Abstract
Acetylation of signaling molecules can lead to apoptosis or differentiation of carcinoma cells. The molecular mechanisms underlying these processes and the biological role of enzymes mediating the transfer or removal of an acetyl-group are currently under intense investigation. Our study shows that Stat1 is an acetylated protein. Stat1 acetylation depends on the balance between Stat1-associated histone deacetylases (HDACs) and histone acetyltransferases (HATs) such as CBP. Remarkably both inhibitors of HDACs and the cytokine interferon α alter this equilibrium and induce Stat1 acetylation. The analysis of Stat1 mutants reveals Lys 410 and Lys 413 as acetylation sites. Experiments with Stat1 mutants mimicking either constitutively acetylated or nonacetylated states show that only acetylated Stat1 is able to interact with NF-κB p65. As a consequence, p65 DNA binding, nuclear localization, and expression of anti-apoptotic NF-κB target genes decrease. These findings show how the acetylation of Stat1 regulates NF-κB activity and thus ultimately apoptosis.
Keywords: Stat1, NF-κB, acetylation, histone deacetylase, Interferon α, HDAC inhibitor
Many signal transduction pathways ultimately result in the post-translational modification of histones, which determines the expression of genes important for cell growth, differentiation, and apoptosis (Wolffe and Hayes 1999; Schreiber and Bernstein 2002). Acetylation of the N-terminal tails of histones correlates with gene activation, while histone deacetylation mediates transcriptional repression (Strahl and Allis 2000). It has also become clear that regulated acetylation of nonhistone proteins determines cellular fate and survival (Blobel 2000; Kouzarides 2000; Cohen et al. 2004). The fine-tuned equilibrium of protein acetylation and deacetylation is maintained by histone acetyltransferases (HATs) and histone deacetylases (HDACs) (Kouzarides 1999). This constitutes a precise and dynamic regulatory system modulating protein assemblies in response to external and internal signals. Protein acetylation therefore appears to play a role that is comparable to phosphorylation (Schreiber and Bernstein 2002).
HDAC inhibitors (HDACi) have been shown to change the expression pattern of genes involved in differentiation, cell cycle arrest, and apoptosis, and they are considered as candidate drugs for cancer therapy (Krämer et al. 2001; Kelly et al. 2002; Melnick and Licht 2002). However, the molecular mechanisms underlying cell-specific modulation of signaling pathways by HDACi and factors determining sensitivity toward these compounds are still subject to intense investigation (Mayo et al. 2003). Several recent reports suggest that HDACi-induced apoptosis depends on the expression of Jun, Bcl-2 proteins, p21WAF/CIP1, p53, NF-κB, and Akt (Vrana et al. 1999; Henderson et al. 2003; Mayo et al. 2003). For some of these proteins the association with HDACs and/or acetylation of lysine residues has been shown (Kouzarides 2000; Chen and Greene 2003; Kiernan et al. 2003; Weiss et al. 2003).
In the case of NF-κB, which contributes significantly to anti-apoptotic signaling (Perkins 2004), HDACi were shown to repress NF-κB signaling and expression of several NF-κB target genes (Huang et al. 1997; Inan et al. 2000; Krämer et al. 2001). Given the critical role of NF-κB in tumorigenesis, several studies were undertaken to identify factors influencing this tumor promoter (Perkins 2004). It became clear that NF-κB signaling is controlled at several levels by regulatory proteins, such as the I-κB protein family. Furthermore, Stat1 has been suggested to repress NF-κB-mediated signaling (Wang et al. 2000; Suk et al. 2001; Shen and Lentsch 2004), and several stimuli induce apoptosis to a significantly greater extent in a Stat1-positive cellular background (Kumar et al. 1997; Meyer et al. 2002). Similar to NF-κB, Stat1 associates with HATs and HDACs (Korzus et al. 1998; Nusinzon and Horvath 2003). Stat1 regulates the expression of gene products mediating various cellular processes constitutively or inducibly by growth factors and cytokines such as interferons in a phosphorylation-dependent manner (Chatterjee-Kishore et al. 2000; Ihle 2001). Induction of interferon signaling has been shown to activate the apoptotic program, which indicates that these cytokines could be useful in cancer therapy (de Vries et al. 2003). Remarkably, tyrosine phosphorylation of Stat1 and its transcriptional activity appear to be dispensable for NF-κB inhibition and apoptosis induction in response to certain stimuli (Wang et al. 2000; Meyer et al. 2002). However, it is still unclear which other post-translational modifications are involved in this process and whether conditions exist in which Stat1 and NF-κB can interact with each other.
We investigated the molecular mechanisms underlying the induction of apoptosis and the modulation of signaling pathways by HDACi and interferon α in human melanoma cell lines. Our results show that cells undergoing apoptosis in response to such substances increase expression and acetylation of Stat1. This leads to altered interactions of Stat1 with HDACs, CBP, and NF-κB. Our results indicate that the acetylation of Stat1 functions as a molecular switch, which permits binding of Stat1 to NF-κB and thus reduces NF-κB signaling. This mechanism appears to be critical for the induction of cell death by pharmacological and physiological stimuli.
Results
Response of human melanoma cells to HDACi
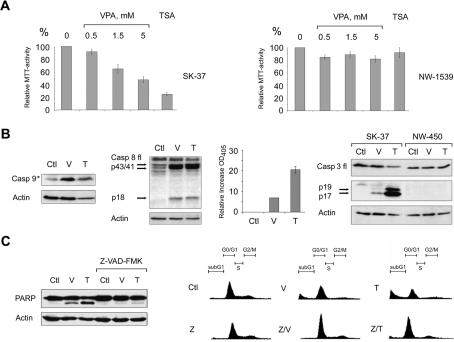
HDACi can induce growth arrest and apoptosis in tumor cells of different origin (Krämer et al. 2001; Kelly et al. 2002). We found significant differences in the sensitivity of various melanoma cell lines toward these compounds. SK-37 cells showed strong growth reduction in the MTT assay upon HDACi treatment, whereas NW-1539 cells were not affected significantly at the inhibitor concentrations used (Fig. 1A). These cell lines are prototypical examples and have characteristics similar to other melanoma cell lines that are either HDACi sensitive (e.g., MZ-19) or resistant (e.g., NW-450).
Figure 1.
HDACi induce apoptosis in SK-37 cells. (A) The proliferation of SK-37 and NW-1539 melanoma cells was determined by MTT test after exposure to VPA (0.5–5 mM) or TSA (100 nM) for 48 h (SK-37) or 72 h (NW-1539); (0) untreated cells. (B) Induction of activated caspase 9 (Casp 9, activation denoted by an asterisk) and cleavage of full-length caspase 8 (Casp 8 fl) into the active subunits p43/41/18 were detected by Western blot after treatment of SK-37 cells with VPA (V, 1.5 mM) or TSA (T, 100 nM) for 48 h. Caspase 3 activity was measured by conversion of Ac-DEVD-pNA to pNA, which has an absorption peak at 405 nm. This increase is given relative to the activity of lysates from untreated cells (Ctl). HDACi-induced conversion of full-length caspase 3 (Casp 3 fl) to the active p17/19 subunits was analyzed in SK-37 and NW-450 cells. (C) Proteolytic cleavage of PARP and apoptotic chromatin fragmentation induced by VPA (1.5 mM) or TSA (100 nM) after 48 h were detected by Western blot and PI FACS analysis. Cotreatment of SK-37 cells with Z-VAD-FMK (Z, 100 μM) blocks HDACi-induced apoptosis.
To investigate the molecular mechanisms underlying this differential response, we evaluated whether the reduced proliferation due to HDACi relies on proapoptotic properties and effects on caspases (Thornberry and Lazebnik 1998). In the HDACi-sensitive SK-37 cell line, we detected activation of the initiator caspases 8 and 9 after treatment (Fig. 1B). Furthermore, we measured caspase 3 activity in extracts from these cells by colorimetric assay. Conversion of the proenzyme form of the executioner caspase 3 (p32) to the catalytically active proteases p17 and p19 was detectable in SK-37 but not in HDACi-resistant NW-450 cells (Fig. 1B).
Activation of caspase 3 during HDACi-mediated apoptosis of SK-37 cells was also verified by examining the cleavage of PARP (116 kDa) into 85- and 28-kDa fragments (Thornberry and Lazebnik 1998). The pan-caspase inhibitor Z-VAD-FMK inhibited PARP cleavage as well as the occurrence of a hypodiploid (sub-G1) fraction resulting from DNA fragmentation in SK-37 cells (Fig. 1C). Analysis of nuclei stained with Hoechst dye gave similar results (data not shown). These observations confirm that HDACi trigger apoptotic, caspase-dependent pathways in SK-37 melanoma cells (Fig. 1C). Furthermore, no signs of nonspecific cell permeabilization and necrotic cell death were found in a PI/Hoechst staining assay (data not shown).
