAUCAAG 5′ | |||
| siAD1b | 5′ CCAGUGAGAGGGAGCUCUGUGTG 3′ | +1527 to +1533, +1572 to +1585 | ADAR1b |
| 3′ GTGGUCACUCUCCCUCGAGACAC 5′ | |||
| siAD2 | 5′ GCCUGGUUUGCAGUACACTT 3′ | +261 to +288 | All ADAR2 mRNAs |
| 3′ TTCGGACCAAACGUCAUGUGU 5′ |
Relative to the AUG start codon for the corresponding mRNA.
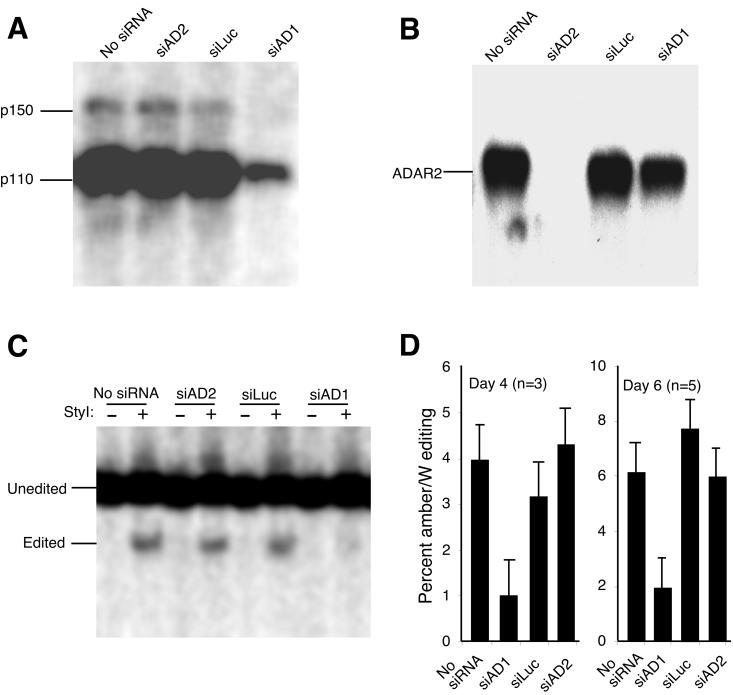
Transfection with siAD1 dramatically inhibited expression of endogenous targeted ADAR1 in Huh-7 cells (Fig. 1A). This suppression was specific: siRNAs shown to be functional against luciferase (10) and ADAR2 (Fig. 1B) did not suppress ADAR1 protein expression, and siAD1 had a minimal effect on ADAR2 expression (Fig. 1B). We also observed that siAD1 could suppress expression of ADAR1 from a cotransfected ADAR1 expression construct (data not shown), further confirming the specificity of ADAR1 suppression by siAD1. Because the level of endogenous ADAR2 expression in Huh-7 cells is too low to accurately quantify the reduction of expression, we demonstrated the efficacy of siAD2, which targets ADAR2, by cotransfection with an ADAR2 expression construct, pMS040 (36). Similar to the effect of siAD1 on ADAR1 expression, siAD2 dramatically and specifically inhibited ADAR2 protein expression (Fig. 1B). Suppression of ADAR1 and ADAR2 by these siRNAs was greatest between days 2 and 4 posttransfection but was still evident 6 days posttransfection.
FIG. 1.
