Abstract
The host factor LSF represses the human immunodeficiency virus type 1 long terminal repeat (LTR) by mediating recruitment of histone deacetylase. We show that pyrrole-imidazole polyamides targeted to the LTR can specifically block LSF binding both in vitro and within cells via direct access to chromatin, resulting in increased LTR expression.
Human immunodeficiency virus type 1 (HIV-1) infection is characterized by cycles of virus production and reinfection within activated CD4+ T lymphocytes. However, HIV establishes a persistent, nonproductive state within a small population of memory CD4+ cells (4, 10, 18). Mechanisms that allow HIV to establish latency are unknown, but modulation of histone architecture within the host genome may participate in this process. The integrated long terminal repeat (LTR) requires histone acetylation prior to activation, implying a role for histone deacetylase (HDAC) in establishing or maintaining quiescence (9). A nucleosome (Nuc-1) protects a region spanning +1 to +155 with respect to the start site of transcription (9, 15, 16).
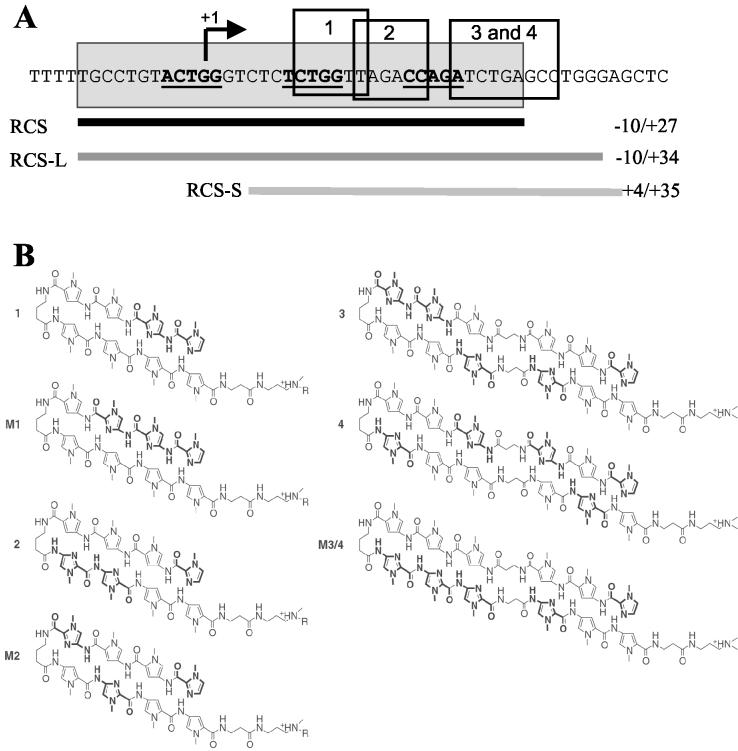
Three binding motifs for LSF exist within the repressor complex sequence (RCS) (Fig. 1A and references 13 and 14), a region from −10 to +27 with respect to the site of LTR transcription initiation. LSF recruits YY1 to the RCS. YY1 cannot bind the RCS in the absence of LSF. YY1 then recruits HDAC1, mediating LTR repression (6, 13, 14).
FIG. 1.