Alteration of Stat1 gene expression after HDAC inhibition
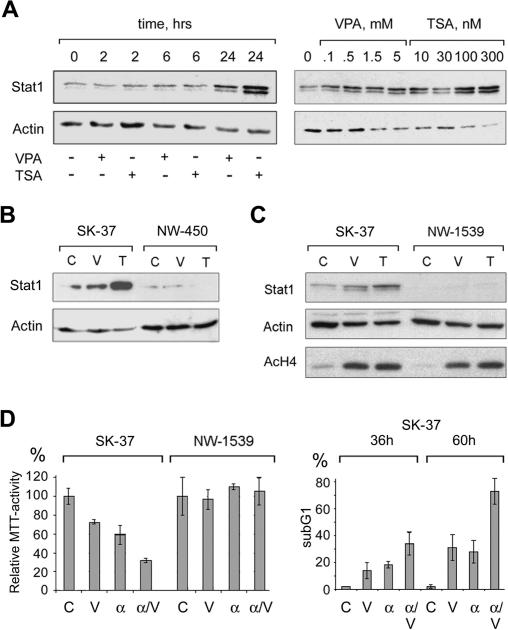
We employed microarray and Western blot analyses to define alterations in gene expression patterns after incubation with HDACi. These assays revealed a time- and dose-dependent increase in Stat1 expression at the mRNA and protein level in SK-37 (Fig. 2A) and several other HDACi-sensitive cell lines. Treatment of SK-37 cells with HDACi and cycloheximide showed that the HDACi-induced increase in Stat1 expression was dependent on de novo protein synthesis (data not shown). Hence, an increase in Stat1 stability due to reduced HDAC activity cannot account for higher Stat1 expression levels. Intriguingly, HDACi-resistant cell lines, such as NW-450 and NW-1539, did not undergo HDACi-induced caspase 3 cleavage and apoptosis (Fig. 1A,B) and expressed very low levels of Stat1, which were not induced by HDACi (Fig. 2B,C). Since no significant difference in HDACi-induced histone hyperacetylation was detected between NW-1539 and SK-37 cells, HDACi were equally effective in blocking HDAC activity in both cell lines (Fig. 2C). This result indicates that not only inhibition of HDACs but also the presence of Stat1 is crucial for HDACi-mediated apoptosis in melanoma cells. Consistent with previous reports (Wong et al. 2002), we detected a strong increase in Stat1 expression in the Stat1-positive SK-37 cell line, but not in NW-1539 cells treated with interferon α. Moreover, cotreatment with HDACi further increased Stat1 expression in SK-37 cells (data not shown). This correlated with enhanced induction of apoptosis as assessed by MTT and FACS analysis (Fig. 2D).
Figure 2.
Correlation of Stat1 expression and apoptosis induction. (A) The time- and dose-dependent increase of Stat1 expression was investigated by Western blot. SK-37 cells were exposed to 1.5 mM VPA or 100 nM TSA for the indicated periods of time. Alternatively, cells were treated for 24 h with different concentrations of VPA (0.1–1.5 mM) or TSA (10–300 nM) as indicated or left untreated (0). (B) Expression of Stat1α in SK-37 and NW-450 melanoma cells treated with 1.5 mM VPA (V) or 100 nM TSA (T) for 24 h or left untreated (C) was analyzed by Western blot. (C) Expression of Stat1 and accumulation of hyperacetylated histone H4 (AcH4) in SK-37 and NW-1539 cells were analyzed after 24 h by Western blot. Cells were treated with VPA (V, 1.5 mM) or TSA (T, 30 nM) or left untreated (C). (D) Sensitivity of melanoma cell lines to VPA (V, 1.5 mM) and interferon α (α, 103 U/mL) was determined by MTT assay. Enhanced induction of apoptosis after treatment of SK-37 cells with VPA and interferon α (α/V) was detected by PI FACS analysis.
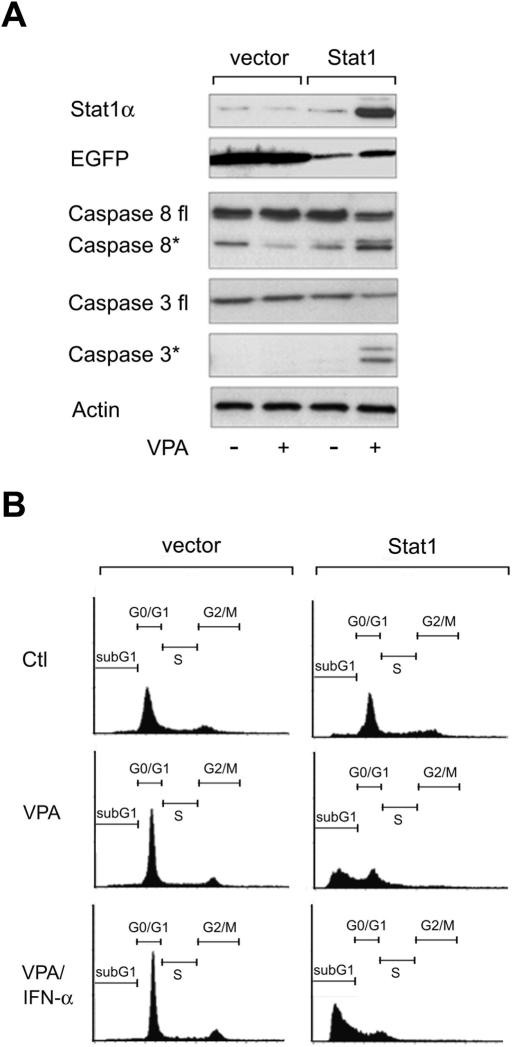
These results prompted us to investigate whether Stat1α is required for HDACi-induced apoptosis in NW-1539 cells. We transduced these cells with a lentiviral vector expressing Stat1α. Indeed, sensitivity toward HDACi was conferred to Stat1α-transduced NW-1539 cells but not to cells which received only the vector encoding GFP (Fig. 3A,B; data not shown). Hence, Stat1 expression levels appear to determine the response of this cell line to HDACi. Furthermore, introduction of Stat1α renders these cells susceptible to enhanced apoptosis induction by VPA and interferon α (Fig. 3B). However, interferon α alone did not induce apoptosis and consistently did not induce Stat1 in this cell line. Similar results were obtained with the Stat1-negative cell line U3A (Müller et al. 1993) reconstituted with Stat1α, albeit with less pronounced apoptosis induction (data not shown).
Figure 3.
Stat1 sensitizes resistant melanoma cells to HDACi and interferon α. (A) Western blot analysis was employed to detect Stat1 expression and induction of apoptosis in NW-1539 cells transduced with SIEW (vector) or S-Stat1α-IEW (Stat1) and treated with VPA (1.5 mM) for 48 h. Asterisks denote activated forms of full-length caspase 3 or caspase 8. (B) DNA fragmentation was analyzed by PI FACS analysis after treatment with VPA (1.5 mM) or VPA and interferon α (103 U/mL) for 60 h. (Ctl) Untreated cells.
Our results clearly show that HDACi induce Stat1 in a cell type-specific manner. However, microarray analyses gave no evidence for increased expression of Stat1 target genes as a result of HDAC inhibition in SK-37 cells. Therefore, the activity of Stat1 in HDACi-induced apoptosis is likely to involve nongenomic effects, such as cross-talk with other signaling pathways.
HDACi modulate NF-κB activity
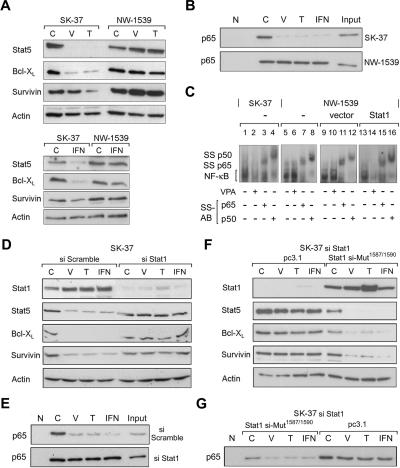
To identify such signaling pathways, gene expression analyses can provide valuable information. Since a critical role of NF-κB for HDACi-induced apoptosis has been described, we analyzed the expression of NF-κB-dependent genes in the HDACi-sensitive cell line SK-37. Our data indicate HDACi-dependent repression of NF-κB target genes such as Bcl-XL, survivin, and Stat5 (Fig. 4A). These results confirm several reports describing effects of HDACi on these genes (Eickhoff et al. 2000; Krämer et al. 2001; Hinz et al. 2002; De Schepper et al. 2003). On the other hand, expression of NF-κB-regulated genes remained unaltered in HDACi-resistant NW-1539 cells, which expressed hardly any Stat1 (Fig. 4A). Treatment with the cytokine interferon α similarly led to repression of these genes in SK37 but not in NW-1539 cells (Fig. 4A).
Figure 4.