Effect of siRNAs on ADAR protein expression and HDV RNA editing. The siRNAs used are described in Table 1. (A) Effect of siAD1 on endogenous ADAR1 protein expression. Huh-7 human hepatoma cells were transfected with the indicated siRNAs at a concentration of 100 nM, as described previously (2). Whole-cell protein lysates were prepared (6) 4 days posttransfection and analyzed by electrophoresis on SDS-8% polyacrylamide gels and immunoblotting with anti-human ADAR1 antibody (kind gift of D. Lazinski), followed by 125I-labeled protein G (New England Nuclear). (B) Effect of siAD2 on ADAR2 protein expression. Huh-7 human hepatoma cells were transfected with 1 μg of pMS040, an expression plasmid for human ADAR2, and the indicated siRNAs, as for panel A. Whole-cell protein lysates prepared 4 days after transfection were analyzed as in panel A, except that an anti-human-ADAR2 antibody (kind gift of D. Lazinski) was used. (C and D) Effect of siRNA transfection on amber/W editing of HDV genotype I. Huh-7 cells were cotransfected with 1 μg of a replicating type I HDV RNA expression construct pHDV · I(+) and the indicated siRNAs. RNAs were harvested on days 4 and day 6 posttransfection and analyzed for amber/W editing by RT-PCR as described previously (18). (C) RT-PCR products from RNAs harvested on day 6. Lanes −, undigested RT-PCR products; lanes +, RT-PCR products digested with StyI. Editing produces a StyI restriction site that is not present in unedited RNA. The undigested band due to unedited RNA and the larger of the two bands due to StyI digestion of cDNA derived from edited RNA are shown. The smaller digestion fragment is less visible and was cut off from the bottom of the gel image. (D) Graph of RT-PCR analysis of amber/W editing results from day 4, left, and day 6, right. Values for day 4 are the average of triplicates, and values for day 6 are the average of quintuplicates. Percent editing (vertical bars) is determined by dividing the sum of the two StyI restriction digestion bands due to editing by the sum of the edited and unedited bands. Thin vertical lines represent standard errors. Note that the vertical scales are not identical for days 4 and 6 because the level of editing for the untreated cells increases between days 4 and 6.
To examine the contributions of ADAR1 and ADAR2 to HDV amber/W site editing, siAD1 and siAD2 were cotransfected with the HDV genotype I replication construct pHDV · I(+) (6). Editing at the amber/W site was assessed on days 4 and 6 posttransfection using a well-characterized RT-PCR restriction digestion assay in which the amount of StyI-digested PCR product indicates the percent editing because editing creates a StyI digestion site that is not present prior to editing (7, 18, 31). Transfection with siAD1, but not siAD2, strongly inhibited amber/W editing (Fig. 1C and D) on days 4 and 6 posttransfection. The extent of this inhibition by siAD1 was similar to the extent to which ADAR1 expression was reduced (Fig. 1A). No inhibition of editing was seen with siLuc, which has been shown to be an effective inhibitor of firefly luciferase expression (10) but has no sequence homology to ADAR1 or ADAR2. This result indicates that the HDV amber/W site is edited primarily by ADAR1 in Huh-7 cells.
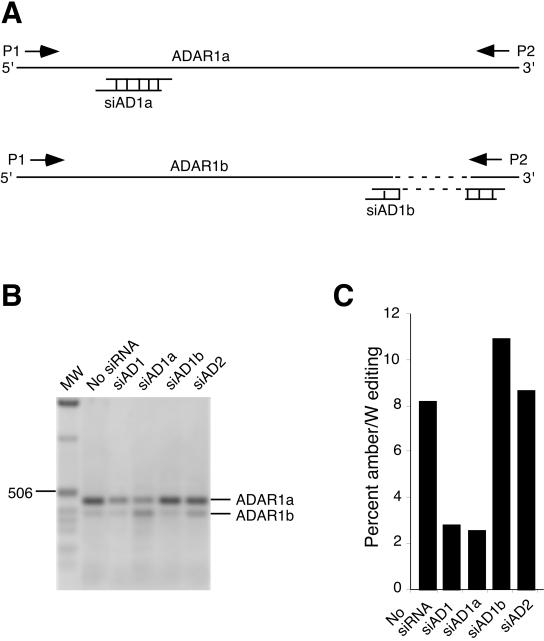
Several splice variants of ADAR1 have been identified that may have different activities on some substrates (24, 25). To determine the roles of ADAR1 splice variants in HDV amber/W editing, we analyzed the relative abundance of the ADAR1a, ADAR1b, and ADAR1c forms by RT-PCR using the primers 5′-CGACCAACTCCATGGCTTCTGA-3′ and 5′-GGTGCTGCCAGTGAGAGGGAG-3′ (nt positions 2238 to 2259 and 2684 to 2704, respectively: GenBank accession no. XM_036845), which amplify a fragment spanning segments that are deleted from ADAR1b and ADAR1c (Fig. 2A and B). The relative abundance of ADAR1a- and ADAR1b-specific PCR products indicated that these variants were present in a ratio of approximately 4:1 (ADAR1a/ADAR1b) in Huh-7 cells and in HDV-infected liver; ADAR1c was not detected. We tested the abilities of siRNAs to selectively target the ADAR1a and ADAR1b splice variants and analyzed their effects on HDV RNA editing (Fig. 2). Transfection with siAD1a and siAD1b, which targeted ADAR1a and ADAR1b, respectively, effectively reduced expression of their respective targets (Fig. 2B). Because ADAR1a is the predominant splice variant, siAD1a had a more pronounced effect on total ADAR protein levels. In accord with this difference, we observed that knockdown of ADAR1a expression substantially reduced HDV amber/W editing, while knockdown of ADAR1b had little effect (Fig. 2C). Thus, ADAR1a is primarily responsible for HDV amber/W editing in Huh-7 cells. It is not possible to tell from these results whether ADAR1b could edit the amber/W site, because the lack of a substantial effect of siAD1b on amber/W editing could simply be due to the lower level of ADAR1b expression.
