Abstract
Nef, an essential pathogenic determinant for human immunodeficiency virus type 1, has multiple functions that include disruption of major histocompatibility complex class I molecules (MHC-I) and CD4 and CD28 cell surface expression. The effects of Nef on MHC-I have been shown to protect infected cells from cytotoxic T-lymphocyte recognition by downmodulation of a subset of MHC-I (HLA-A and -B). The remaining HLA-C and -E molecules prevent recognition by natural killer (NK) cells, which would otherwise lyse cells expressing small amounts of MHC-I. Specific amino acid residues in the MHC-I cytoplasmic tail confer sensitivity to Nef, but their function is unknown. Here we show that purified Nef binds directly to the HLA-A2 cytoplasmic tail in vitro and that Nef forms complexes with MHC-I that can be isolated from human cells. The interaction between Nef and MHC-I appears to be weak, indicating that it may be transient or stabilized by other factors. Supporting the fact that these molecules interact in vivo, we found that Nef colocalizes with HLA-A2 molecules in a perinuclear distribution inside cells. In addition, we demonstrated that Nef fails to bind the HLA-E tail and also fails to bind HLA-A2 tails with deletions of amino acids necessary for MHC-I downmodulation. These data provide an explanation for differential downmodulation of MHC-I allotypes by Nef. In addition, they provide the first direct evidence indicating that Nef functions as an adaptor molecule able to link MHC-I to cellular trafficking proteins.
Despite a strong immune response, individuals infected with human immunodeficiency virus (HIV) are unable to eradicate the virus and become chronically infected. This is due at least in part to HIV's ability to evade the immune system. One important mechanism of viral immune evasion is downmodulation of cell surface major histocompatibility complex class I (MHC-I) molecules by HIV Nef (5, 38), which leads to protection of infected cells from cytotoxic T-lymphocyte (CTL) killing in vitro (5). In addition, MHC-I downmodulation has recently been shown to be an important component of Nef's in vivo activities in a simian model system (30).
HIV-1 Nef is a 27-kDa myristylated protein expressed early in the viral life cycle. It affects the cell surface expression of a number of molecules, disrupts cellular signaling, inhibits apoptosis, and promotes virus production (2, 9, 12, 27, 28, 34, 36, 40, 42, 45, 49, 50). MHC-I downmodulation by Nef is specific in that it affects HLA-A and HLA-B, but not HLA-C (4, 22) or HLA-E (4), the class I molecules necessary for protection from natural killer cells. Although this allotype-specific downmodulation can be mapped to individual amino acid residues in the MHC-I cytoplasmic tail (4, 22), the function of these residues is unknown. One model is that Nef acts as an adaptor protein that selectively binds this region of the cytoplasmic tail and recruits other cellular factors to target MHC-I molecules to the trans-Golgi network (TGN) (14, 22, 33, 44). Consistent with this model, Nef has been shown to associate with PACS-1, a molecule that recycles proteins containing acidic cluster motifs to the TGN (33, 48). Additionally, expression of a dominant-negative PACS-1 mutant antagonizes the downmodulation of MHC-I expression by Nef (7). However, no interaction between Nef and MHC-I molecules that could link MHC-I to PACS-1 has yet been demonstrated.
We present evidence that MHC-I interacts directly with Nef and colocalizes with it in a perinuclear distribution. The interaction between Nef and MHC-I depends on cytoplasmic tail sequences, because Nef does not bind to the HLA-E tail or to an MHC-I molecule lacking a cytoplasmic tail. Although the interaction appears to be weak and requires a cross-linker for efficient detection, it is highly specific and correlates with the functional effects of Nef on MHC-I downmodulation. These data provide an explanation for allotype-specific downmodulation of MHC-I expression by Nef. Additionally, they are the first direct evidence that Nef can bind to MHC-I molecules and function as an adaptor molecule linking MHC-I molecules to cellular trafficking proteins.
MATERIALS AND METHODS
Cell lines and culture conditions.
HeLa cells were obtained from the American Type Culture Collection (ATCC); the HeLa-A2 cell line is described elsewhere (10); 373mg cells were received from Hidde Ploegh (Harvard Medical School). Cells were maintained in Dulbecco's modified Eagle medium supplemented with penicillin, streptomycin, 2 mM glutamine, and 10% fetal bovine serum. To create the H2-Kd and H2-Kd/A2 tail astrocytic cell lines, 373mg cells were transfected using calcium phosphate. They were selected in 1 mg of Geneticin (Life Technologies)/ml and then sorted by fluorescence-activated cell sorting (FACS) for the highest-expression cells. Retrovirus transduction was used to create stable cell lines expressing H2-Kd/E tail and H2-Kd/none as previously described (31), except that viruses were pseudotyped by cotransfection with 0.25 μg of vesicular stomatitis virus G protein (18). Cell lines prepared in this manner were selected in 1 mg of Geneticin/ml but did not require sorting.
Antibodies.
Antibodies to HLA-A2 (One Lambda) or H2-Kd (PharMingen) were used for flow cytometry. For fluorescence microscopy, BB7.2 (ATCC) was used. This antibody was purified from ascites by using a Hitrap Protein A FF column (Amersham Pharmacia Biotech) according to the manufacturer's instructions. For immunoprecipitations, purified BB7.2 was cross-linked to protein A/G agarose by using DMP (dimethylpimelimidate). Briefly, protein A/G beads were equilibrated in 0.2 M sodium borate, pH 9.0. Two milligrams of antibody was incubated per milliliter of protein A/G slurry at room temperature for 1 h. The beads were washed twice with sodium borate, and DMP was then added to 20 mM. The beads were incubated for 30 min at room temperature, and the reaction was stopped by washing once and then incubating for 2 h at room temperature with 0.2 M ethanolamine, pH 8.0. Nef antibodies were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH). For immunoblots, AE6 ascites from James Hoxie was used (46), and for immunoprecipitations, an HIV-1 Nef antiserum from Ronald Swanstrom was used (39).
DNA constructs.
To construct the Nef-DsRed fusion protein, NL4-3 nef was amplified by PCR using oligonucleotides 5′-ATAAGAATGCGGCCGCCGCCACCATGGGTGGCAAGTGGTCAAAAAGT-3′ and 5′-CCCAAGCTTTCAACGCGTACTAGTGCAGTTCTTGAAGTACTCCGGATGC-3′. The product was then digested with NotI and HindIII and was cloned into pShuttle-CMV (Stratagene). DsRed fused to an N-terminal pentaglycine linker was made by amplifying the DsRed open reading frame from pDsRed2-N1 (Clontech) by using oligonucleotides 5′-GACTAGTGGAGGTGGTGGTGGAATGGCCTCCTCCGAGAACGTCATC-3′ and 5′-CGACGCGTCAGGAACAGGTGGTGGCGGCCCTC-3′. This product was then digested with SpeI and MluI and cloned into the SpeI/MluI sites of pShuttle-CMV-Nef to generate a Nef-pentaglycine-DsRed fusion protein (Nef-DsRed). A control vector was constructed by subcloning a SalI-NotI fragment of pDsRed2-N1 into the SalI/NotI sites of pShuttle-CMV. The HindIII (filled in)/SalI fragments encoding DsRed or Nef-DsRed were then subcloned from pShuttle-CMV into the BamHI (filled in)/SalI sites of pEB4. This vector contains transcriptional regulatory elements from pAdEF1αloxP (University of Michigan Vector Core) including the EF1α promoter, bovine growth hormone polyadenylation sequence, and 4F2 enhancer element. The elements were removed as a 2.7-kb cassette by SspI digestion, followed by a partial NotI digestion. The cassette was cloned into the HindIII and EcoRI sites (filled in) of pUC19 to generate pEB4.