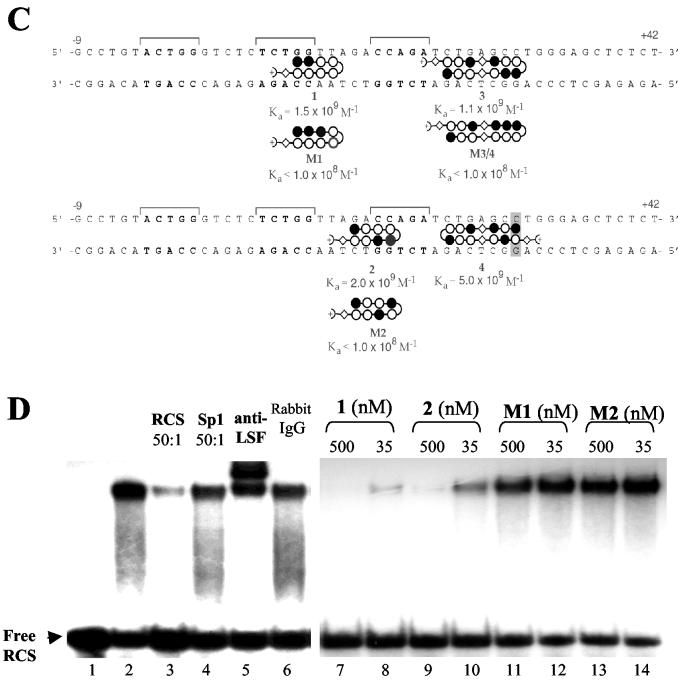
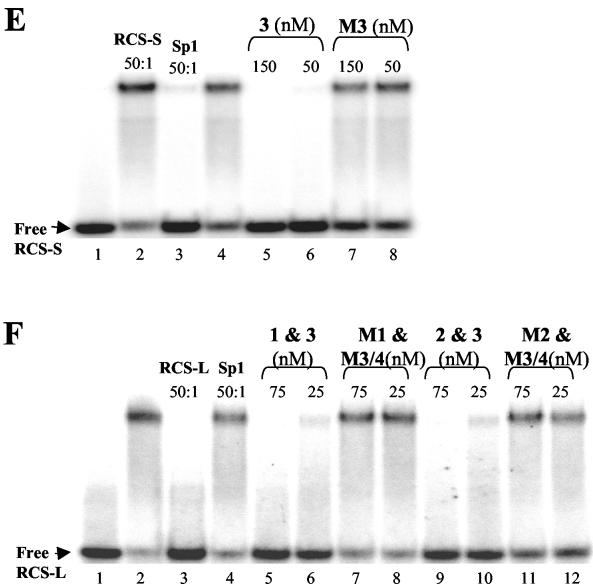
RCS-binding polyamides specifically inhibit LSF binding to the RCS of the HIV-1 LTR in vitro. (A) Sequences of the RCS (gray)- and LSF-binding motifs (underlined), sequences targeted by polyamides 1, 2, 3 and 4 (boxed), and oligonucleotide probes (thick lines below) used in the EMSA. (B) Chemical structure of each polyamide (1, 2, 3, and 4) and their mismatched controls (M1, M2, and M3/4). (C) Ball-and-stick model of polyamides juxtaposed to their target sites; the Ka value for each molecule is indicated. Polyamide 4 binds in a 3′ to 5′, N to C orientation as a single mismatch at a discrete site with high affinity and selectivity. (D) EMSA of RCS alone (lane 1) and that retarded by his-LSF (lane 2). Lanes 3 and 4 show competition by unlabeled RCS or Sp1 consensus binding sequence oligonucleotides (50:1 ratio of competitor to probe), and lanes 5 and 6 show supershift induced by the addition of antibody to LSF or nonspecific immunoglobulin G (IgG). Lanes 7 to 14 show competition by polyamides 1, 2, M1, and M2 (the concentration of the polyamide is shown). (E) EMSA of RCS-S (+4 to +35) alone (lane 1) and that retarded by his-LSF (lane 2), and competition by RCS or Sp1 (lanes 3 and 4), polyamide 3 (lanes 5 and 6), or M3/4 (lanes 7 and 8). (F) EMSA of RCS-L (−10 to +34) alone (lane 1) and that retarded by his-LSF (lane 2), and competition by RCS or Sp1 (lanes 3 and 4), polyamides 1 and 3 (lanes 5 and 6), M1 and M3/4 (lanes 7 and 8), 2 and 3 (lanes 9 and 10), or M2 and M3/4 (lanes 11 and 12).
Polyamides are synthetic small molecules containing N-methylpyrrole and N-methylimidazole amino acids, designed to bind to specific DNA sequences with affinities comparable to those of natural DNA-binding transcriptional regulatory proteins (1-3). Pyrrole-imidazole polyamides can modulate gene expression by preventing the binding of transcriptional activators or repressors in vitro (7, 8, 12). By using polyamides, we now directly demonstrate that blockade of LSF binding in the context of host chromatin increases LTR expression.