FIG. 2.
Effects of ADAR1 splice variants on HDV amber/W editing. (A) Illustration of the structure of ADAR1 splice variants ADAR1a and ADAR1b (24). ADAR1b is generated by alternative splicing at exon 7 and has a deletion of 78 nucleotides (shown by the dashed line) compared with ADAR1a. Locations of primers P1 and P2 used for RT-PCR analysis of ADAR1a and ADAR1b expression are indicated by leftward and rightward arrows. Locations of ADAR1a- and ADAR1b-specific siRNAs are indicated schematically. Note that the sketch is not drawn to scale. (B) RT-PCR analyses to detect the efficiency of ADAR gene targeting by siRNAs. Huh-7 cells were transfected with siAD1, siAD1a, siAD1b, or siAD2, as in Fig. 1. RNAs were harvested 4 days posttransfection and analyzed by RT-PCR. Products were run on 1% agarose gels, stained with ethidium bromide, and photographed. The image shown is an inverted image of a scanned photograph, which better illustrates the reduction of intensity of several bands. MW, molecular weight standards (1-kb ladder; Invitrogen, Carlsbad, Calif.). Sizes of selected molecular weight markers are indicated, as are the locations of the predicted RT-PCR products for ADAR1a and ADAR1b. (C) Effect of siRNA-targeted reduction of ADAR1a and ADAR1b on HDV amber/W editing. Huh-7 cells were transfected with pHDV · I(+) and the indicated siRNAs; RNAs were harvested 6 days posttransfection and analyzed for HDV amber/W editing as in Fig. 1.
We also attempted to use siRNAs to suppress expression of another variant of ADAR1, p150 (13, 19, 29), but were unable to do so. This variant is expressed at approximately 1/10 the level of the predominant form, p110, both in Huh-7 cells and in infected liver tissue, and is not preferentially associated with either the ADAR1a or ADAR1b splice variant. Although overexpression studies suggest that the p150 form is not more active than the p110 form, a more definitive study will be required to determine the role of ADAR1 p150 in HDV amber/W editing.
Three genotypes of HDV have been identified, each with different geographic distributions and associated disease severities (5, 17, 35, 37). These genotypes exhibit functional differences in RNA replication and in RNA editing (3, 6, 17, 37). In particular, the RNA structures required for amber/W editing are dramatically different for genotypes I and III (3). For example, the A-C mismatch that is required for editing in the genotype I site occurs as an A-U pair in genotype III, and a highly conserved A-U or G-C base pair in genotype I (4, 31) occurs as an A-A pair in genotype III (3). The structure around the editing site in HDV genotype II has not yet been defined, but there are fewer base pairs in the predicted structure in a region that is important for editing in genotype I (17). These structural variations raise the question of whether the same deaminase is active for all three genotypes (3).