H2-Kd with the HLA-A2 tail (H2-Kd/A2) was constructed as described elsewhere (10). To generate H2-Kd/E, a pRSV H2-Kd/A2 SalI-AgeI fragment and an AgeI-digested PCR product amplified from GST-E (see below) were cloned into MSCV 2.2 digested with XhoI and HpaI in a three-way ligation. To generate H2-Kd/A2 Y320A, a pRSV H2-Kd/A2 SalI-Age I fragment and an Age I-EcoRI-digested PCR product amplified from GST-A2 Y320A (see below) were cloned into MSCV 2.2 digested with XhoI and EcoRI in a three way ligation. The oligonucleotides used to generate the E-tail from GST-E were as follows: 5′ oligonucleotide, 5′-CAGGTGACCGGTGCTGTGGTCGCTGCTGTGATGTGGAGGAAGAAGAGCTCAGGTGGA-3′; 3′ oligonucleotide, 5′-CACCTGCAGCTGTTACAAGCTGTGAGACTCAGACCC-3′. The oligonucleotides used to generate the A2 Y320A-tail were as follows: 5′ oligonucleotide, 5′-CAGGTGACCGGTGCTGTGGTCGCTGCTGTGATGTGGAGGAGGAAGAGCTCAGATAGA-3′; 3′ oligonucleotide, 5′-GGTTTTCACCGTCATCACCG-3′. H2-Kd/none was generated by introducing a premature stop codon via PCR mutagenesis at position 306.
Nef (E62-65A) was made by site-directed PCR mutagenesis of NL4-3 nef. The 5′ fragment was generated using 5′-CGGGATCCATGGGTGGCAAGTGGTCAAAA-3′ and 5′-CTGAGGTGTGACTGGAAAACCCACCGCTGCCGCCGCTTGTGCTTCTAGCCAGGCACAAGCAGC-3′, and the 3′ fragment was generated using 5′-GCTGCTTGTGCCTGGCTAGAAGCACAAGCGGCGGCAGCGGTGGGTTTTCCAGTCACACCTCAG-3′ and 5′-GGAATTCTCAGCAGTTCTTGAAGTACTCGGG-3′. To generate the GST-Nef (wild type) or GST-Nef (E62-65A) fusion protein, NL4-3 nef or a combination of the 5′ and 3′ fragments described above was amplified by PCR using oligonucleotides 5′-CGGGATCCATGGGTGGCAAGTGGTCAAAA-3′ and 5′-GGAATTCTCAGCAGTTCTTGAAGTACTCGGG-3′. The GST-Nef core domain (amino acid codons 58 to 206) was also amplified by PCR using oligonucleotides 5′-CGGAATTCTCACCAGGCACAAGCAGCATTGTT-3′ and 5′-GGAATTCTCAGCAGTTCTTGAAGTACTCGGG-3′. Nef (wild type, E62-65A, and Core) PCR products were then cloned into the BamHI/EcoRI sites of pGEX-2T (AP Biotech).
To generate the GST-A2, GST-A2 Y320A, and GST-E fusion proteins, PCR was used to amplify the cytoplasmic domains of MHC-I molecules by using pcDNA HLA-A2 (Hidde Ploegh), pcDNA HLA-A2 Y320A (44), and HLA-E cDNA (Pamela Bjorkman, Caltech) as templates. The following oligonucleotides were used: for HLA-A2 and HLA-A2 Y320A, 5′-CGGGATCCAGGAGGAAGAGCTCAGATAGA-3′ and 5′-GGAATCTCACACTTTACAAGCTGTGAG-3′; for HLA-E, 5′-CGGGATCCAGGAAGAAGAGCTCAGGTGGA-3′ and 5′-GGAATTCTTACAAGCTGTGAGACTCAGA-3′. PCR products were digested with BamHI and EcoRI and cloned into the same sites in pGEX-2T.
GST-A2 carboxy-terminal truncation constructs were generated by introduction of a premature termination codon through PCR amplification using GST-A2 as a template. The oligonucleotides used were 5′-GCGATGCTGGTTGCCAACGATCAGA-3′ coupled with: 5′-GGAATTCTCAACTGCTTGCAGCCTG-3′ (for A2ΔD), 5′-GGAATTCTCAGCTCCCTCCTTTTCT-3′ (for A2ΔY), and 5′-GGAATTCTCATGAGCTCTTCCTCCT-3′ (for A2ex5). The PCR products were digested with EcoRI and EcoRV and were cloned into the same sites in pGEX-2T.
GST-PACSfbr was constructed by PCR amplification of the furin binding region of PACS-1a from a human brain cDNA library (Clontech) by use of oligonucleotides XY010 and YX111, which have been described previously (7). The product was then digested with BamHI and cloned into the BamHI site of pGEX 5X-1 (AP Biotech).
FACS analysis.
To determine cell surface expression of the indicated MHC-I molecules, cells were stained in FACS buffer (phosphate-buffered saline [PBS], 2% human serum, 1% HEPES, 1% NaN3) with an antibody against HLA-A2 (dilution, 1:200; One Lambda) or H2-Kd (dilution, 1:200; PharMingen), followed by a goat anti-mouse secondary antibody conjugated to phycoerythrin (PE) (dilution, 1:250; Biosource International) or fluorescein isothiocyanate (FITC) (dilution, 1:250; Molecular Probes). Cells were analyzed on a Becton Dickinson FACScan using CELLQuest software.
Immunofluorescence.
At 24 h posttransfection, cells were fixed in PBS plus 2% paraformaldehyde for 15 min at room temperature and then permeabilized in PBS plus 0.4% Tween 20 for 15 min at 37°C. Cells were blocked in goat wash (PBS, 10 mM HEPES buffer, 2% goat serum, and 0.025% sodium azide), followed by staining with an HLA-A2-specific monoclonal antibody (BB7.2; 20 μg/ml) and a goat anti-mouse antibody conjugated with AlexaFluor 488 (dilution, 1:100; Molecular Probes). Images were collected using a Zeiss LSM 510 confocal laser scanning microscope equipped with a 63× objective lens. Images were processed using Adobe Photoshop 5.5 imaging software.
Adenovirus transductions.
A replication-defective adenovirus with deletions in the E2A, E1B, and E3 regions, expressing HIV-1 Nef, was constructed by the University of Michigan Vector Core facility and used to transduce cells as previously described (44). The multiplicity of infection (MOI) was estimated based on the infectivity of 293 cells. In astrocytic cells the lowest MOI able to yield downmodulation of MHC-I expression equivalent to that in HIV-infected T cells was used.
HIV transductions.
T1 T cells were transduced with virus prepared from HIV-1 molecular clones (NL-PI env frameshift mutant [5; M. R. Kasper and K. L. Collins, submitted for publication]) as previously described (5).
Metabolic labeling and immunoprecipitations.
Twenty-two to 24 h after adenovirus transduction or 48 h after HIV-1 transduction, cells were depleted of methionine and cysteine by incubation for 15 min in prelabel medium (Dulbecco's modified Eagle medium lacking methionine and cysteine, supplemented with 10% dialyzed fetal bovine serum). Cells were labeled for 4 h in prelabel medium plus 0.2 mCi of 35S-Pro-Mix (Amersham)/ml and 25 mM NH4Cl (Fisher) where indicated. Intact cells were treated with 1 mM dithiobis(succinimidylpropionate) (DSP), a reversible, homobifunctional cross-linking reagent (Pierce) (41, 43), as recommended by the manufacturer and were lysed in PBS (pH 8) plus 0.3% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) (Fisher), 0.1% sodium dodecyl sulfate (SDS), and 1 mM phenylmethylsulfonyl fluoride (PMSF). Lysates were precleared overnight with a control antibody (mouse immunoglobulin G [IgG] or normal rabbit immunoglobulin fraction) and protein A and protein G plus agarose (Oncogene). Immunoprecipitations were performed for 2 h with an antibody against either β2 microglobulin (β2M) (Dako), H2-Kd (PharMingen), Nef, or matched control antibodies. Samples were washed six times with Tris-buffered saline,-0.3% CHAPS-0.1% SDS. Samples were separated by SDS-15% polyacrylamide gel electrophoresis (SDS-15% PAGE), and the gel was dried and exposed to Kodak Biomax MS film. For double immunoprecipitations, the first immunoprecipitation was performed with an anti-β2M antibody or a normal rabbit immunoglobulin fraction (control), and beads were resuspended in 1% SDS and boiled for 10 min. The supernatant was precleared with protein A/G agarose and then used for a second immunoprecipitation with a Nef antibody or normal rabbit serum. For immunoprecipitation and Western blot analysis, cell lysates were generated as described above without metabolic labeling. Lysates were immunoprecipitated with an anti-HLA-A2 antibody (BB7.2) that was cross-linked to protein A/G or beads alone as described above. Western blotting was then performed with a polyclonal anti-Nef antibody.