Stat1 interferes with NF-κB function. (A) Expression of NF-κB target genes after HDAC inhibition and treatment with interferon α was investigated by Western blot analysis of SK-37 and NW-1539 cell lysates. Cells were incubated with 1.5 mM VPA (V), 30 nM TSA (T), or 103 U interferon α (IFN), or left untreated (C) for 48 h. (B) ABCD-assay with a biotinylated NF-κB consensus oligo was used to detect NF-κB–DNA binding under conditions described in A. (N) Nonrelevant biotinylated oligos. (C) NF-κB DNA binding was analyzed by EMSA of lysates from SK-37, NW-1539, and transduced NW-1539 cells (vector or Stat1) that were either untreated or treated with VPA (1.5 mM) for 48 h. Identity of the NF-κB–DNA complex was verified by p65 and p50 antibody supershifts (SS-AB). (D) NF-κB target gene expression was investigated in lysates of SK-37 cells transduced with an siRNA vector encoding scrambled RNA or an si-sequence against Stat1. Experimental conditions are as described in A. (E) NF-κB–DNA binding was investigated in lysates of SK-37 cells by ABCD-assay. Cells and conditions are as described in A. (F) NF-κB target gene expression was investigated in lysates of SK-37 cells transduced as stated in D and transfected with empty vector or pc3 HA-Stat1 containing mutations conferring resistance against the siRNA. Experimental conditions are as described in A. (G) NF-κB–DNA binding was investigated in lysates of SK-37 cells by ABCD-assay. Cells and conditions are as described in E.
Data shown in Figures 2C and 4A indicate that Stat1α expression inversely correlates with the activity of NF-κB after HDACi treatment or interferon α stimulation. Therefore, we employed a binding assay with a biotinylated NF-κB consensus site oligonucleotide to investigate whether DNA binding of NF-κB might be affected in cells expressing different amounts of Stat1. Decreased affinity of NF-κB for a cognate DNA sequence was observed after treatment with HDACi or interferon α only in cells expressing Stat1 (Fig. 4B). EMSAs confirmed functional impairment of NF-κB p65/p50 binding to its DNA sequence in extracts from HDACi-treated Stat1-positive SK-37 cells (Fig. 4C, left). EMSAs performed with the same lysates and an oligonucleotide specific for Stat3 showed no decrease in DNA binding, which rules out nonspecific effects of HDACi treatment on the DNA binding of transcription factors (data not shown). Reduced affinity of NF-κB for DNA after HDAC inhibition was also observed with Stat1α-transduced NW-1539 cells, but not in parental or vector-transduced Stat1-negative NW-1539 cells (Fig. 4C, cf. lanes 5,9,13 and 6,10,14). Hence, DNA binding of NF-κB after treatment with HDACi is only reduced in the presence of Stat1α, suggesting a link of both signaling pathways.
We challenged the proposed role of Stat1 in regulating NF-κB function by transduction of SK-37 cells with lentiviral vectors encoding small interfering RNA (siRNA) directed against Stat1 or a nonrelevant sequence. We observed that basal and induced Stat1 levels were reduced only in cells that received the siStat1 vector. In control-transduced SK-37 cells, HDACi or interferon α still decreased the expression of NF-κB target genes as well as NF-κB DNA binding. However, NF-κB functions were no longer affected under identical conditions in SK-37 cells subjected to a constitutive siRNA-mediated knock-down of Stat1 levels (Fig. 4D,E). In a rescue experiment we transfected a mutated siRNA-resistant Stat1 vector into the Stat1 knock-down SK-37 cells. Consequently, a strong repression of NF-κB functions by HDACi and interferon α could again be induced. Moreover, high levels of Stat1 expression caused a reduction of NF-κB activity independent of these substances. These data demonstrate that Stat1 is a crucial regulator of NF-κB in vivo (Fig. 4F,G).
The localization of nuclear p65 is modulated by Stat1 and HDACi
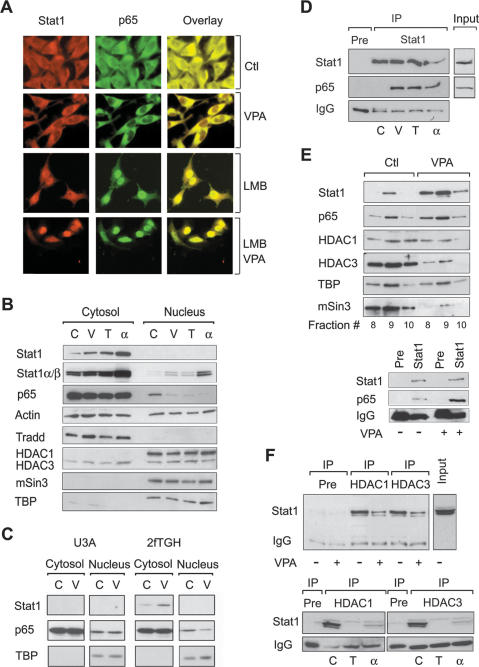
We gained further insights into the mechanism underlying the regulation of NF-κB in SK-37 cells by analyzing its subcellular distribution. In situ immunofluorescence analysis showed a shift of NF-κB p65 from the nucleus to the cytosol and an increased colocalization with Stat1α in the cytosol when HDACs were inhibited or cells were treated with interferon α (Fig. 5A; data not shown). Treatment of cells with the nuclear export inhibitor LMB prevented basal and HDACi-induced export of NF-κB p65 and caused nuclear accumulation of Stat1α and p65. Again, colocalization of these proteins was enhanced (Fig. 5A).
Figure 5.
Complex formation and localization of Stat1α and NF-κB p65. (A) Interaction and colocalization of Stat1α and NF-κB p65 in SK-37 cells treated with VPA (1.5 mM, 24 h) and/or LMB (10 nM) were analyzed by immunofluorescence microscopy. (Ctl) Untreated. (B) Exclusion of p65 from the nuclear compartment after treatment of SK-37 cells with VPA (V, 1.5 mM), TSA (T, 100 nM), or interferon α (α, 103 U) for 24 h was confirmed by cellular fractionation and p65 Western blot. (C) Untreated. Reprobing was done with antibodies against Stat1. The affinity of the Stat1α antibody is not sufficient to detect nuclear Stat1. All other proteins detected serve as loading and fractionation controls. (C) U3A and 2fTGH cells were analyzed for cytoplasmic retention of p65 after incubation with 1.5 mM VPA (V) for 24 h by Western blot of cytosolic and nuclear fractions. (D) Interaction of Stat1α and NF-κB p65 in SK-37 cell lysates was investigated by Western blot of specific IPs as described in B (IP, Pre [preimmune serum], Input). (E) The composition of Stat1 complexes in SK-37 cells after treatment with VPA (1.5 mM, 48 h) was investigated by Western blot analysis of Superose 6 column fractions. (Lower panel) IP was used to verify the interaction of Stat1α with NF-κB. (F) HDAC1 and HDAC3 were precipitated from whole-cell extracts (IP). Western blot analysis was performed with an antibody against Stat1α/β. Treatment conditions are as described in D.
The analysis of cytosolic and nuclear fractions of SK-37 cells by Western blot indicates that Stat1 expression increased both in the cytosol and in the nucleus (Fig. 5B). In these cells, as in many other cancer cell lines, low constitutive amounts of NF-κB p65 reside in the nucleus and help to attenuate proapoptotic signaling. For p65 a clear reduction in the nuclear compartment was observed after treatment with HDACi as well as interferon α, which confirms our microscopy results. These data suggest that removal of p65 from the nucleus and interaction with Stat1 might be key steps in the HDACi-induced repression of NF-κB target genes and apoptosis induction. Another possible explanation, changes in NF-κB p65 expression, can be ruled out since p65 levels were not altered significantly (Fig. 5B). Western blot analysis of 2fTGH cells and their derived Stat1-negative cell line U3A confirmed the dependence on Stat1 for nuclear export of p65 upon HDAC inhibition (Fig. 5C). Hence, it appears plausible that HDACi and interferon α inhibit nuclear localization of p65 only in cells expressing Stat1.
HDACi induce the interaction of Stat1α and NF-κB
Having established a role of Stat1α in NF-κB signaling, we investigated whether these transcription factors could interact physically. First, we tested if this interaction is mediated by TRADD, which was shown to associate with Stat1α under certain conditions (Wang et al. 2000). However, in several immunoprecipitation (IP) experiments, we could not detect an HDACi-dependent interaction of Stat1α with TRADD in SK-37 cells (data not shown). On the other hand, precipitation of Stat1α or NF-κB p65 revealed an association of these proteins upon HDAC inhibition or interferon stimulation (Fig. 5D). Coimmunoprecipitation (co-IP) experiments performed with cytosolic and nuclear fractions confirmed our microscopy data showing that such complexes are located in the cytosol. Moreover, Stat1–NF-κB complex formation is likely to be DNA independent, since addition of ethidium bromide did not alter its stability (data not shown). Next, we analyzed Stat1 complexes by Superose 6 column fractionation of SK-37 cell extracts and found that in high molecular weight fractions the amount of Stat1 increased together with NF-κB p65 after VPA treatment (Fig. 5E). Notably, this complex accumulated in a time-dependent manner that paralleled apoptosis induction after HDAC inhibition. IP of Stat1α from these fractions followed by Western blotting against p65 showed that a weak basal interaction of these proteins increased upon HDAC inhibition (Fig. 5E).