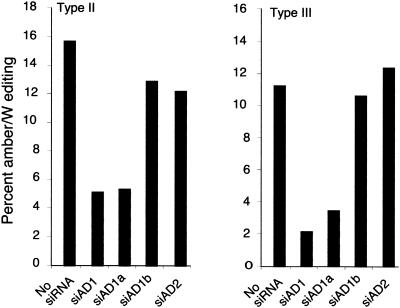
To determine the roles of ADAR1, ADAR2, and ADAR1 splice variants in amber/W editing for HDV genotypes II and III, we cotransfected Huh-7 cells with either replication-competent genotype II constructs or genotype III constructs and our panel of ADAR-targeted siRNAs. In results similar to those for genotype I (Fig. 1), transfection with siAD1 and siAD1a inhibited amber/W editing in HDV genotypes II and III, whereas siAD2 had no detectable effect (Fig. 3). Thus, despite structural variations around the amber/W site, ADAR1a is primarily responsible for HDV amber/W editing for all three HDV genotypes. It is worth noting that the levels of amber/W editing differ among the three genotype clones tested. It is not yet clear to what extent these differences are due to the intrinsic activity of the different editing substrates or other factors, such as different regulatory mechanisms. Overall, these data indicate that the sequence and structural determinants for RNA editing are complex. Examination of the predicted structure around the HDV amber/W site (3, 4, 17) and several cellular (34) and synthetic (23) substrates for RNA editing suggests that primary sequence, base pairing, and internal bulges and loops can all contribute to highly specific editing. Clearly, a more complete understanding of these determinants will require further study.
FIG. 3.
Effect of ADAR1 and ADAR2 siRNAs on amber/W editing in HDV genotypes II and III. Huh-7 cells were transfected with HDV genotype II or genotype III expression constructs pHDV · II(+) (unpublished data) or pHDV · III(+) (3) and the indicated siRNAs. RNAs were harvested 6 days posttransfection and analyzed for amber/W editing as in Fig. 1 except that for genotype II the restriction enzyme BtgI was used (16). Values shown are the averages from duplicate experiments. Note that because the intrinsic levels of editing are different for genotypes II and III, the vertical scales have been adjusted to give similar maximum heights.
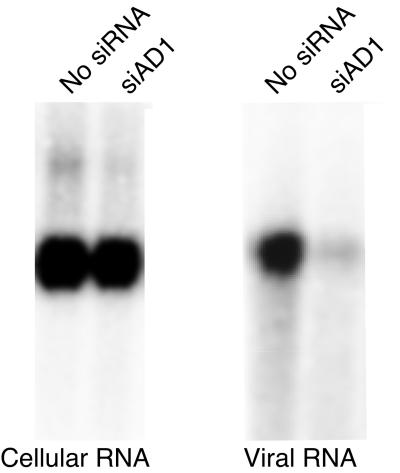
Editing at the amber/W site is required for the production of HDAg-L, which is necessary for the formation of infectious HDV virions with a hepatitis B virus surface antigen envelope (8). To examine the effect of inhibiting editing on virion production, we cotransfected Huh-7 cells with pHDV · I(+), siAD1, and a hepatitis B virus surface antigen expression construct; RNAs were harvested from cells and from exported viral particles 6 days posttransfection and analyzed by Northern blot hybridization (3). Whereas cotransfection of siAD1 had no effect on HDV RNA replication, virion production was strongly inhibited (Fig. 4), in accord with the suppression of amber/W editing (Fig. 1).
FIG. 4.
Inhibition of virion secretion by siAD1. Huh-7 cells were cotransfected with 1 μg of the replicating Type I HDV RNA expression construct pHDV · I(+) and the hepatitis B surface antigen expression construct pGEM3-HBV(BspEI) (3) with or without siAD1, as indicated. Total cellular RNA and viral RNA were harvested 6 days posttransfection and analyzed for HDV RNA replication and virion secretion by Northern blot hybridization, as described previously (3). Left, HDV genomic RNA in transfected cells; right, HDV genomic RNA in virus particles.
We have shown here that ADAR1a is primarily responsible for editing HDV RNA at the amber/W site in Huh-7 cells. Because the relative levels of ADAR1 and ADAR2 expression and the relative levels of ADAR1 splice variants are similar in Huh-7 cells and infected liver tissue, it is likely that ADAR1a is also primarily responsible for editing in HDV-infected liver tissue. While our results indicate a correlation between ADAR protein expression levels and participation in HDV amber/W editing during replication in Huh-7 cells and infected liver tissue, they do not directly address the relative specific activities of ADAR1, ADAR2, and their splice variants on the HDV amber/W sites. Further studies in this area could provide valuable information on the determinants of editing activity and specificity for these enzymes.
Our results further emphasize the effects of various ADAR levels on HDV and underscore the central role of RNA editing in the HDV replication cycle. Previously we showed that overexpression of ADARs inhibits HDV RNA replication by increased HDAg-L production and hyperediting of HDV RNA (18). The observed marked decrease in virus production (Fig. 4) that accompanies suppression of ADAR1 expression (because HDAg-L synthesis is inhibited) complements those previous findings. Finally, it is conceivable that targeted disruption of host functions, such as ADAR, by siRNA could be used as therapy against viruses dependent on those functions. Such an approach may avoid problems posed by virus genetic variability and genetic escape mutants.