GST binding assays.
Glutathione S-transferase (GST) fusion proteins were expressed in BL21(DE3)pLysS cells and purified according to the manufacturer's instructions (AP Biotech). Recombinant Nef proteins without the GST tag were prepared by incubating GST-Nef with 0.14 U of thrombin (Sigma)/μl for 2 h at room temperature, followed by inactivation with 2.5 mM PMSF. Purified proteins were stored in small aliquots at −80°C. For binding assays, GST fusion proteins (6 to 12 μg) were incubated with recombinant Nef protein (0.5 to 2 μg) in binding buffer (PBS, 0.05% NP-40, 0.16 mg of bovine serum albumin [New England Biolabs]/μl, 2.5 mM PMSF) for 1 h on ice. GST proteins were captured by incubation with 12.5 μl of glutathione Sepharose beads (AP Biotech) for 1.5 h at 4°C. Proteins were cross-linked by addition of 0.25 to 1 mM DSP (Pierce) for 30 min, followed by incubation with 50 mM Tris, pH 7.4, for 15 min at room temperature. The beads were then washed three times with PBS plus 0.05% NP-40 and twice with 50 mM Tris, pH 8. Bound proteins were eluted for 30 min at 4°C with elution buffer (10 mM reduced glutathione, 50 mM Tris [pH 8], 50 mM dithiothreitol), and recovered Nef was assayed by Western blotting with an anti-Nef monoclonal antibody (AE6 Ascites). Immunoblots were quantitated by densitometric analysis of scanned images using ImageQuant software (Molecular Dynamics).
RESULTS
HIV-1 Nef colocalizes with MHC-I in the perinuclear region.
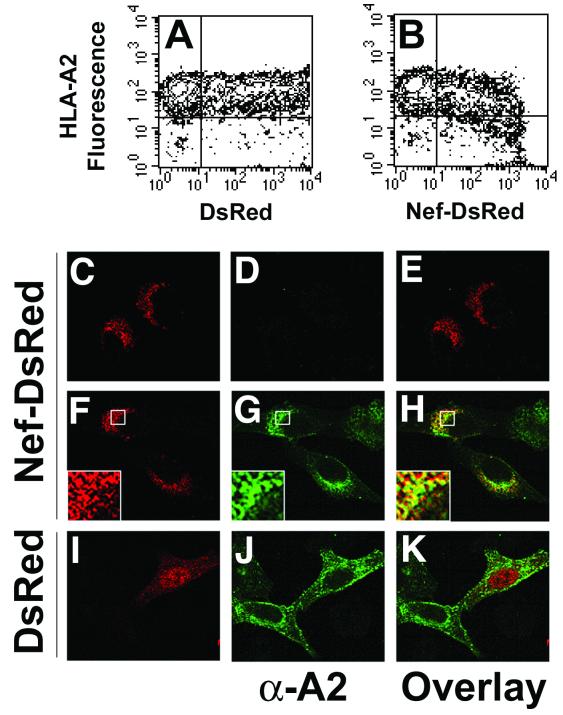
As a first step to determining whether Nef and MHC-I could interact physically, we tested whether HIV-1 Nef and MHC-I were located in the same region of the cell. To accomplish this, the fluorescent molecule DsRed was fused to the C terminus of HIV-1 Nef (Nef-DsRed). Because Nef is an adaptor protein containing multiple surfaces for protein-protein interactions, a pentaglycine linker was inserted at the fusion site to allow steric mobility between the two proteins. To test the functional activity of the fusion protein, plasmids encoding DsRed or the Nef-DsRed fusion protein were transiently transfected into a HeLa cell line stably expressing HLA-A2 (HeLa-A2). Two-color flow cytometry was utilized to monitor the level of HLA-A2 molecules expressed on the cell surface as a function of DsRed expression. We routinely observed HLA-A2 downmodulation (up to 3.5-fold) in cells transfected with Nef-DsRed, compared to cells transfected with DsRed (Fig. 1 A and B). These results are similar to what has been previously reported for untagged Nef (38, 44). Interestingly, a threshold level of Nef-DsRed was required to achieve efficient MHC-I downmodulation, a phenomenon that is also consistent with previous reports (24, 47).
FIG. 1.
HIV-1 Nef-DsRed fusion protein causes reduction of cell surface MHC-I antigens, and colocalizes with MHC-I in the perinuclear area. (A and B) Nef-DsRed fusion protein downmodulates MHC-I. HeLa cells stably expressing HLA-A2 (HeLa-A2) were transiently transfected with plasmids encoding either DsRed (A) or a Nef-DsRed fusion protein (B). At 48 h posttransfection, HLA-A2 cell surface expression and DsRed protein expression were analyzed simultaneously by two-color flow cytometry. Crosshairs denote maximum fluorescence of negative controls for HLA-A2 staining (HeLa parental cells) and DsRed protein expression (mock-transfected HeLa-A2 cells). Results shown are representative of three independent experiments. (C through K) Nef-DsRed and MHC-I colocalize in the perinuclear region of cells. HeLa cells (C through E) or HeLa-A2 cells (F through K) were grown on glass slides and transiently transfected with plasmids encoding DsRed (I through K) or Nef-DsRed (C through H). At 24 h posttransfection, DsRed protein was detected by direct immunofluorescence (C, F, and I), and HLA-A2 was detected by indirect immunofluorescence using the HLA-A2-specific monoclonal antibody BB7.2 (D, G, and J). Enlargements of boxed regions are displayed as insets in the lower left corners of panels F through H. Images were collected using a Zeiss LSM 510 confocal laser scanning microscope, and single Z sections are displayed. Overlays and insets of DsRed and HLA-A2 images were generated using Adobe Photoshop imaging software. Results shown are representative of three independent experiments.
To examine the subcellular distribution of Nef and HLA-A2, HeLa or HeLa-A2 cells were grown on glass slides, transfected with plasmids encoding DsRed or Nef-DsRed, and stained with an antibody specific for HLA-A2. Consistent with previous studies, Nef-DsRed was localized to punctate structures primarily in the perinuclear region of the cell (6, 13, 14, 33) (Fig. 1C and F), and Nef induced a dramatic intracellular accumulation of HLA-A2 in the perinuclear region (14, 22, 33, 44) (Fig. 1G). An overlay of Nef and HLA-A2 fluorescence revealed a significant amount of colocalization (Fig. 1H), whereas no substantial colocalization was observed between DsRed and HLA-A2 (Fig. 1K). The colocalization of MHC-I and Nef was only partial, probably because Nef has other cellular functions besides MHC-I downmodulation and may only associate with MHC-I transiently. These data demonstrate that when Nef and HLA-A2 are coexpressed, they are targeted to the same perinuclear region of the cell, which has previously been shown to colocalize with TGN markers (data not shown) (14, 22, 33, 44).
HIV-1 Nef associates with MHC-I in vivo.