We also analyzed the presence of HDACs in the Stat1 complex before and after HDAC inhibition or treatment with interferon α by IP and Western blot. The addition of HDACi to SK-37 cells led to reduced association of Stat1 with HDAC1 and HDAC3 (Fig. 5F). No binding of Stat1 to other class I HDACs (2 and 8) was observed (data not shown). Superose 6 fractionation substantiated these results and also revealed decreased comigration of Stat1α complexes with the corepressor mSin3 (Fig. 5E). Our observations not only confirm that Stat1 can interact with repressive cofactors but also indicate that HDACs dissociate upon interferon α or HDACi treatment.
Acetylation of Stat1
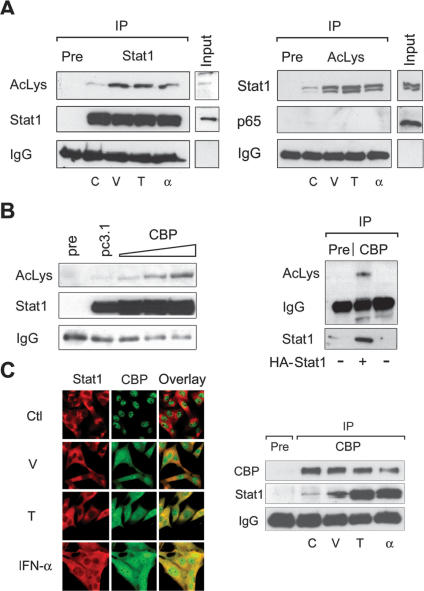
Recent publications show that the acetylation of proteins regulates multiple cellular processes (Kouzarides 2000; Cohen et al. 2004). We therefore tested whether Stat1 undergoes acetylation. Stat1α was immunoprecipitated from SK-37 whole-cell extracts with a monoclonal antibody under stringent lysis conditions in RIPA buffer. A pan-acetyl-lysine antibody recognized a band corresponding to the molecular weight of Stat1α (Fig. 6A). Reprobing with the monoclonal Stat1α antibody confirmed the acetylation signal as Stat1α. As expected, the basal acetylation level of endogenous Stat1α was increased after HDAC inhibition. Remarkably, long-term stimulation with interferon α also enhanced Stat1 acetylation (Fig. 6A). To obtain further evidence for the acetylation of Stat1, an anti-acetyl-lysine antibody was used for IP and Western blots were probed with an antibody against Stat1. In this experiment, the pan-acetyl-lysine antibody precipitated Stat1. Again, the acetylation level of Stat1 increased after HDACi or interferon α treatment and allowed recovery of increasing amounts of Stat1 from treated cells (Fig. 6A, right panel). These findings indicate that endogenous Stat1 is acetylated in vivo and that this modification can be increased by HDACi or interferon α (Fig. 6A). No acetylation of endogenous NF-κB p65 was detectable under these conditions, and p65 could not be precipitated with the pan-acetyl-lysine antibody (Fig. 6A; data not shown). This is consistent with reports stating the need for overexpression of a HAT to detect NF-κB acetylation in vivo (Chen and Greene 2003).
Figure 6.
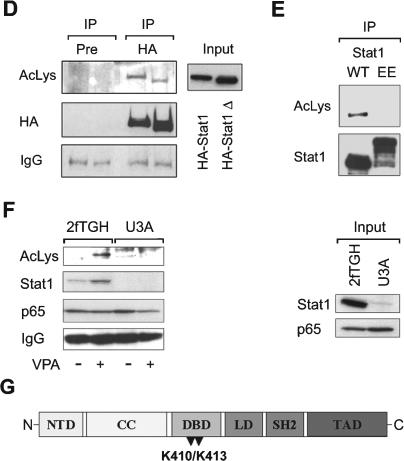
Acetylation of Stat1. (A) SK-37 cells were either treated with VPA (V, 1.5 mM), TSA (T, 30 nM), or interferon α (α, 103 U), or left untreated for 48 h. Endogenous Stat1α was immunoprecipitated from RIPA lysates and analyzed by Western blot with an antibody recognizing acetylated lysines (anti-AcLys, left). Reprobing of the same membrane confirms that the acetylation signal corresponds to Stat1α and shows efficacy and specificity of the IP. Anti-AcLys immunoprecipitates from RIPA lysates were probed with antibodies recognizing Stat1α/β or NF-κB p65. Preimmune serum was used as a control. Input lanes show 2% of the extract used for IP. (B) Increasing amounts of a CBP expression vector (1, 5, or 10 μg) were transfected into 293T cells. Stat1α was precipitated from RIPA lysates and probed with anti-AcLys. IPs with preimmune serum and IPs from cells transfected with the empty vector pc3.1 (10 μg) are controls. TNT-translated HA-Stat1 was acetylated in vitro as described (Gu and Roeder 1997) using immunoprecipitated CBP. (C) Interaction and colocalization of Stat1 and CBP in SK-37 cells were analyzed by immunofluorescence microscopy and IP of CBP. Cells were either treated with VPA (V, 1.5 mM), TSA (T, 30 nM), or interferon α (α, 103 U), or left untreated (C) for 48 h. (D) Acetylation levels of HA-Stat1ΔXbaI compared with full-length HA-Stat1α were determined by IP from 293T cell lysates as described in A. Cells were transfected with recombinant Stat1 and CBP vectors at a ratio of 5:1. (E) The experiment was performed as in D, except that HA-Stat1α or GFP-Stat1 410,413K → E were transfected. (F) NF-κB p65 was immunoprecipitated from 2fTGH or U3A cell extracts. The presence and acetylation of Stat1α were detected by Western blot as described in A. Cells were treated with 1.5 mM VPA for 24 h or left untreated. (G) Schematic representation of Stat1α showing positions of acetylated lysines and mutants generated. (NTD) N-terminal domain; (CC) coiled coil; (DBD) DNA-binding domain; (LD) linker domain; (TAD) transcriptional activation domain. Mutants are designated QQ (mutation of both K410 and K413 to Q) and RR (mutation of both K410 and K413 to R).
Transfection experiments with 293T cells revealed that increased expression of CBP, though not of p300, enhances Stat1 acetylation (Fig. 6B, left panel; data not shown). Moreover, we were able to acetylate Stat1α in vitro using immunoprecipitated CBP as an acetyltransferase (Fig. 6B, right panel). Ectopically expressed Stat1 was also found to be acetylated upon cotransfection of CBP (Fig. 6C). Considering that CBP can mediate the transfer of an acetyl-group to Stat1, we analyzed potential interactions of these proteins by microscopy and IP analysis. In untreated cells, CBP resided in the nucleus. However, upon treatment with HDACi and especially with interferon α, CBP translocated from the nucleus to the cytosol and displayed increased association with Stat1 (Fig. 6C).
A deletion mutant of Stat1 (ΔXbaI) lacking the C terminus including the Ser727 phosphorylation site retained acetylation, as indicated by a corresponding smaller band detected with an anti-acetyl-lysine antibody (Fig. 6D). Hence, Stat1 serine phosphorylation (Ihle 2001), which can be induced by VPA (Gurvich et al. 2004), is not critical for Stat1 acetylation. Considering that the Stat1 ΔXbaI mutant resembles Stat1β and the fact that Stat1α/β are precipitated with the anti-acetyl-lysine antibody (Fig. 6A, left panel), it is evident that both Stat1 splice variants can be acetylated.
To identify lysine residues in Stat1α that are subject to acetylation, several Stat1 lysine mutants (Horvath et al. 1996; Yang et al. 1999, 2002; Meyer et al. 2002) were expressed in 293T cells and immunoprecipitated. Western blot analysis with an antibody against acetylated lysine showed that only the Stat1 410,413K → E mutant (Meyer et al. 2002) was not acetylated under conditions in which wild-type Stat1 became strongly acetylated (Fig. 6E). Since this was not due to decreased interaction of this mutant with CBP (data not shown), Lys 410 and Lys 413 located within the Stat1 DNA-binding domain (DBD) are likely to be the major sites of acetylation.
Acetylation of Stat1α mediates p65-binding and confers susceptibility to apoptosis
Given that the acetylation of Stat1 correlates with the induction of apoptosis, interaction with p65, and repression of NF-κB signaling, we tested whether p65-associated Stat1α is acetylated in vivo. Indeed, an acetylated protein corresponding in size to Stat1α coprecipitated with NF-κB p65 from 2fTGH cell lysates after VPA treatment. We confirmed that this acetylated protein was indeed Stat1 by reprobing the membrane with a Stat1 antibody. Additionally, both the acetylation signal and the signal for Stat1 were not detectable in the Stat1-negative U3A cell line (Fig. 6F). Thus, we conclude that at least a fraction of p65-associated Stat1α is acetylated in vivo.
We hypothesized that acetylation of Stat1α might render cells sensitive to HDACi-induced apoptosis. Therefore, we tested whether induction of apoptosis is specifically due to the acetylation of lysines within the DBD of Stat1α. Lys 410 and Lys 413 were replaced either with glutamine (K → Q) or arginine (K → R) resembling constitutively acetylated or nonacetylated states, respectively (Fig. 6G). These experiments were conducted in NW-1539 and U3A cells and treatment with VPA was included as an additional control.