Acknowledgments
This work was supported by grant R01-AI42324 from the National Institutes of Health.
We thank David Lazinski for the plasmids pMS040 and PDL700 and rabbit anti-human ADAR1 and ADAR2 antibodies. We thank Vinod Rustgi for providing the liver sample from an HDV-infected patient and for comments on the manuscript.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caplen, N. J., S. Parrish, F. Imani, A. Fire, and R. A. Morgan. 2001. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc. Natl. Acad. Sci. USA 98:9742-9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casey, J. L. 2002. RNA editing in hepatitis delta virus genotype III requires a branched double-hairpin RNA structure. J. Virol. 76:7385-7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey, J. L., K. F. Bergmann, T. L. Brown, and J. L. Gerin. 1992. Structural requirements for RNA editing in hepatitis delta virus: evidence for a uridine-to-cytidine editing mechanism. Proc. Natl. Acad. Sci. USA 89:7149-7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casey, J. L., T. L. Brown, E. J. Colan, F. S. Wignall, and J. L. Gerin. 1993. A genotype of hepatitis D virus that occurs in northern South America. Proc. Natl. Acad. Sci. USA 90:9016-9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casey, J. L., and J. L. Gerin. 1998. Genotype-specific complementation of hepatitis delta virus RNA replication by hepatitis delta antigen. J. Virol. 72:2806-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey, J. L., and J. L. Gerin. 1995. Hepatitis D virus RNA editing: specific modification of adenosine in the antigenomic RNA. J. Virol. 69:7593-7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, F. L., P. J. Chen, S. J. Tu, C. J. Wang, and D. S. Chen. 1991. The large form of hepatitis delta antigen is crucial for assembly of hepatitis delta virus. Proc. Natl. Acad. Sci. USA 88:8490-8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, C. X., D. S. Cho, Q. Wang, F. Lai, K. C. Carter, and K. Nishikura. 2000. A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. RNA 6:755-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 11.Elbashir, S. M., W. Lendeckel, and T. Tuschl. 2001. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 15:188-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elbashir, S. M., J. Martinez, A. Patkaniowska, W. Lendeckel, and T. Tuschl. 2001. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. EMBO J. 20:6877-6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George, C. X., and C. E. Samuel. 1999. Characterization of the 5′-flanking region of the human RNA-specific adenosine deaminase ADAR1 gene and identification of an interferon-inducible ADAR1 promoter. Gene 229:203-213. [DOI] [PubMed] [Google Scholar]
- 14.Gerin, J. L., J. L. Casey, and R. H. Purcell. 2001. Hepatitis delta virus, p. 3037-3050. In D. M. Knipe, P. M. Howley, et al. (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, New York, N.Y.
- 15.Gott, J. M., and R. B. Emeson. 2000. Functions and mechanisms of RNA editing. Annu. Rev. Genet. 34:499-531. [DOI] [PubMed] [Google Scholar]
- 16.Hsu, S. C., W. J. Syu, I. J. Sheen, H. T. Liu, K. S. Jeng, and J. C. Wu. 2002. Varied assembly and RNA editing efficiencies between genotypes I and II hepatitis D virus and their implications. Hepatology 35:665-672. [DOI] [PubMed] [Google Scholar]
- 17.Ivaniushina, V., N. Radjef, M. Alexeeva, E. Gault, S. Semenov, M. Salhi, O. Kiselev, and P. Deny. 2001. Hepatitis delta virus genotypes I and II cocirculate in an endemic area of Yakutia, Russia. J. Gen. Virol. 82:2709-2718. [DOI] [PubMed] [Google Scholar]
- 18.Jayan, G. C., and J. L. Casey. 2002. Increased RNA editing and inhibition of hepatitis delta virus replication by high-level expression of ADAR1 and ADAR2. J. Virol. 76:3819-3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawakubo, K., and C. E. Samuel. 2000. Human RNA-specific adenosine deaminase (ADAR1) gene specifies transcripts that initiate from a constitutively active alternative promoter. Gene 258:165-172. [DOI] [PubMed] [Google Scholar]