Polyamides (Fig. 1A to C) were synthesized as described previously (1). Quantitative DNase I footprinting titration assays (data not shown) demonstrated that polyamides 1, 2, 3, and 4 exclusively bound the target sites with equilibrium association constant (Ka) values of >109 M−1, while mismatch polyamides M1, M2, and M3/4 containing a single altered residue bound with Ka values of ≪108 M−1. Electrophoresis mobility shift assays (EMSA) performed as previously described (6, 14) demonstrated that 35 to 50 nM of match polyamides each specifically inhibited the binding of 60 ng of his-LSF to the RCS oligonucleotide, while mismatched control polyamides did not (Fig. 1D and E). As polyamides bind poorly near the ends of oligonucleotides, RCS-S was used to study polyamides 3 and 4 and RCS-L was used to study the combination of polyamide 3 or 4 with polyamide 1 or 2. The potency of inhibition was increased when polyamides were used in combination (Fig. 1F). A total of 0.5 μM match polyamides were required to inhibit complex formation at the RCS when EMSAs were performed with nuclear extract (data not shown). Multiprotein complexes may be more resistant to inhibition by polyamides.
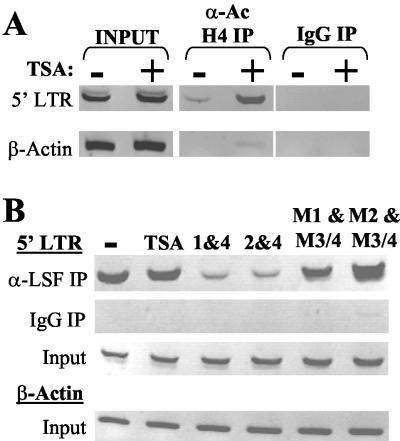
We then directly measured the ability of polyamides to specifically alter promoter occupancy by using chromatin immunoprecipitation (ChIP) assays (11, 17). These were performed as described previously (11) by using a HeLa cell line containing a single chromosomally integrated copy of the LTR linked to a chloramphenicol acetyltransferase (CAT) reporter gene (5). Consistent with results of previous DNase protection studies (9, 16), ChIP repeatedly demonstrated that histone acetylation near Nuc-1 and the RCS increased following treatment with the HDAC inhibitor trichostatin (TSA) (Fig. 2A). Serial dilutions of input DNA demonstrated that doubling of the DNA input results in a less than twofold increase in ChIP PCR product (11). Therefore, any change of more than twofold was deemed significant. A greater than fourfold increase in acetylated histone H4 near the LTR Nuc-1 site, as measured by titration of DNA input, was induced by TSA treatment. The β-actin promoter, an additional input control, was little affected by the global effects of TSA and showed changes of twofold or less (Fig. 2A and data not shown).
FIG. 2.
Polyamides block binding of LSF to the RCS in vivo. ChIP assays show an increase of acetylated histone H4 near Nuc-1 of the LTR after treatment with TSA and a decrease in LSF at this region of the LTR after exposure to RCS-binding polyamides. (A) PCR amplification of the 5′ LTR or β-actin promoter performed by using cell extracts (−) and cells exposed to TSA (+). Shown are products of ChIP and PCR amplification of the 5′ LTR or β-actin promoter using anti-acetylated histone H4 or mock immunoprecipitation (IP) with rabbit immunoglobulin G (IgG) and control PCR amplification of the 5′ LTR or β-actin promoter using extract prior to immunoprecipitation. Results are representative of three independent experiments. (B) ChIP with anti-LSF. PCR products of extracts of untreated cells, cells exposed to TSA, and polyamides 1 and 4, 2 and 4, M1 and M3/4, or M2 and M3/4. Shown are products of ChIP and PCR amplification of the 5′ LTR using anti-LSF or mock immunoprecipitation with rabbit immunoglobulin G and control PCR amplification of the 5′ LTR or β-actin promoter using extract prior to immunoprecipitation. PCR amplification of the β-actin promoter with extract following immunoprecipitation with anti-LSF yielded no product (data not shown). These two experiments are representative of four independent experiments.