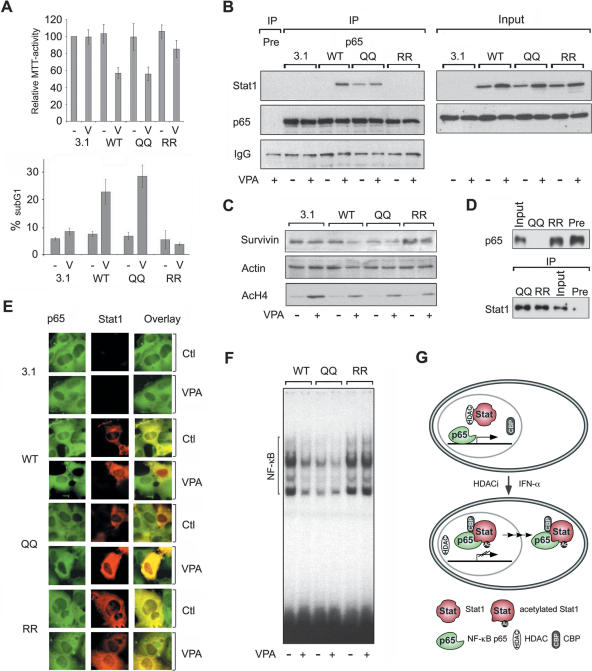
Proliferation and apoptosis of NW-1539 cells transfected with wild-type and mutant Stat1α expression vectors were scored by MTT and FACS analysis (Fig. 7A). Overexpression of wild-type Stat1 together with VPA treatment led to reduced proliferation and apoptosis induction (Fig. 7A). Transfection of the Stat1 mutant, in which K410 and K413 were replaced by glutamine (410,413K → Q) also reduced proliferation and even enhanced the rate of apoptosis of NW-1539 cells in response to HDACi treatment. In contrast, substitution of K410 and K413 with arginine (410,413K → R) could not render cells sensitive to apoptosis induction by HDACi (Fig. 7A). These data indicate that the acetylation of Stat1 and inhibition of NF-κB are required, though not sufficient, for the induction of apoptosis.
Figure 7.
Identification of Stat1α acetylation as critical regulator of HDACi-induced apoptosis. (A) NW-1539 cells were transfected with Stat1α (WT, wild type), lysine mutants, or equal amounts of empty vector (pc3.1). Proliferation and apoptosis were scored 72 h later by MTT and PI FACS analysis, respectively. (WT) Wild type; (QQ) 410,413K → Q; (RR) 410,413K → R; (–) untreated; (V) 1.5 mM VPA. (B) Interaction of overexpressed wild-type (WT) and mutant Stat1α (QQ, RR) with NF-κB p65 in U3A cells was analyzed by IP and Western blot. Cells were incubated with 1.5 mM VPA for 48 h or left untreated. Input lanes are 2% of the lysate used for IP and are shown at expositons allowing signal comparison. (C) U3A cells were transfected and treated as described in B. Survivin expression was analyzed by Western blot. Detection of actin and AcH4 serve as loading and treatment controls, respectively. (D) SK-37 nuclear lysates were incubated with HA-Stat1 (QQ, 410,413K → Q; RR, 410,413K → R) immunoprecipitated in RIPA buffer or a precipitate formed with a control antibody (pre). Ten microliters of input and 20 μL of depleted nuclear extract were loaded (upper panel). The lower panel shows equal Stat1 IP efficiencies. (E) NF-κB p65 localization was analyzed by immunofluorescence microscopy of NW-1539 cells transfected and treated as described in B. Note: Compare transfected and nontransfected cells within each field. (F) DNA binding of NF-κB was investigated by EMSA with cell lysates of NW-1539 cells transfected and treated as described in B. (G) Model for acetylation-dependent Stat1–NF-κB cross-talk.
Next, we examined whether ectopically expressed wild-type and mutant Stat1 proteins interact differentially with endogenous NF-κB p65. Equal expression of all Stat1 constructs was verified by Western blot (Fig. 7B). Immunoprecipitates of p65 were analyzed for the presence of Stat1 by Western blot. Results shown in Figure 7B confirm that treatment with VPA significantly enhances the association of wild-type Stat1α and p65 (see Fig. 5). Furthermore, the pseudo-acetylated Stat1α mutant 410,413K → Q constitutively bound p65 in vivo, whereas Stat1α 410,413K → R did not associate with p65 even after treatment with VPA (Fig. 7B).
When we analyzed the effect of Stat1 lysine mutations on the expression of the NF-κB target gene survivin, we found that Stat1α 410,413K → Q caused a significant decrease. In contrast, Stat1α 410,413K → R failed to reduce survivin expression under identical conditions (Fig. 7C). Figures 5A,B and 7E show that NF-κB p65 is depleted from the nucleus upon acetylation of Stat1 in vivo. We could confirm this result in vitro by demonstrating the efficient depletion of p65 from nuclear lysates by a pseudo-acetylated mutant of Stat1 (Fig. 7D).
Furthermore, immunofluorescence analysis showed that nuclear p65 was no longer detectable in NW-1539 cells if wild-type Stat1 was expressed ectopically and cells were treated with HDACi. Stat1α 410,413K → Q mimicked these effects, whereas expression of Stat1α 410,413K → R failed to increase cytoplasmic localization of p65 (Fig. 7E, cf. transfected and untransfected cells within each field). Moreover, expression of Stat1α 410,413K → Q in NW-1539 cells reduced DNA binding of NF-κB similar to overexpressed wild-type Stat1 in cells treated with VPA, whereas expression of Stat1α 410,413K → R did not (Fig. 7F). Thus, neither treatment of cells with an HDACi nor mere expression of Stat1 exerted unspecific effects on NF-κB. Based on these results, we propose a model in which acetylated Stat1α binds to and sequesters NF-κB p65 in the cytoplasm, thereby interfering with NF-κB function (Fig. 7G). Consequently, the susceptibility of cells to apoptosis induction depends on the presence and acetylation status of Stat1.
Discussion
Currently, the specific roles of acetyltransferases and deacetylases as well as their substrates are under intense investigation. Results based on these studies allow insights into the molecular mechanisms determining whether and how particular cell lines and types respond to changes in protein acetylation. Here, we show that in melanoma cell lines, resistance toward HDACi and interferon α-induced apoptosis inversely correlates with Stat1 expression and acetylation. The acetylation of Lys 410 and Lys 413 within the DNA-binding domain of Stat1 triggers its interaction with NF-κB p65. As a consequence, the level of nuclear p65 decreases significantly and DNA binding of NF-κB is inhibited. This leads to the down-regulation of anti-apoptotic NF-κB target genes, thus shifting the balance toward cell death. Based on our results, we propose a model of altered cross-talk between Stat1 and NF-κB signal transduction pathways that provides an explanation of how HDACi and interferon α down-regulate NF-κB activity.
HDACi resistant and sensitive melanoma cell lines
When we started to study the effects of HDACi on melanoma cell lines, we realized that they can be divided into sensitive and resistant subclasses. This allowed us to investigate the underlying molecular mechanisms in a set of cell lines derived from the same type of tumor. Our data indicate that sensitive cell lines (e.g., SK-37) undergo programmed cell death via both the extrinsic and the intrinsic apoptotic pathways (Fig. 1). In sensitive cell lines HDACi treatment significantly decreases the expression of anti-apoptotic genes such as Bcl-XL, Stat5, and survivin, which are bona fide target genes of NF-κB (Eickhoff et al. 2000; Krämer et al. 2001; Hinz et al. 2002; De Schepper et al. 2003). In resistant cell lines, on the other hand, neither changes in expression levels of these genes nor apoptosis induction are detectable, although hyperacetylation of histones is readily apparent (Figs. 2C, 4A).
A microarray analysis revealed that Stat1 is among those genes that are significantly up-regulated in sensitive melanoma cell lines in response to the HDACi VPA and TSA. These findings were confirmed at the protein level (Fig. 2A,B). Interestingly, Stat1 expression was very low (close to the detection limit) and not inducible in the HDACi-resistant cell lines NW-450 and NW-1539. Furthermore these cell lines, in contrast to HDACi-sensitive cells, were not affected by interferon α (Fig. 2C,D). To investigate whether Stat1 plays indeed a causative role in the induction of apoptosis in response to HDACi, we introduced Stat1α into NW-1539 cells by lentiviral transduction. Our results show that expression of Stat1 in NW-1539 restored sensitivity of this cell line toward HDACi and interferon α (Fig. 3A,B). Further experiments are required to establish whether this Stat1-dependent mechanism determining resistance or sensitivity toward HDACi represents a general principle relevant to many types of tumor cells. The HDACi-resistant cell lines were originally established from patients who had undergone immunotherapy including interferon α treatment. We speculate that during this process interferon α resistant cells with defects in Stat1 signaling were selected. In principle, both mutations within the Stat1 gene as well as epigenetic silencing could shut down Stat1 expression. Our observation that the resistant cell lines NW-450 and NW-1539 re-express Stat1 when treated with 5-aza-cytidine highlights the relevance of DNA methylation in this context (O.H. Krämer and T. Heinzel, unpubl.). HDACi and interferon α are being considered as candidate drugs for cancer therapy (Krämer et al. 2001; Kelly et al. 2002; de Vries et al. 2003). According to our data, the combination of HDACi and interferon α or demethylating agents could be particularly effective in the treatment of melanomas. If this were the case, Stat1 expression might serve as a useful marker for the prediction of clinical response.