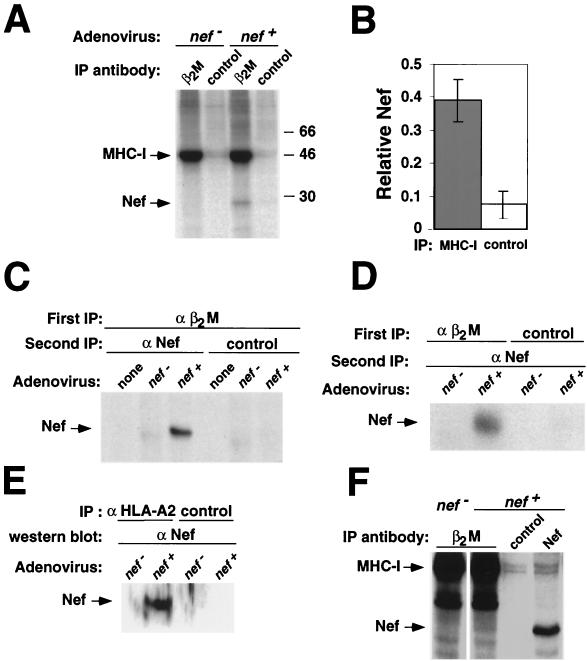
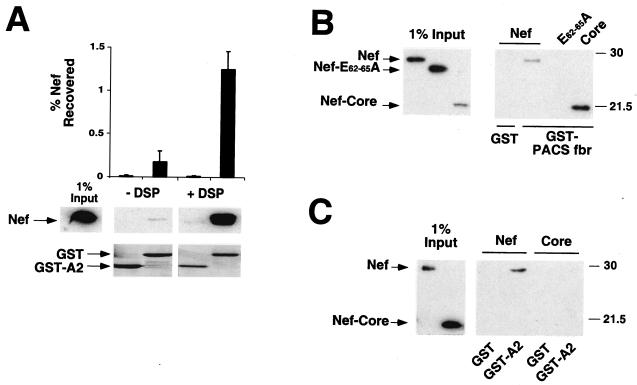
One way that Nef may alter MHC-I localization is through a direct or indirect interaction with MHC-I. To examine this possibility, we used an adenovirus system to express Nef in an astrocytic cell line (373mg). This system was chosen because astrocytic cells are a natural target for HIV infection, and we have previously shown that they respond well to Nef (44). These cells were transduced with a replication-defective adenoviral vector expressing Nef (adeno-Nef) or control-adeno (an identical adenovirus that lacks the Nef open reading frame). Twenty-four hours later, they were metabolically labeled and treated with the cell-permeant cross-linker DSP (41, 43). Total MHC-I was then immunoprecipitated from lysates prepared from these cells by using an antibody directed against the MHC-I light chain (β2M). Interestingly, we found that a Nef-sized band coprecipitated with MHC-I from lysates containing Nef, but not from control lysates that did not express Nef, or when a control antibody was used (Fig. 2A and B). Quantitation of these data revealed that the amount of Nef coprecipitating with MHC-I was sixfold above background levels (Fig. 2B).
FIG. 2.
Nef interacts with MHC-I in vivo. (A) MHC-I coimmunoprecipitates a 27-kDa protein in Nef-expressing cells. 373mg astrocytic cells were transduced with adeno-Nef or control-adeno at an estimated MOI of 300:1 (see Materials and Methods). The next day they were metabolically labeled, treated with the cross-linker DSP, and immunoprecipitated with either an antibody against β2M or normal rabbit immunoglobulin fraction (control). Results shown are representative of three separate experiments. (B) Quantitation of binding. The relative amount of 27-kDa protein coprecipitating with a β2M antibody versus a control antibody was determined using a phosphorimager. Results shown were normalized for the amount of total Nef immunoprecipitated with an anti-Nef antibody. Means ± standard deviations for three separate experiments are shown. (C, D, and E) MHC-I coimmunoprecipitates Nef. (C) β2M containing complexes were immunoprecipitated as described in the legend to panel A. After the first immunoprecipitation, the agarose beads were boiled in 1% SDS to destroy the antibody and protein complexes from the first immunoprecipitation. The supernatant was then reimmunoprecipitated with either an antibody against Nef or normal serum (control). (D) A double immunoprecipitation was performed as in the experiment for which results are shown in panel C, except that complexes were first immunoprecipitated with an antibody against β2M or with a control antibody (normal rabbit immunoglobulin fraction), followed by immunoprecipitation with an antibody against Nef. (E) Immunoprecipitations were performed as described in the legend to panel A, except that cells were not metabolically labeled and either an anti-HLA-A2 antibody (BB7.2) cross-linked to protein A/G agarose or protein A/G agarose alone was used. Immunoprecipitations were run on SDS-PAGE and Western blotted for Nef. (F) Nef coprecipitates with MHC-I in HIV-1-infected T cells. T1 T cells were transduced with an HIV molecular clone (NL-PI env frameshift mutant) pseudotyped with vesicular stomatitis virus protein G. Forty-eight hours later, the cells were metabolically labeled, treated with DSP, and immunoprecipitated as for panel A.
In order to clearly identify the 27-kDa protein coimmunoprecipitating with MHC-I in Nef-expressing cells, a double immunoprecipitation was performed. Using metabolically labeled lysates as described above, a first immunoprecipitation was performed with an antibody against the MHC-I light chain (β2M). The beads from this immunoprecipitation were then resuspended in 1% SDS and boiled to release the bound proteins. The released proteins were then reimmunoprecipitated with an antibody against Nef. The 27-kDa band was detected only in lysates containing Nef that were first immunoprecipitated with an anti-β2M antibody and then reimmunoprecipitated with an anti-Nef antibody (Fig. 2C and D). Additionally, the Nef-sized band was not present when the first immunoprecipitation was performed with a control antibody instead of an antibody against β2M (Fig. 2D). Finally, Western blot analysis detected Nef in anti-MHC-I, but not control, immunoprecipitations (Fig. 2E). Thus, the 27-kDa band coimmunoprecipitating with MHC-I in Nef-expressing cells is indeed Nef.
The converse immunoprecipitation using an antibody directed against Nef coprecipitated bands that were similar in size to MHC-I (data not shown). However, there was insufficient recovery to clearly determine their identity using the double immunoprecipitation protocol described above (41). This may be because only a small fraction of total cellular Nef associates with MHC-I, or because Nef complexed with MHC-I is not accessible to anti-Nef antibodies.
We also tested the ability of Nef to associate with MHC-I in HIV-1-infected T cells. For this experiment T1 T cells were transduced with HIV-1 (NL-PI env frameshift mutant). Forty-eight hours later, metabolically labeled lysates were generated as described above. A band the size of Nef was present in Nef-containing lysates immunoprecipitated with an antibody against β2M but not in control antibody immunoprecipitations or in anti-β2M immunoprecipitations from lysates that did not contain Nef (Fig. 2F). While the amount of coprecipitating Nef appears to be less than what was recovered from astrocytic cells, this result is not surprising. The interaction between Nef and MHC-I may be transient, and trafficking of MHC-I in T cells has been reported to occur more rapidly than in other cell types (26). Additionally, only a percentage (∼50%) of the T1 cells were transduced with HIV-1, so not all the cells were expressing Nef.
Nef selectively binds the cytoplasmic tail of HLA-A2, but not H2-Kd.