To document that polyamides act directly to antagonize LSF binding to the LTR RCS site in the natural context of host chromatin, we then performed ChIP assays by using an anti-LSF antibody (14). Cells were either treated with 400 nM TSA or were exposed to 2 μM polyamides, and then ChIP assays were performed. The β-actin PCR product was equivalent before ChIP but, presumably due to the lack of LSF sites at the β-actin promoter, no product was detected after ChIP with α-LSF (data not shown).
As expected, TSA treatment resulted in no change in ChIP with anti-LSF (Fig. 2B). However, binding polyamides alone (data not shown) and in combination (Fig. 2B) reproducibly displaced LSF, as evidenced by a decrease of up to sixfold in ChIP. A modest, nonspecific increase in ChIP was sometimes observed when cells are treated with mismatch control polyamides. This two- to threefold increase was also observed by using polyamides targeted to the Ets-1 site of the LTR (8), a factor that binds in an upstream region of the LTR (data not shown). This nonspecific effect is most likely due to low-affinity interactions between polyamides and DNA fragments during ChIP. Nevertheless, the potent ability of specific RCS-binding polyamides to inhibit LSF binding results in an overall decrease in ChIP. Transfection of cells with a dominant-negative LSF construct, capable of forming inactive LSF heterodimers and blocking repression at the RCS (6), induced markedly diminished LSF occupancy at the RCS in ChIP assays (11). Therefore, ChIP of LSF at the RCS is inhibited both by specific polyamide blockade of the LSF binding site and by inhibiting the ability of LSF to bind this DNA site. These findings provide further evidence not only of the sequence-specific nature of polyamides and the specificity of the ChIP assay but they are also the first direct evidence that polyamides access chromatin and affect transcription factor occupancy within a living cell.
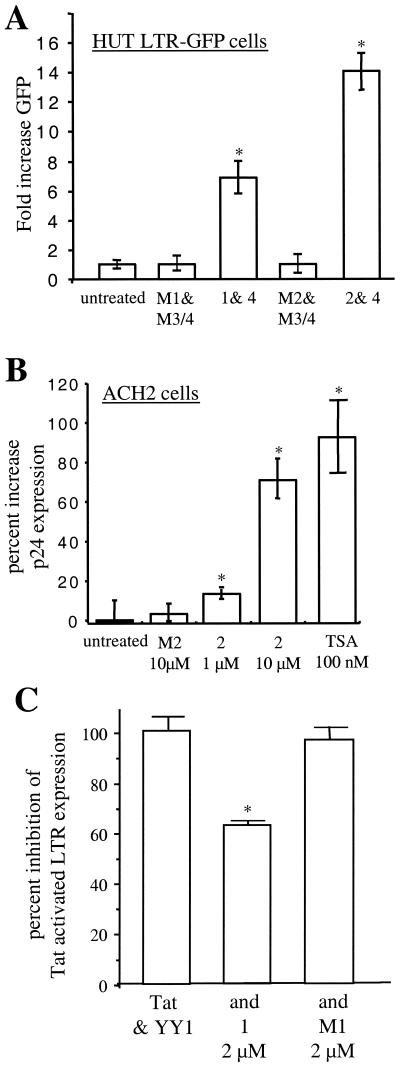
To illustrate that RCS-binding polyamides could affect basal LTR expression we studied the T-cell line HUT78-LTR-GFP (provided by J. V. Garcia), which contains an integrated HIV-1 LTR promoter linked to a green fluorescence protein (GFP) reporter gene. Cells were synchronized in media with 0.5% serum overnight, refed, and exposed to RCS-binding polyamides or mismatch control polyamides for 4 h. Mean GFP fluorescence intensity was then calculated. Fluorescence was not increased significantly (<5%) by mismatch control polyamides but was increased by up to 14-fold by RCS-binding polyamides (Fig. 3A ).
FIG.3.