- 20.Keegan, L. P., A. Gallo, and M. A. O'Connell. 2001. The many roles of an RNA editor. Nat. Rev. Genet. 2:869-878. [DOI] [PubMed] [Google Scholar]
- 21.Kim, U., Y. Wang, T. Sanford, Y. Zeng, and K. Nishikura. 1994. Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proc. Natl. Acad. Sci. USA 91:11457-11461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai, F., C. X. Chen, K. C. Carter, and K. Nishikura. 1997. Editing of glutamate receptor B subunit ion channel RNAs by four alternatively spliced DRADA2 double-stranded RNA adenosine deaminases. Mol. Cell. Biol. 17:2413-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehmann, K. A., and B. L. Bass. 1999. The importance of internal loops within RNA substrates of ADAR1. J. Mol. Biol. 291:1-13. [DOI] [PubMed] [Google Scholar]
- 24.Liu, Y., R. B. Emeson, and C. E. Samuel. 1999. Serotonin-2C receptor pre-mRNA editing in rat brain and in vitro by splice site variants of the interferon-inducible double-stranded RNA-specific adenosine deaminase ADAR1. J. Biol. Chem. 274:18351-18358. [DOI] [PubMed] [Google Scholar]
- 25.Liu, Y., C. X. George, J. B. Patterson, and C. E. Samuel. 1997. Functionally distinct double-stranded RNA-binding domains associated with alternative splice site variants of the interferon-inducible double-stranded RNA-specific adenosine deaminase. J. Biol. Chem. 272:4419-4428. [DOI] [PubMed] [Google Scholar]
- 26.Luo, G. X., M. Chao, S. Y. Hsieh, C. Sureau, K. Nishikura, and J. Taylor. 1990. A specific base transition occurs on replicating hepatitis delta virus RNA. J. Virol. 64:1021-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melcher, T., S. Maas, A. Herb, R. Sprengel, M. Higuchi, and P. H. Seeburg. 1996. RED2, a brain-specific member of the RNA-specific adenosine deaminase family. J. Biol. Chem. 271:31795-31798. [DOI] [PubMed] [Google Scholar]
- 28.Melcher, T., S. Maas, A. Herb, R. Sprengel, P. H. Seeburg, and M. Higuchi. 1996. A mammalian RNA editing enzyme. Nature 379:460-464. [DOI] [PubMed] [Google Scholar]
- 29.Patterson, J. B., and C. E. Samuel. 1995. Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: evidence for two forms of the deaminase. Mol. Cell. Biol. 15:5376-5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polson, A. G., B. L. Bass, and J. L. Casey. 1996. RNA editing of hepatitis delta virus antigenome by dsRNA-adenosine deaminase. Nature 380:454-456. [DOI] [PubMed] [Google Scholar]
- 31.Polson, A. G., H. L. Ley III, B. L. Bass, and J. L. Casey. 1998. Hepatitis delta virus RNA editing is highly specific for the amber/W site and is suppressed by hepatitis delta antigen. Mol. Cell. Biol. 18:1919-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rizzetto, M. 1983. The delta agent. Hepatology 3:729-737. [DOI] [PubMed] [Google Scholar]
- 33.Sato, S., S. K. Wong, and D. W. Lazinski. 2001. Hepatitis delta virus minimal substrates competent for editing by ADAR1 and ADAR2. J. Virol. 75:8547-8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seeburg, P. H., M. Higuchi, and R. Sprengel. 1998. RNA editing of brain glutamate receptor channels: mechanism and physiology. Brain Res. Rev. 26:217-229. [DOI] [PubMed] [Google Scholar]
- 35.Shakil, A. O., S. Hadziyannis, J. H. Hoofnagle, A. M. Di Bisceglie, J. L. Gerin, and J. L. Casey. 1997. Geographic distribution and genetic variability of hepatitis delta virus genotype I. Virology 234:160-167. [DOI] [PubMed] [Google Scholar]
- 36.Wong, S. K., S. Sato, and D. W. Lazinski. 2001. Substrate recognition by ADAR1 and ADAR2. RNA 7:846-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu, J. C., T. Y. Chiang, and I. J. Sheen. 1998. Characterization and phylogenetic analysis of a novel hepatitis D virus strain discovered by restriction fragment length polymorphism analysis. J. Gen. Virol. 79:1105-1113. [DOI] [PubMed] [Google Scholar]