Stat1–NF-κB cross-talk
We observed that the expression of a subset of NF-κB target genes after treatment with HDACi or interferon α inversely correlates with Stat1 levels. This prompted us to analyze the affinity of NF-κB for DNA in cells with different Stat1 expression statuses. A reduction of NF-κB DNA binding was only observed with HDACi-treated or interferon α-stimulated Stat1-positive but not with Stat1-negative cell extracts (Fig. 4). This is consistent with the cell type-specific repression of NF-κB-dependent genes by this cytokine and HDACi (Fig. 4A,B). Moreover, in cells expressing Stat1, the amount of p65 in the nucleus drops significantly in response to such treatment, and this effect can be inhibited by the nuclear export inhibitor LMB (Fig. 5A).
Since these results could be due to a physical interaction of Stat1 and p65, we performed co-IP experiments. Indeed, we detected the formation of a Stat1–NF-κB complex upon treatment with HDACi and interferon α (Fig. 5D). Gel filtration experiments indicate that the molecular weight of this complex is in the mega-Dalton range. Therefore, the complex is likely to contain several additional proteins (Fig. 5). Although a potential cross-talk of Stat1 and NF-κB signaling pathways has been discussed in several reports (Chatterjee-Kishore et al. 2000; Suk et al. 2001; Shen and Lentsch 2004; Sizemore et al. 2004), evidence for the physical association of these factors has not been published. We speculate that this is due to the fact that we observed their interaction only upon treatment of cells with HDACi or extended stimulation with interferon α (Fig. 5D).
Regulation of Stat1 acetylation status
The coincidence of cellular susceptibility against HDACi and the cytokine interferon α points out common actions of these drugs, and indeed both induce acetylation of Stat1. The dynamic control of Stat1 acetylation is reflected by its association with acetyltransferases and deacetylases. Increased expression of the acetyltransferase CBP promotes Stat1 acetylation in vivo and this HAT can also acetylate Stat1 in vitro (Fig. 6B). Furthermore, in response to treatment with interferon α, CBP can translocate to the cytosol, which permits physical interaction with the major cellular pool of Stat1 (Fig. 6C). This correlates with the occurrence of acetylated Stat1 (Fig. 6A,C). Therefore, CBP is likely to mediate Stat1 acetylation. Moreover, since the CBP bromodomain could dock directly to acetylated Stat1, this could impose a positive feedback on the acetylation of Stat1. This would be consistent with the notion that Nε-acetylated proteins stably associate with acetyltransferases and deacetylases in a signal-dependent manner (Yang 2004).
Based on interactions of endogenous proteins in co-IP experiments, HDAC1 and HDAC3, but not HDAC2 and HDAC8, presumably deacetylate Stat1. Inhibition of these enzymes by HDACi is an obvious mechanism by which these compounds could induce Stat1 acetylation (Fig. 6A). However, acetylation of Stat1 also correlates with the dissociation of HDACs from Stat1 (Fig. 5F) and an increased interaction with CBP (Fig. 6C), which would both favor Stat1 acetylation. This implies that we identified a regulatory system that allows convergence of multiple inputs on protein acetylation and deacetylation. Moreover, cell type-specific differences are likely to affect the dynamic equilibrium of HDACs and HATs controlling Stat1 acetylation.
Acetylated Stat1 mediates suppression of anti-apoptotic NF-κB target genes
Acetylation is considered as a covalent modification that could, similar to phosphorylation, affect the activity of a wide range of proteins by altering intermolecular interactions. Transcription factors such as NF-κB and p53 were shown to be acetylated in their DNA-binding domains (Yang 2004). Here, we show that acetylation of Stat1 lysine residues 410 and 413 within its DBD regulates its interaction with NF-κB p65. Since the cells used in our analysis are characterized by a low constitutive activity of NF-κB, the majority of p65 molecules should exist in cytoplasmic I–κB complexes. The formation of Stat1–NF-κB complexes correlates with the depletion of residual transcriptionally active p65 from the nucleus. This interferes with NF-κB function and renders cells permissive to apoptosis induction (Figs. 4, 7). Our data indicate that acetylation of Stat1 and inhibition of NF-κB is required, though not sufficient, for this process. Such an observation is consistent with data describing increased susceptibility to apoptosis upon introduction of dominant-negative I-κBs, yet no apoptosis induction solely by expression of these proteins in solid tumors (Mayo et al. 2003). Additional actions of HDACi such as the modulation of other signaling pathways and altered cell cycle regulation appear to be necessary. Consistent with this hypothesis, low levels of acetylated Stat1 are detectable in untreated cells but do not lead to spontaneous apoptosis.
Interestingly, microarray experiments revealed that in cells exposed to HDACi about one-third of significant changes in gene expression consist of repression rather than activation events (Van Lint et al. 1996; Mitsiades et al. 2004). This initially unexpected observation could be due to the induction of transcriptional repressors that do not require HDAC activity to function. Such an indirect mechanism would require de novo protein synthesis. On the other hand, the modulation of cross-talk between Stat1 and NF-κB signaling pathways we discovered is independent of protein synthesis and provides a plausible explanation for the suppression of NF-κB target genes by both interferons and the inhibition of HDAC activity. The resulting down-regulation of anti-apoptotic genes could be a prototypical example for the HDACi- and cytokine-mediated inhibition of gene expression.
Our data generated with HDACi, experiments with pseudo-acetylated and nonacetylated Stat1 mutant molecules, ectopic expression, siRNA, and rescue approaches show how acetyl-lysine moieties may contribute to the temporal and spatial regulation of protein function. An interesting future challenge will be to understand which other signaling networks rely on protein acetylation events and how they generate in vivo responses to external and internal signals.
Materials and methods
Drugs and chemicals
Valproic acid, TSA, prodidium iodide, LMB, Hoechst 33258, trypan blue, and MTT were purchased from Sigma, interferon α was purchased from Roche, and Z-VAD-FMK and Ac-DEVD-pNA were purchased from Alexis.
Cell lines, transfections, and microscopy
SK-Mel-37, Mz-Mel-19, NW-Mel-1539, NW-Mel-450 (Jäger et al. 2002) (abbreviated as SK-37, Mz-19, NW-1539, or NW-450), Mz-Mel-5, Mz-Mel-7, NW-Mel-726, NW-Mel-745, 293T, 2fTGH, and U3A cells were maintained in DMEM supplemented with 10% FCS, 1% penicillin/streptomycin, and 5% L-glutamine at 37°C in a 5% CO2 atmosphere. SK-Mel-28 and Malme-3-M cell lines were grown in RPMI containing the same additives. Cells were transfected using PEI (Sigma) or lipofectamine (Invitrogen). Preparation and image analysis of cells were performed as described (Heger et al. 2001). Cy3- and FITC-labeled secondary antibodies were used for immunofluorescence.
Preparation of cell lysates and immunoblotting
Lysate preparation and Western blot procedures were carried out as described (Standke et al. 1994; Krämer et al. 2003). Antibodies were obtained from Santa Cruz Biotechnology (Stat1, p65, p50, HDAC1, HDAC3, CBP, survivin, caspase 3, Tradd, TBP, HA, GFP, mSin3), Sigma (actin), Pharmingen (Bcl-XL, PARP), Transduction labs (Stat5), and NEB (caspase 8, caspase 9, AcLys). The AcH4 antibody has been described (Göttlicher et al. 2001). Co-IP experiments were performed as described (Heinzel et al. 1997). For direct IP of Stat1α and NF-κB, cells were lysed in RIPA buffer. To detect interactions, NETN buffer containing 0.1% NP-40 was used. TSA (1 μM) was added to preserve acetylation.
Measurement of proliferation and apoptosis
MTT assays were performed as described (Denizot and Lang 1986). The cellular DNA content was determined by PI flow cytometry (Göttlicher et al. 2001). Cell viability was also determined by trypan blue exclusion and a PI/Hoechst staining assay (Suk et al. 2001). Caspase 3 assays were performed with 200 μL of caspase 3 cleavage buffer (100 mM Tris at pH 8.0, 10% sucrose, 150 mM NaCl, 0.1% CHAPS, 10 mM DTT), 2.5 μL of 2 mM Ac-DEVD-pNA, and 50 μg protein.
Production of lentiviral particles
HA-Stat1α was cloned into the SacII site of the pHR′cPPT SIEW Sin vector to yield pS-Stat1α-IEW. Lentiviral vector stocks were produced as described (Zufferey et al. 1997; Baus and Pfitzner 2005). Effective transduction was confirmed by fluorescence microscopy, FACS, and Western blot.