RCS-binding polyamides modulate expression of an integrated HIV-1 LTR in tissue culture model systems. (A) RCS-binding polyamides (5 μM) induce an increase in expression of an integrated LTR-GFP reporter gene in HUT78 cells in the absence of Tat, as shown by an increased GFP fluorescence. Significant changes in mean fluorescence are indicated by an asterisk (P < 0.01); the mean of five independent samples was compared by using the unpaired t test. (B) HIV-1 p24 gag production was measured in triplicate cultures of ACH2 cells after 24 h of exposure to polyamide 2 or M2, 100 nM TSA, or medium control. An asterisk indicates statistically significant difference from the results of the control culture (P < 0.05 by unpaired t test). (C) Expression of the integrated reporter gene in HeLa-LTR-CAT can be activated by transfection of Tat, and this activation is blunted by cotransfection of YY1 (6, 11, 14). Inhibition of pAR-Tat activation (50 ng) by CMV-YY1 (1.5 μg) is expressed as 100%. The effect of exposure of cells to binding polyamide 1 or mismatch polyamide M1 is displayed. An asterisk indicates statistically significant difference from 100% repression (P < 0.05). Results of four independent transfections are shown.
To verify that polyamides induced a corresponding increase in viral production we tested the chronically infected ACH2 cell line (Fig. 3B). Cells were again synchronized in 0.5% serum overnight, refed, and exposed to RCS-binding polyamides, mismatch control polyamides, or TSA for 24 h. RCS-binding polyamides, but not mismatch polyamides, resulted in an increase in p24 production comparable to the effect of TSA.
Finally, as overexpression of YY1 can inhibit Tat-mediated LTR activation (6, 13, 14) we tested whether RCS-binding polyamides could block repression mediated by YY1. HeLa cells containing a single integrated LTR-CAT reporter gene were transfected as previously described (6) and were incubated with 2 μM polyamides. RCS-binding polyamides specifically blunted the ability of YY1 to block Tat activation (Fig. 3C).
In summary, we show that RCS-binding polyamides targeted to the HIV LTR can inhibit LSF binding to the RCS in vitro and can increase expression of the LTR. RCS-binding polyamides selectively upregulate expression of the integrated HIV-1 promoter in three different model systems. We observed increasing expression in HUT78 T cells in the absence of Tat and in viral expression in the ACH2 model of persistent infection, and we observed relief of YY1-induced repression in the HeLa LTR-CAT reporter system. Pyrrole-imidazole polyamides, which are synthetic cell-permeable small molecules, are novel and useful tools to inhibit the DNA-binding activity of transcription factors and directly modulate target gene activity in a living cell.
Our findings suggest that YY1, LSF, and HDAC1 may play an ongoing, active role in the establishment or maintenance of viral latency. Occupancy of the repressor complex at the LTR may exist in a state of equilibrium, with increased occupancy resulting in HDAC1 recruitment, nucleosome deacetylation, and transcriptional repression. Studies of primary cells obtained from HIV-infected donors to validate this model are ongoing.
FIG. 1—Continued.FIG. 1—Continued.
Acknowledgments
We thank H. Johnson for excellent technical assistance. We thank R. Koup and R. Gaynor for advice and J. V. Garcia, D. Sodora, and U. Hansen for advice and careful review. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH): HeLaCD4-CAT from Barbara K. Felber and George N. Pavlakis and ACH-2 from Thomas Folks.
This work was supported by an NIH postdoctoral grant (GM19789-02) to C.M., an NIH grant (AI 45297) to D.M.M., and an NIH grant (GM51747) to P.B.D.