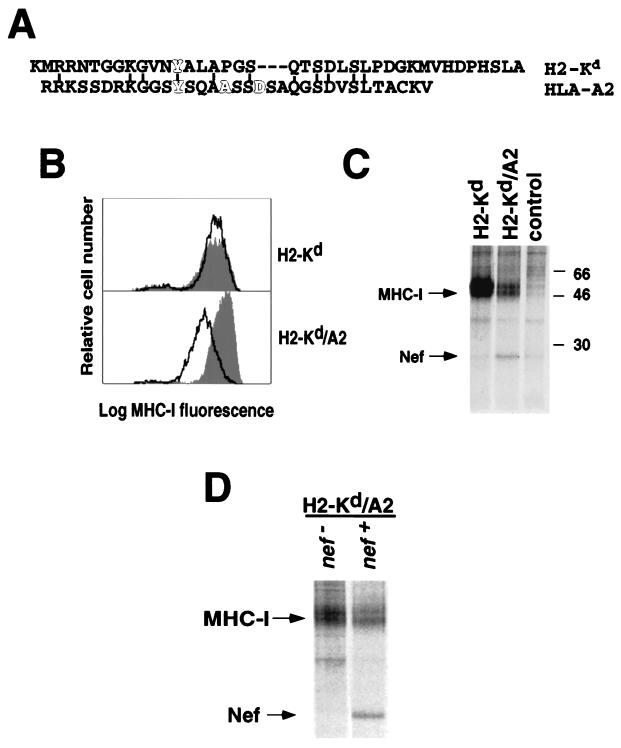
To determine the domain of MHC-I that associates with Nef, we examined Nef binding to chimeric MHC-I molecules. We had previously determined that the mouse class I molecule, H2-Kd, was not efficiently downmodulated by Nef because it lacked necessary amino acids in the cytoplasmic tail (10) (Fig. 3A). However, a chimeric molecule composed of the H2-Kd extracellular domain fused to the HLA-A2 transmembrane domain and cytoplasmic tail (H2-Kd/A2) was efficiently downmodulated (10) (Fig. 3B). Therefore, we predicted that Nef would specifically bind to the H2-Kd/A2 chimera and not to wild-type H2-Kd. To test this hypothesis, astrocytic cell lines stably expressing these molecules were transduced at an estimated MOI of 100:1 with adeno-Nef and immunoprecipitated with an antibody against H2-Kd. We found that Nef coimmunoprecipitated with the H2-Kd/A2 chimera much more efficiently than with wild-type H2-Kd (Fig. 3C). Control immunoprecipitations using an antibody directed against Nef revealed similar levels of Nef expression in these two cell lines (data not shown), indicating that differential binding is not an artifact of different Nef expression levels. Because the H2-Kd/A2 chimera differs from H2-Kd only in that the transmembrane and cytoplasmic tail are derived from HLA-A2, the above data indicate that these domains are necessary and sufficient for binding to Nef.
FIG. 3.
Interaction of Nef and MHC-I is dependent on specific amino acid sequences in the cytoplasmic tail. (A) Amino acid alignment of the cytoplasmic tails of the murine MHC-I molecule H2-Kd and the human MHC-I molecule HLA-A2. Amino acids that are important for downmodulation of MHC-I by Nef are highlighted (4, 22). Conserved amino acids between H2-Kd and HLA-A2 are also indicated. (B) Nef downmodulates H2-Kd with HLA-A2 transmembrane and cytoplasmic domains more efficiently than wild-type H2-Kd. 373mg astrocytic cells stably expressing either wild-type H2-Kd or H2-Kd with an HLA-A2 tail (H2-Kd/A2) were transduced with adeno-Nef or control-adeno at an estimated MOI of 100:1. Twenty-four hours later, the cells were stained with an antibody against H2-Kd and analyzed by flow cytometry. The filled curve represents MHC-I levels of cells treated with the control virus or nontransduced cells (there was no significant difference in MHC-I expression between untreated cells and cells treated with control-adeno). The solid line represents the results of Nef expression. Data presented are representative of at least three experiments. (C) Nef specifically binds the HLA-A2 cytoplasmic tail. The astrocytic cell lines described above were transduced with Nef-adeno or control-adeno at an estimated MOI of 100:1, metabolically labeled for 4 h, treated with DSP, and immunoprecipitated using an antibody against H2-Kd or mouse IgG. Results shown are representative of six separate experiments. (D) Reduced yield of H2-Kd/A2 compared with H2-Kd is dependent on Nef expression. 373mg stable cell lines expressing the MHC-I H2-Kd/A2 chimera were transduced with control-adeno or Nef-adeno at an estimated MOI of 300:1. They were metabolically labeled and immunoprecipitaited as in the experiment for which results are shown in panel C. Results shown are representative of two experiments.
It has been reported that Nef reduces the half-life of MHC-I by about twofold after 8 h (38). Consistent with these reports, a Nef-dependent loss of MHC-I stability was not apparent after a shorter (4-h) labeling period when total MHC-I was immunoprecipitated with an antibody directed against β2M (Fig. 2A). However, when an antibody specific for the H2-Kd heavy chain was used, we consistently detected a relatively small Nef-dependent loss of H2-Kd/A2, but not H2-Kd (Fig. 3C and D). An effect of Nef at earlier time points was probably more evident in immunoprecipitations of H2-Kd/A2 than in immunoprecipitations of total MHC-I, because not all MHC-I allotypes immunoprecipitated by the anti-β2M antibody are affected by Nef, and because the effect was not large (twofold or less). In a previous study, Nef-dependent degradation was reversed with ammonium chloride (38)—suggesting that it occurs in acidic organelles. In agreement with these results, addition of ammonium chloride increased the yield of Nef-MHC-I complexes (data not shown), and ammonium chloride was added in subsequent experiments (see below).
Nef binds to the HLA-A2 but not the HLA-E cytoplasmic tail.
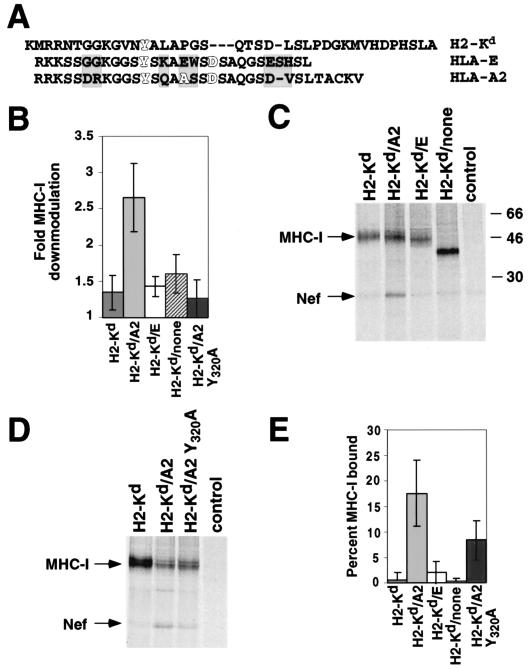
There are also amino acid differences between HLA-A2 and HLA-E that result in differential downmodulation of these molecules (Fig. 4A) (4). To determine whether Nef binding explains the differential downmodulation of HLA-A2 and HLA-E, we replaced the HLA-A2 cytoplasmic tail in H2-Kd/A2 with the HLA-E cytoplasmic tail (H2-Kd/E). As an additional control, the HLA-A2 cytoplasmic tail was removed from H2-Kd/A2 (H2-Kd/none). These molecules were expressed in astrocytic cells, and tested for sensitivity to Nef as described above. As predicted, Nef only downmodulated chimeric MHC-I molecules with a wild-type HLA-A2 tail (Fig. 4B). Correspondingly, Nef associated with MHC-I molecules that were downmodulated (HLA-A2) but not with molecules resistant to downmodulation (H2-Kd, HLA-E) (Fig. 4C and E). We found that Nef bound to ∼18% of the chimera with the wild-type HLA-A2 tail, whereas it bound to ≤1% of the molecules with the H2-Kd or HLA-E tail or no tail (Fig. 4E). The differential binding of these molecules did not result from different Nef expression levels, because anti-Nef immunoprecipitations indicated that similar levels of Nef were expressed in each of these cell lines (data not shown). Thus, the specific downmodulation conferred by the HLA-A2 cytoplasmic tail can be explained by its ability to bind Nef (compare Fig. 4B with Fig. 4E). These data are particularly interesting, as the HLA-E tail differs from the HLA-A2 tail by only a few amino acids (Fig. 4A, shaded regions).
FIG. 4.