EMSA (gel retardation assay) and ABCD-Assay (Avidin–Biotin-coupled DNA assay)
Radioactive DNA-binding assays were performed as described (Garcia et al. 1997). The NF-κB oligonucleotide (sc-2505) and supershift antibodies were purchased from Santa Cruz. ABCD-assays were performed as described (Baumann et al. 2005) with the NF-κB oligonucleotides Bio 5′-GGAATTTCCGGGAA TTTCCGGGAATTTCCGGGAATTTCCC-3′ and 5′-GGGAA ATTCCCGGAAATTCCCGGAAATTCCCGGAAATTCC-3′ or biotinylated nonrelevant oligos.
Plasmids
Stat1α was mutagenized by overlap extension PCR (Ho et al. 1989). The primers used were KK, 5′-AAAAGATCTATGTCT CAGTGGTACGAACTTCAGCAGC-3′, 5′-AAAGAATTCGT ACTGTGTTCATCATACTGTCGAACTCTAC-3′; QQ, 5′-G CAATTGCAAGAACAGCAAAATGCTGG-3′, 5′-CCAGCAT TTTGCTGTTCTTGCAATTGC-3′; and RR, 5′-GCAATTGC GAGAACAGCGAAATGCTGG-3′, 5′-CCAGCATTTCGCTG TTCTCGCAATTGC-3′.
PCR products were cloned into pc3.1 TOPO (Invitrogen). Alternatively, HA-Stat1 was mutagenized using the Quick change site-directed mutagenesis kit (Stratagene). The siRNA sequences for Stat1 were described (Higashi et al. 2003). Mutagenesis of this site toward RNA interference (RNAi)-resistance was carried out by creating silent mutations with the QuickChange kit using the primers 5′-GAGAAGCTTCTTGGTCCGAATG CCAGCCCCGATGG-3′ and 5′-CCATCGGGGCTGGCATTC GGACCAAGAAGCTTCTC-3′.
Acknowledgments
We thank G. Carra, A. Schimpf, S. Reichardt, and H. Kunkel for excellent technical assistance; D. Zimmermann, I. Oehme, B. Dälken, N. Novac, M. Landersz, U. Dietrich (GSH), B. Schlott, and K.H. Gührs (FLI Jena, Dept. F. Grosse) for experimental support; and M. Göttlicher for critical reading of the manuscript. M. Zörnig and S. Hövelmann were invaluable discussion partners throughout the project. G. Stark, M. Müller, and P. Heinrich generously provided cell lines; M. Scherr, K. Brocke-Heidrich, and F. Horn provided the lentiviral expression vector; and C. Glass, J. Darnell, and U. Vinkemeier provided Stat1 expression vectors. This work was supported by NGFN grants to T.H. (N1KR-S31T30), E.P. (N1KR-S31T21), R.S. (N1KR-S31T24), and M.G. (N1KR-S31T19), in addition to funding through DFG SFB 604 (T.H.).
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.364306.
References
- Baumann S., Dostert, A., Novac, N., Bauer, A., Schmid, W., Fas, S.C., Krueger, A., Heinzel, T., Kirchhoff, S., Schütz, G., et al. 2005. Glucocorticoids inhibit activation-induced cell death (AICD) via direct DNA-dependent repression of the CD95 ligand gene by a glucocorticoid receptor dimer. Blood 106: 617–625. [DOI] [PubMed] [Google Scholar]
- Baus D. and Pfitzner, E. 2005. Specific function of STAT3, SOCS1 and SOCS3 in the regulation of proliferation and survival of classical Hodgkin lymphoma cells. Int. J. Can. (Epub ahead of print October 4, 2005. PMID: 16206268] [DOI] [PubMed]
- Blobel G.A. 2000. CREB-binding protein and p300: Molecular integrators of hematopoietic transcription. Blood 95: 745–755. [PubMed] [Google Scholar]
- Chatterjee-Kishore M., Wright, K.L., Ting, J.P., and Stark, G.R. 2000. How Stat1 mediates constitutive gene expression: A complex of unphosphorylated Stat1 and IRF1 supports transcription of the LMP2 gene. EMBO J. J 19: 4111–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.F. and Greene, W.C. 2003. Regulation of distinct biological activities of the NF-κB transcription factor complex by acetylation. J. Mol. Med. 81: 549–557. [DOI] [PubMed] [Google Scholar]
- Cohen H.Y., Lavu, S., Bitterman, K.J., Hekking, B., Imahiyerobo, T.A., Miller, C., Frye, R., Ploegh, H., Kessler, B.M., and Sinclair, D.A. 2004. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol. Cell 13: 627–638. [DOI] [PubMed] [Google Scholar]
- De Schepper S., Bruwiere, H., Verhulst, T., Steller, U., Andries, L., Wouters, W., Janicot, M., Arts, J., and Van Heusden, J. 2003. Inhibition of histone deacetylases by chlamydocin induces apoptosis and proteasome-mediated degradation of survivin. J. Pharmacol. Exp. Ther. 304: 881–888. [DOI] [PubMed] [Google Scholar]
- de Vries E.G., Timmer, T., Mulder, N.H., van Geelen, C.M., van der Graaf, W.T., Spierings, D.C., de Hooge, M.N., Gietema, J.A., and de Jong, S. 2003. Modulation of death receptor pathways in oncology. Drugs Today (Barc) 39, Suppl C: 95–109. [PubMed] [Google Scholar]
- Denizot F. and Lang, R. 1986. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 89: 271–277. [DOI] [PubMed] [Google Scholar]
- Eickhoff B., Ruller, S., Laue, T., Kohler, G., Stahl, C., Schlaak, M., and van der Bosch, J. 2000. Trichostatin A modulates expression of p21waf1/cip1, Bcl-xL, ID1, ID2, ID3, CRAB2, GATA-2, hsp86 and TFIID/TAFII31 mRNA in human lung adenocarcinoma cells. Biol. Chem. 381: 107–112. [DOI] [PubMed] [Google Scholar]
- Garcia R., Yu, C.L., Hudnall, A., Catlett, R., Nelson, K.L., Smithgall, T., Fujita, D.J., Ethier, S.P., and Jove, R. 1997. Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ. 8: 1267–1276. [PubMed] [Google Scholar]
- Göttlicher M., Minucci, S., Zhu, P., Krämer, O.H., Schimpf, A., Giavara, S., Sleeman, J.P., Lo, C.F., Nervi, C., Pelicci, P.G., et al. 2001. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 20: 6969–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W. and Roeder, R.G. 1997. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90: 595–606. [DOI] [PubMed] [Google Scholar]
- Gurvich N., Tsygankova, O.M., Meinkoth, J.L., and Klein, P.S. 2004. Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 64: 1079–1086. [DOI] [PubMed] [Google Scholar]
- Heger P., Lohmaier, J., Schneider, G., Schweimer, K., and Stauber, R.H. 2001. Qualitative highly divergent nuclear export signals can regulate export by the competition for transport cofactors in vivo. Traffic 2: 544–555. [DOI] [PubMed] [Google Scholar]
- Heinzel T., Lavinsky, R.M., Mullen, T.M., Söderström, M., Laherty, C.D., Torchia, J., Yang, W.M., Brard, G., Ngo, S.D., Davie, J.R., et al. 1997. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature 387: 43–48. [DOI] [PubMed] [Google Scholar]
- Henderson C., Mizzau, M., Paroni, G., Maestro, R., Schneider, C., and Brancolini, C. 2003. Role of caspases, Bid, and p53 in the apoptotic response triggered by histone deacetylase inhibitors trichostatin-A (TSA) and suberoylanilide hydroxamic acid (SAHA). J. Biol. Chem. 278: 12579–12589. [DOI] [PubMed] [Google Scholar]
- Higashi K., Inagaki, Y., Fujimori, K., Nakao, A., Kaneko, H., and Nakatsuka, I. 2003. Interferon-γ interferes with transforming growth factor-β signaling through direct interaction of YB-1 with Smad3. J. Biol. Chem. 278: 43470–43479. [DOI] [PubMed] [Google Scholar]
- Hinz M., Lemke, P., Anagnostopoulos, I., Hacker, C., Krappmann, D., Mathas, S., Dorken, B., Zenke, M., Stein, H., and Scheidereit, C. 2002. Nuclear factor κB-dependent gene expression profiling of Hodgkin's disease tumor cells, pathogenetic significance, and link to constitutive signal transducer and activator of transcription 5a activity. J. Exp. Med. 196: 605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S.N., Hunt, H.D., Horton, R.M., Pullen, J.K., and Pease, L.R. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77: 51–59. [DOI] [PubMed] [Google Scholar]
- Horvath C.M., Stark, G.R., Kerr, I.M., and Darnell Jr., J.E. 1996. Interactions between STAT and non-STAT proteins in the interferon-stimulated gene factor 3 transcription complex. Mol. Cell. Biol. 16: 6957–6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N., Katz, J.P., Martin, D.R., and Wu, G.D. 1997. Inhibition of IL-8 gene expression in Caco-2 cells by compounds which induce histone hyperacetylation. Cytokine 9: 27–36. [DOI] [PubMed] [Google Scholar]
- Ihle J.N. 2001. The Stat family in cytokine signaling. Curr. Opin. Cell Biol. 13: 211–217. [DOI] [PubMed] [Google Scholar]