REFERENCES
- 1.Baird, E. E., and P. B. Dervan. 1996. Solid-phase synthesis of polyamides containing imidazole and pyrrole amino acids. J. Am. Chem. Soc. 118:6141-6146. [Google Scholar]
- 2.Bremer, R. E., E. E. Baird, and P. B. Dervan. 1998. Inhibition of major-groove-binding proteins by pyrrole-imidazole polyamides with an Arg-Pro-Arg positive patch. Chem. Biol. 5:119-133. [DOI] [PubMed] [Google Scholar]
- 3.Cho, J., M. E. Parks, and P. B. Dervan. 1995. Cyclic polyamides for recognition in the minor groove of DNA. Proc. Natl. Acad. Sci. USA 92:10389-10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chun, T.-W., L. Carruth, D. Finzi, X. Shen, J. A. DiGiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T. C. Quinn, Y. H. Kuo, R. Brookmeyer, M. A. Zeiger, P. Barditch-Crovo, and R. F. Siliciano. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183-188. [DOI] [PubMed] [Google Scholar]
- 5.Ciminale, V., B. K. Felber, M. Campbell, and G. N. Pavlakis. 1990. A bioassay for HIV-1 based on Env-CD4 interaction. AIDS Res. Hum. Retrovir. 6:1281-1287. [DOI] [PubMed] [Google Scholar]
- 6.Coull, J., F. Romerio, J.-M. Sun, J. M. Volker, K. M. Galvin, J. R. Davie, Y. Shi, U. Hansen, and D. M. Margolis. 2000. The human factors YY1 and LSF repress the human immunodeficiency virus type-1 long terminal repeat via recruitment of histone deacetylase 1. J. Virol. 74:6790-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickinson, L. A., J. W. Trauger, E. E. Baird, P. B. Dervan, B. J. Graves, and J. M. Gottesfeld. 1999. Inhibition of Ets-1 DNA binding and ternary complex formation between Ets-1, NF-κB, and DNA by a designed DNA-binding ligand. J. Biol. Chem. 274:12765-12773. [DOI] [PubMed] [Google Scholar]
- 8.Dickinson, L. A., J. W. Trauger, E. E. Baird, P. Ghazal, P. B. Dervan, and J. M. Gottesfeld. 1999. Anti-repression of RNA polymerase II transcription by pyrrole-imidazole polyamides. Biochemistry 38:10801-10807. [DOI] [PubMed] [Google Scholar]
- 9.El Kharroubi, A., and E. Verdin. 1994. Protein-DNA interactions within DNase I-hypersensitive sites located downstream of the HIV-1 promoter. J. Biol. Chem. 269:19916-19924. [PubMed] [Google Scholar]
- 10.Finzi, D., M. Hermankova, T. Pierson, L. M. Carruth, C. Buck, R. E. Chaisson, T. C. Quinn, K. Chadwick, J. Margolick, R. Brookmeyer, J. Gallant, M. Markowitz, D. D. Ho, D. D. Richman, and R. F. Siliciano. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295-1300. [DOI] [PubMed] [Google Scholar]
- 11.He, G., and D. M. Margolis. 2002. Counter-regulation of chromatin acetylation and histone deacetylase occupancy at the integrated promoter of human immunodeficiency virus type 1 (HIV-1) by the HIV-1 activator Tat and the HIV-1 repressor YY1. Mol. Cell. Biol. 22:2965-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mapp, A. K., A. Z. Ansari, M. Ptashne, and P. B. Dervan. 2000. Activation of gene expression by small molecule transcription factors. Proc. Natl. Acad. Sci. USA 97:3930-3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margolis, D. M., M. Somasundaran, M. R. Green. 1994. Human transcription factor YY1 represses human immunodeficiency virus type-1 transcription and virion production. J. Virol. 68:905-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romerio, F., M. Gabriel, and D. M. Margolis. 1997. Repression of human immunodeficiency virus type-1 through the novel cooperation of the human factors YY1 and LSF. J. Virol. 71:9375-9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheridan, P. L., T. P. Mayall, E. Verdin, and K. A. Jones. 1997. Histone acetyltransferases regulate HIV-1 enhancer activity in vitro. Genes Dev. 11:3327-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verdin, E., P. Paras, Jr., and C. Van Lint. 1993. Chromatin disruption in the promoter of human immunodeficiency virus type 1 during transcriptional activation. EMBO J. 12:3249-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orlando, V., H. Strutt, and R. Paro. 1997. Analysis of chromatin structure by in vivo formaldehyde cross-linking. Methods 11:205-214. [DOI] [PubMed] [Google Scholar]
- 18.Wong, J. K., M. Hezareh, H. F. Gunthard, D. V. Havlin, C. I. Ignacio, C. A. Spina, and D. D. Richman. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278:1291-1295. [DOI] [PubMed] [Google Scholar]