Nef binds HLA-A2 but not HLA-E. (A) Amino acid alignment of the cytoplasmic tails of the murine MHC-I molecule H2-Kd and the human MHC-I molecules HLA-A2 and HLA-E. Amino acids that are important for downmodulation of MHC-I by Nef are highlighted (4, 22). Shaded residues indicate the differences between HLA-A2 and HLA-E. (B) The HLA-A2 cytoplasmic tail is necessary for Nef-mediated downmodulation. Astrocytic cell lines expressing the indicated MHC-I molecule were transduced with adeno-Nef or control-adeno at an estimated MOI of 100:1. After 24 h, the cells were stained with an antibody against H2-Kd and analyzed by flow cytometry. Fold downmodulation was calculated by comparing the mean fluorescence intensity of cells transduced with control-adeno to that of those transduced with adeno-Nef (nontransduced cells had fluorescence similar to that of control-adeno-transduced cells). (C) The HLA-A2 cytoplasmic tail is necessary for Nef binding. Astrocytic cells stably expressing the indicated MHC-I molecule were transduced with adeno-Nef at an estimated MOI of 100:1. They were metabolically labeled for 4 h in the presence of 25 mM NH4Cl, treated with DSP, and immunoprecipitated using an antibody against H2-Kd or mouse IgG. Results shown are representative of six independent experiments. (D) Mutation of Y320 disrupts Nef binding. Coimmunoprecipitation experiments were performed as described for panel C, using astrocytic cells stably expressing MHC-I molecule H2-Kd, H2-Kd/A2, or H2-Kd/A2 Y320A. The results are representative of three independent experiments. (E) Quantitation of Nef binding. The percentage of MHC-I molecules bound to Nef was quantitated as follows: the number of Nef counts specifically coprecipitating with MHC-I was determined using a phosphorimager. Nef counts were then corrected for the different numbers of methionines and cysteines in Nef versus MHC-I (7 versus 10 to 14 depending upon the chimera). Assuming a 1:1 ratio of Nef to MHC-I molecules, the percentage of MHC-I molecules bound to Nef was determined by dividing the counts from bound MHC-I molecules (equivalent to corrected Nef counts) by the total MHC-I counts × 100. Results shown are means ± standard deviations from six independent experiments.
Nef binding to the HLA-A2 Y320A mutant is impaired.
It has been reported that specific mutations within the HLA-A2 tail confer resistance to downmodulation by Nef (4, 22). For example, HLA-A2 molecules containing a tyrosine mutation Y320A (14, 22) are not downmodulated by Nef. In agreement with these results, we found that a chimeric molecule containing the Y320A mutation (H2-Kd/A2 Y320A) was also resistant to downmodulation by Nef (Fig. 4B). To determine if a defect in Nef binding could explain its resistance to downmodulation, we measured the ability of this molecule to coprecipitate Nef. We found that Nef bound to ∼18% of wild-type HLA-A2, whereas the Y320A mutation decreased this binding to ∼8% (Fig. 4D and E). Thus, the resistance of the Y320A mutation to downmodulation by Nef was partially explained by a binding defect. However, because the defect was only partial, this residue may have an additional function important for Nef-mediated downmodulation of HLA-A2.
HIV-1 Nef binds to the cytoplasmic tail of HLA-A2 in vitro by use of purified proteins.
To determine whether the binding of Nef to the cytoplasmic tail of HLA-A2 was direct, we tested binding of the purified proteins in vitro. For these experiments, the HLA-A2 cytoplasmic tail was purified as a GST fusion protein (GST-A2), mixed with purified Nef, and precipitated using glutathione beads. A weak but specific coprecipitation of Nef with GST-A2 was observed without a cross-linker (approximately 0.2% of the Nef input [Fig. 5A]). Addition of the cross-linking reagent DSP stabilized the specific Nef-MHC-I cytoplasmic tail interaction, increasing recovery approximately 10-fold (Fig. 5A). Therefore, DSP was included in subsequent experiments. It should be noted that the percentage of Nef recovered underestimates the amount of complex formed because we recover only a fraction of the GST fusion protein when it is rebound to the beads and eluted. Also, the cross-linker treatment reduces overall GST protein recovery (Fig. 5A).
FIG. 5.
HIV-1 Nef directly binds to the cytoplasmic domain of HLA-A2 in vitro. (A) Nef association with the HLA-A2 cytoplasmic tail is specifically stabilized by cross-linking. The cytoplasmic tail of HLA-A2 (A2) and HIV-1 Nef were overexpressed and purified from Escherichia coli as GST fusion proteins. GST was cleaved from Nef by using thrombin, as described in Materials and Methods. Purified GST and GST-A2 were then incubated with recombinant Nef, with or without treatment with a cross-linking reagent (DSP), and proteins were then precipitated with glutathione beads. The mean percentage of Nef recovered in the pulldown assay was determined by densitometry of immunoblots from three independent experiments (graph). A representative anti-Nef immunoblot (middle panel) and a Coomassie stain of the membrane (bottom panel) are shown. All images in the middle panel were taken from the same exposure of an anti-Nef immunoblot. (B) Nef core domain is functional for binding to GST-PACSfbr. The furin binding region (fbr) of human PACS-1a (amino acids 117 to 266) was fused to GST, purified, and tested for in vitro binding to either NL4-3 Nef, a Nef acidic domain mutant (E62-65A), or the Nef core domain. Results shown are representative of two independent experiments, each performed in triplicate. (C) The Nef core domain (amino acids 58 to 206) is not sufficient to bind GST-A2. GST pulldown assays were performed as described above. A fraction of Nef protein input (1%) was immunoblotted (left panel) to control for equal protein concentration and antibody recognition. Results shown are representative of three independent experiments, each performed in duplicate.
To confirm the specificity of the in vitro binding assay, and to determine which domain of Nef bound HLA-A2, we purified the globular core domain of Nef (amino acids 58 to 206) (1, 16, 20) as described in Materials and Methods. However, repeated attempts to purify the N-terminal anchor domain of Nef (amino acids 1 to 57) were unsuccessful because of its apparent instability. To demonstrate that the core domain was functionally active, we tested whether it was able to specifically bind the PACS-1a furin binding region (PACSfbr) (Fig. 5B) (33). We found that PACSfbr bound to wild-type Nef and to the Nef core domain, but not to a mutant Nef protein (Nef E62-65A) previously shown to be defective in PACSfbr binding (33) (Fig. 5B). Under the same conditions, wild-type Nef, but not the Nef core domain, specifically associated with the cytoplasmic domain of HLA-A2 (Fig. 5C). These data indicate that our assay is specific and that amino acid residues in the Nef anchor domain are needed for MHC-I, but not PACS-1a, binding.
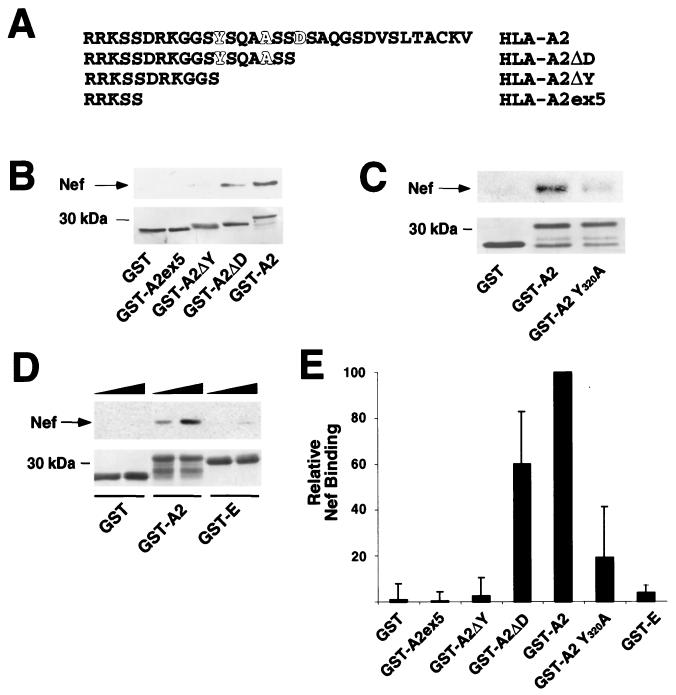
HIV-1 Nef directly binds to a sequence in the cytoplasmic domain of HLA-A2 that is required for downmodulation.