- Inan M.S., Rasoulpour, R.J., Yin, L., Hubbard, A.K., Rosenberg, D.W., and Giardina, C. 2000. The luminal short-chain fatty acid butyrate modulates NF-κB activity in a human colonic epithelial cell line. Gastroenterology 118: 724–734. [DOI] [PubMed] [Google Scholar]
- Jäger E., Karbach, J., Gnjatic, S., Jäger, D., Maeurer, M., Atmaca, A., Arand, M., Skipper, J., Stockert, E., Chen, Y.T., et al. 2002. Identification of a naturally processed NY-ESO-1 peptide recognized by CD8+ T cells in the context of HLA-B51. Cancer Immun. 2: 12. [PubMed] [Google Scholar]
- Kelly W.K., O'Connor, O.A., and Marks, P.A. 2002. Histone deacetylase inhibitors: From target to clinical trials. Expert Opin. Investig. Drugs 11: 1695–1713. [DOI] [PubMed] [Google Scholar]
- Kiernan R., Bres, V., Ng, R.W., Coudart, M.P., El Messaoudi, S., Sardet, C., Jin, D.Y., Emiliani, S., and Benkirane, M. 2003. Post-activation turn-off of NF-κB-dependent transcription is regulated by acetylation of p65. J. Biol. Chem. 278: 2758–2766. [DOI] [PubMed] [Google Scholar]
- Korzus E., Torchia, J., Rose, D.W., Xu, L., Kurokawa, R., McInerney, E.M., Mullen, T.M., Glass, C.K., and Rosenfeld, M.G. 1998. Transcription factor-specific requirements for coactivators and their acetyltransferase functions. Science 279: 703–707. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. 1999. Histone acetylases and deacetylases in cell proliferation. Curr. Opin. Genet. Dev. 9: 40–48. [DOI] [PubMed] [Google Scholar]
- ____. 2000. Acetylation: A regulatory modification to rival phosphorylation? EMBO J. 19: 1176–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer O.H., Göttlicher, M., and Heinzel, T. 2001. Histone deacetylase as a therapeutic target. Trends Endocrinol. Metab. 12: 294–300. [DOI] [PubMed] [Google Scholar]
- Krämer O.H., Zhu, P., Ostendorff, H.P., Golebiewski, M., Tiefenbach, J., Peters, M.A., Brill, B., Groner, B., Bach, I., Heinzel, T., et al. 2003. The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. EMBO J. 22: 3411–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Commane, M., Flickinger, T.W., Horvath, C.M., and Stark, G.R. 1997. Defective TNF-α-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science 278: 1630–1632. [DOI] [PubMed] [Google Scholar]
- Mayo M.W., Denlinger, C.E., Broad, R.M., Yeung, F., Reilly, E.T., Shi, Y., and Jones, D.R. 2003. Ineffectiveness of histone deacetylase inhibitors to induce apoptosis involves the transcriptional activation of NF-κB through the Akt pathway. J. Biol. Chem. 278: 18980–18989. [DOI] [PubMed] [Google Scholar]
- Melnick A. and Licht, J.D. 2002. Histone deacetylases as therapeutic targets in hematologic malignancies. Curr. Opin. Hematol. 9: 322–332. [DOI] [PubMed] [Google Scholar]
- Meyer T., Begitt, A., Lodige, I., van Rossum, M., and Vinkemeier, U. 2002. Constitutive and IFN-γ-induced nuclear import of STAT1 proceed through independent pathways. EMBO J. 21: 344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiades C.S., Mitsiades, N.S., McMullan, C.J., Poulaki, V., Shringarpure, R., Hideshima, T., Akiyama, M., Chauhan, D., Munshi, N., Gu, X., et al. 2004. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: Biological and clinical implications. Proc. Natl. Acad. Sci. 101: 540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Laxton, C., Briscoe, J., Schindler, C., Improta, T., Darnell Jr., J.E., Stark, G.R., and Kerr, I.M. 1993. Complementation of a mutant cell line: Central role of the 91 kDa polypeptide of ISGF3 in the interferon-α and -γ signal transduction pathways. EMBO J. 12: 4221–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusinzon I. and Horvath, C.M. 2003. Interferon-stimulated transcription and innate antiviral immunity require deacetylase activity and histone deacetylase 1. Proc. Natl. Acad. Sci. 100: 14742–14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins N.D. 2004. NF-κB: Tumor promoter or suppressor? Trends Cell Biol. 14: 64–69. [DOI] [PubMed] [Google Scholar]
- Schreiber S.L. and Bernstein, B.E. 2002. Signaling network model of chromatin. Cell 111: 771–778. [DOI] [PubMed] [Google Scholar]
- Shen H. and Lentsch, A.B. 2004. Progressive dysregulation of transcription factors NF-κB and STAT1 in prostate cancer cells causes proangiogenic production of CXC chemokines. Am. J. Physiol. Cell Physiol. 286: C840–C847. [DOI] [PubMed] [Google Scholar]
- Sizemore N., Agarwal, A., Das, K., Lerner, N., Sulak, M., Rani, S., Ransohoff, R., Shultz, D., and Stark, G.R. 2004. Inhibitor of κB kinase is required to activate a subset of interferon γ-stimulated genes. Proc. Natl. Acad. Sci. 101: 7994–7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standke G.J., Meier, V.S., and Groner, B. 1994. Mammary gland factor activated by prolactin on mammary epithelial cells and acute-phase response factor activated by interleukin-6 in liver cells share DNA binding and transactivation potential. Mol. Endocrinol. 8: 469–477. [DOI] [PubMed] [Google Scholar]
- Strahl B.D. and Allis, C.D. 2000. The language of covalent histone modifications. Nature 403: 41–45. [DOI] [PubMed] [Google Scholar]
- Suk K., Chang, I., Kim, Y.H., Kim, S., Kim, J.Y., Kim, H., and Lee, M.S. 2001. Interferon γ (IFNγ) and tumor necrosis factor α synergism in ME-180 cervical cancer cell apoptosis and necrosis. IFNγ inhibits cytoprotective NF-κB through STAT1/IRF-1 pathways. J. Biol. Chem. 276: 13153–13159. [DOI] [PubMed] [Google Scholar]
- Thornberry N.A. and Lazebnik, Y. 1998. Caspases: Enemies within. Science 281: 1312–1316. [DOI] [PubMed] [Google Scholar]
- Van Lint C., Emiliani, S., and Verdin, E. 1996. The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Expr. 5: 245–253. [PMC free article] [PubMed] [Google Scholar]
- Vrana J.A., Decker, R.H., Johnson, C.R., Wang, Z., Jarvis, W.D., Richon, V.M., Ehinger, M., Fisher, P.B., and Grant, S. 1999. Induction of apoptosis in U937 human leukemia cells by suberoylanilide hydroxamic acid (SAHA) proceeds through pathways that are regulated by Bcl-2/Bcl-XL, c-Jun, and p21CIP1, but independent of p53. Oncogene 18: 7016–7025. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wu, T.R., Cai, S., Welte, T., and Chin, Y.E. 2000. Stat1 as a component of tumor necrosis factor α receptor 1–TRADD signaling complex to inhibit NF-κB activation. Mol. Cell. Biol. 20: 4505–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C., Schneider, S., Wagner, E.F., Zhang, X., Seto, E., and Bohmann, D. 2003. JNK phosphorylation relieves HDAC3-dependent suppression of the transcriptional activity of c-Jun. EMBO J. 22: 3686–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe A.P. and Hayes, J.J. 1999. Chromatin disruption and modification. Nucleic Acids Res. 27: 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L.H., Sim, H., Chatterjee-Kishore, M., Hatzinisiriou, I., Devenish, R.J., Stark, G., and Ralph, S.J. 2002. Isolation and characterization of a human STAT1 gene regulatory element. Inducibility by interferon (IFN) types I and II and role of IFN regulatory factor-1. J. Biol. Chem. 277: 19408–19417. [DOI] [PubMed] [Google Scholar]
- Yang X.J. 2004. Lysine acetylation and the bromodomain: A new partnership for signaling. Bioessays 26: 1076–1087. [DOI] [PubMed] [Google Scholar]
- Yang E., Wen, Z., Haspel, R.L., Zhang, J.J., and Darnell Jr., J.E. 1999. The linker domain of Stat1 is required for γ interferon-driven transcription. Mol. Cell. Biol. 19: 5106–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E., Henriksen, M.A., Schaefer, O., Zakharova, N., and Darnell Jr., J.E. 2002. Dissociation time from DNA determines transcriptional function in a STAT1 linker mutant. J. Biol. Chem. 277: 13455–13462. [DOI] [PubMed] [Google Scholar]
- Zufferey R., Nagy, D., Mandel, R.J., Naldini, L., and Trono, D. 1997. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15: 871–875. [DOI] [PubMed] [Google Scholar]