To identify the binding site for Nef on HLA-A2, we constructed a series of carboxy-terminal truncation mutants (Fig. 6A). The first truncation, A2ΔD, retained the ability to associate with Nef (Fig. 6B and E) and is known to support MHC-I downmodulation (21). However, when the sequence Y320SQAASS326, which is necessary for MHC-I downmodulation (4, 14, 22), was deleted (A2ΔY), Nef binding was abolished (Fig. 6B and E). Furthermore, a point mutation in this region of the tail (A2 Y320A) that disrupts the ability of Nef to downmodulate MHC-I (14, 22) also significantly decreased the binding of Nef (Fig. 6C and E), in agreement with the in vivo binding assay (Fig. 4D and E). In addition, the HLA-E tail, which does not support Nef-mediated downmodulation and does not bind Nef in vivo (Fig. 4B, C, and E), did not efficiently interact with Nef in this assay system (Fig. 6D and E). Taken together, our studies using purified Nef and MHC-I cytoplasmic tails indicate that Nef directly binds residues in the cytoplasmic tail that are necessary for downmodulation and that the Nef anchor domain is necessary for this interaction.
FIG. 6.
HIV-1 Nef directly binds to a sequence in the cytoplasmic domain of MHC-I that is necessary for downmodulation. (A) Cytoplasmic tail sequences of deletion mutants used in Nef binding assays. Amino acids that are important for downmodulation of MHC-I by Nef are highlighted (4, 22). (B) Nef binds to a region of the cytoplasmic tail of HLA-A2 that contains amino acid residues required for downmodulation. C-terminal truncations of the HLA-A2 cytoplasmic domain were fused to GST and subjected to the in vitro binding assay described in the legend to Fig. 5. A representative immunoblot (upper panel) and Coomasie stain of the membrane (lower panel) are shown. (C) Tyrosine 320 is required for efficient Nef binding. A point mutation in the cytoplasmic tail of HLA-A2 (GST-A2 Y320A) was tested in the in vitro binding assay. A representative immunoblot (upper panel) and Coomasie stain of the membrane (lower panel) are shown. (D) The cytoplasmic domain of HLA-E does not efficiently bind Nef. The cytoplasmic tail of HLA-E was fused to GST (GST-E) and was tested in the in vitro binding assay. An anti-Nef immunoblot (upper panel) and Coomasie stain of the membrane (lower panel) are shown. In this experiment, Nef protein was added at increasing amounts (1 to 2 μg), indicated by the solid triangles. (E) Quantitation of the ability of GST proteins to bind Nef in vitro. To assess the relative binding of the cytoplasmic tail constructs, the amount of Nef coprecipitating was determined by densitometry using immunoblots from a minimum of three independent experiments. Data presented are the mean percentages of wild-type binding ± standard deviations for each tail construct.
DISCUSSION
Differential binding of MHC-I allotypes.
Our results indicate that Nef downmodulates MHC-I through a direct physical interaction. This conclusion is based on the facts that Nef and MHC-I colocalized in the perinuclear region of the cell, Nef-MHC-I complexes were isolated from living cells, and purified Nef bound the purified HLA-A2 cytoplasmic tail in vitro. The yield of complexes was relatively small in vitro and required a cross-linker, suggesting that additional factors (e.g., membrane association, multiprotein complex formation, or oligomerization) (23, 32, 33) might be involved in stabilizing the Nef-MHC-I interaction in vivo or that the interaction is transient.
In addition, we demonstrated that HIV-1 Nef bound to the cytoplasmic domain of a subset of MHC-I molecules that are downmodulated by Nef (HLA-A2 but not HLA-E, H2-Kd, or an MHC-I molecule lacking a cytoplasmic tail). Moreover, Nef binding required the same amino acid sequence (YSQAASS) in the cytoplasmic domain of HLA-A2 that is necessary for MHC-I downmodulation. The corresponding amino acid sequence in HLA-E (YSKAEWS) differs by three amino acids, explaining the inability of Nef to bind this molecule. These data provide a molecular basis for the differential downmodulation of HLA-E and HLA-A2 by HIV-1 Nef.
Mechanism for MHC-I downmodulation by Nef.
The data presented here and elsewhere (4, 6, 14, 21, 22, 33, 38, 44) support a model in which Nef binds to MHC-I and serves as an adaptor to recruit other cellular factors that promote intracellular accumulation and accelerated degradation of MHC-I. Nef may accomplish this task by promoting protein-protein interactions with MHC-I and a number of trafficking molecules reported to interact with Nef (PACS-1a, phosphatidylinositol 3-kinase [PI 3-kinase], adaptor protein complexes, and a subunit of a vacuolar ATPase [NBP1]) (6, 19, 25, 33). Indeed, our data indicate that the MHC-I cytoplasmic tail and PACS-1 interact with functionally distinct domains of Nef and suggests that both MHC-I and PACS-1 could bind Nef simultaneously. However, additional experiments aimed at identifying three-way complexes are required to prove which protein complexes are specifically involved in the disruption of MHC-I transport by HIV-1 Nef.
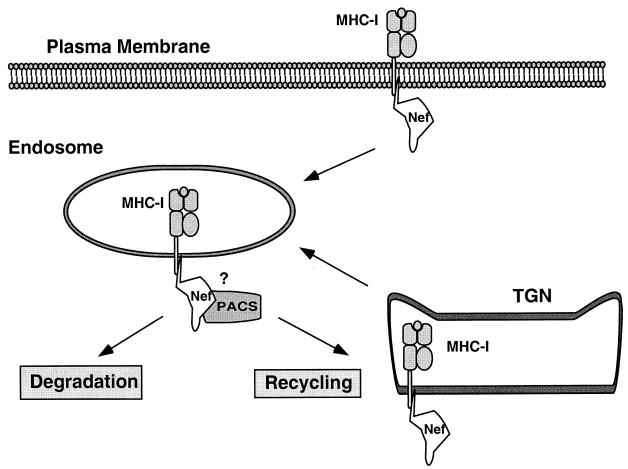
Data from a number of laboratories indicate that Nef may affect MHC-I at two different cellular locations to achieve efficient downregulation and targeting of MHC-I to the TGN (Fig. 7). The first pathway involves acceleration of endocytosis of MHC-I at the plasma membrane (14, 38). The second pathway is independent of endocytosis, as it is not blocked by dominant-negative dynamin (K44A) in HeLa cells (21, 44). There is evidence that the latter pathway involves redirecting MHC-I transport from the TGN (44); however, the specific details remain relatively undefined. The data presented here indicate that Nef most likely disrupts MHC-I at these two locations by directly binding the MHC-I cytoplasmic tail, and promoting its association with other cellular proteins. Interestingly, vesicles from the TGN and plasma membrane may intersect at the endosomal-TGN network—the site of Nef/MHC-I accumulation (14, 22, 33, 44), and a known site of PACS-1 activity (29, 48). PI 3-kinase, a regulator of TGN-to-endosome/lysosome trafficking (3, 8, 11), is necessary for Nef's effects on MHC-I (44), providing additional evidence for the role of this pathway. The ability of Nef to affect the trafficking of MHC-I at both the TGN and the plasma membrane would maximize the effects of Nef on antigen presentation and escape of HIV-infected cells from CTL killing.
FIG. 7.
Model for Nef-induced MHC-I downmodulation. HIV-1 Nef binds to the cytoplasmic domain of MHC-I, resulting in both accelerated internalization from the plasma membrane (38) and a block of transport to the cell surface. Subsequently, MHC-I is degraded in an acidic compartment (38) or recycled from endosomes to the TGN (14, 22, 33, 44). Nef may accomplish this by acting as an adaptor protein that links MHC-I to other molecules involved in protein transport such as PACS-1 (33), adaptor protein complexes (AP-1, -2, or -3) (22), vacuolar ATPase (25), or PI 3-kinase (44). However, the existence of a complex between MHC-I, Nef, and another Nef-binding protein has not yet been demonstrated.
For several years, models of MHC-I downmodulation by HIV-1 Nef in which Nef physically associates with MHC-I have been proposed. However, this study is the first to provide direct experimental evidence of this. In addition, data presented here provide the first evidence that complexes of Nef bound to cytoplasmic tails exist in vivo. Previous studies that have examined binding of Nef to other cytoplasmic tails (CD4 and the ζ chain of the T-cell receptor) demonstrated binding in vitro or by using insect cells (15-17, 35, 37). Our demonstration that Nef binds MHC-I both in vitro and in vivo provides strong evidence that this complex has biological significance. Disruption of the Nef-MHC-I complex is an appropriate goal for therapeutic drug design because it should inhibit Nef-mediated MHC-I downmodulation and allow the immune system to function more effectively.
Acknowledgments
M. Williams and J. F. Roeth contributed equally to this work.
We thank Tracey Filzen for exceptional technical support and Lezlie Siegel-Hopkins for assistance with manuscript preparation. We are grateful to the University of Michigan Gene Vector Core facility for supplying recombinant adenovirus, the Flow Cytometry Core for assistance in developing cell lines, the Microscopy and Image-Analysis laboratory for assistance with microscopy, and the Hybridoma Core facility for providing the BB7.2 ascites.
This work was supported by NIH grant RO1 AI46998, the Pew Charitable Trusts, and the University of Michigan Biological Scholars program. M.W. and J.F.R. were supported by an NIH predoctoral traineeship in cellular and molecular biology (GM07315). M.W. was also supported by a Molecular Mechanisms of Microbial Pathogenesis Training Grant (5T32 AI07528). M.R.K. was supported by a Genetics Training Grant (5T32 GM07544).
REFERENCES
- 1.Arold, S., P. Franken, M. P. Strub, F. Hoh, S. Benichou, R. Benarous, and C. Dumas. 1997. The crystal structure of HIV-1 Nef protein bound to the Fyn kinase SH3 domain suggests a role for this complex in altered T cell receptor signaling. Structure 5:1361-1372. [DOI] [PubMed] [Google Scholar]
- 2.Bell, I., T. M. Schaefer, R. P. Trible, A. Amedee, and T. A. Reinhart. 2001. Down-modulation of the costimulatory molecule, CD28, is a conserved activity of multiple SIV Nefs and is dependent on histidine 196 of Nef. Virology 283:148-158. [DOI] [PubMed] [Google Scholar]
- 3.Brown, W. J., D. B. DeWald, S. D. Emr, H. Plutner, and W. E. Balch. 1995. Role for phosphatidylinositol 3-kinase in the sorting and transport of newly synthesized lysosomal enzymes in mammalian cells. J. Cell Biol. 130:781-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen, G. B., R. T. Gandhi, D. M. Davis, O. Mandelboim, B. K. Chen, J. L. Strominger, and D. Baltimore. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661-671. [DOI] [PubMed] [Google Scholar]
- 5.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 6.Craig, H. M., T. R. Reddy, N. L. Riggs, P. P. Dao, and J. C. Guatelli. 2000. Interactions of HIV-1 nef with the mu subunits of adaptor protein complexes 1, 2, and 3: role of the dileucine-based sorting motif. Virology 271:9-17. [DOI] [PubMed] [Google Scholar]
- 7.Crump, C. M., Y. Xiang, L. Thomas, F. Gu, C. Austin, S. A. Tooze, and G. Thomas. 2001. PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. EMBO J. 20:2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson, H. W. 1995. Wortmannin causes mistargeting of procathepsin D: evidence for the involvement of a phosphatidylinositol 3-kinase in vesicular transport to lysosomes. J. Cell Biol. 130:797-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doms, R. W., and D. Trono. 2000. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 14:2677-2688. [DOI] [PubMed] [Google Scholar]
- 10.Fleis, R., T. Filzen, and K. L. Collins. Species-specific effects of HIV-1 Nef-mediated MHC-I downmodulation. Virology, in press. [DOI] [PubMed]
- 11.Gaffet, P., A. T. Jones, and M. J. Clague. 1997. Inhibition of calcium-independent mannose 6-phosphate receptor incorporation into trans-Golgi network-derived clathrin-coated vesicles by wortmannin. J. Biol. Chem. 272:24170-24175. [DOI] [PubMed] [Google Scholar]
- 12.Geleziunas, R., W. Xu, K. Takeda, H. Ichijo, and W. C. Greene. 2001. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 410:834-838. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg, M. E., S. Bronson, M. Lock, M. Neumann, G. N. Pavlakis, and J. Skowronski. 1997. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 16:6964-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg, M. E., A. J. Iafrate, and J. Skowronski. 1998. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 17:2777-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenway, A., A. Azad, and D. McPhee. 1995. Human immunodeficiency virus type 1 Nef protein inhibits activation pathways in peripheral blood mononuclear cells and T-cell lines. J. Virol. 69:1842-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grzesiek, S., S. J. Stahl, P. T. Wingfield, and A. Bax. 1996. The CD4 determinant for downregulation by HIV-1 Nef directly binds to Nef. Mapping of the Nef binding surface by NMR. Biochemistry 35:10256-10261. [DOI] [PubMed] [Google Scholar]
- 17.Harris, M. P., and J. C. Neil. 1994. Myristoylation-dependent binding of HIV-1 Nef to CD4. J. Mol. Biol. 241:136-142. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins, N. 1993. High titers of retrovirus (vesicular stomatitis virus) pseudotypes, at last. Proc. Natl. Acad. Sci. USA 90:8759-8760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, Y. H., S. H. Chang, J. H. Kwon, and S. S. Rhee. 1999. HIV-1 Nef plays an essential role in two independent processes in CD4 down-regulation: dissociation of the CD4-p56 (lck) complex and targeting of CD4 to lysosomes. Virology 257:208-219. [DOI] [PubMed] [Google Scholar]
- 20.Lee, C. H., K. Saksela, U. A. Mirza, B. T. Chait, and J. Kuriyan. 1996. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell 85:931-942. [DOI] [PubMed] [Google Scholar]
- 21.Le Gall, S., F. Buseyne, A. Trocha, B. D. Walker, J. M. Heard, and O. Schwartz. 2000. Distinct trafficking pathways mediate Nef-induced and clathrin-dependent major histocompatibility complex class I down-regulation. J. Virol. 74:9256-9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Gall, S., L. Erdtmann, S. Benichou, C. Berlioz-Torrent, L. Liu, R. Benarous, J. M. Heard, and O. Schwartz. 1998. Nef interacts with the mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity 8:483-495. [DOI] [PubMed] [Google Scholar]
- 23.Liu, L. X., N. Heveker, O. T. Fackler, S. Arold, S. Le Gall, K. Janvier, B. M. Peterlin, C. Dumas, O. Schwartz, S. Benichou, and R. Benarous. 2000. Mutation of a conserved residue (D123) required for oligomerization of human immunodeficiency virus type 1 Nef protein abolishes interaction with human thioesterase and results in impairment of Nef biological functions. J. Virol. 74:5310-5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, X., J. A. Schrager, G. D. Lange, and J. W. Marsh. 2001. HIV Nef-mediated cellular phenotypes are differentially expressed as a function of intracellular Nef concentrations. J. Biol. Chem. 276:32763-32770. [DOI] [PubMed] [Google Scholar]
- 25.Lu, X., H. Yu, S. H. Liu, F. M. Brodsky, and B. M. Peterlin. 1998. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity 8:647-656. [DOI] [PubMed] [Google Scholar]
- 26.Machy, P., A. Truneh, D. Gennaro, and S. Hoffstein. 1987. Major histocompatibility complex class I molecules internalized via coated pits in T lymphocytes. Nature 328:724-726. [DOI] [PubMed] [Google Scholar]
- 27.Mahlknecht, U., C. Deng, M. C. Lu, T. C. Greenough, J. L. Sullivan, W. A. O'Brien, and G. Herbein. 2000. Resistance to apoptosis in HIV-infected CD4+ T lymphocytes is mediated by macrophages: role for Nef and immune activation in viral persistence. J. Immunol. 165:6437-6446. [DOI] [PubMed] [Google Scholar]
- 28.Manninen, A., and K. Saksela. 2002. HIV-1 Nef interacts with inositol trisphosphate receptor to activate calcium signaling in T cells. J. Exp. Med. 195:1023-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molloy, S. S., E. D. Anderson, F. Jean, and G. Thomas. 1999. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis Trends Cell Biol. 9:28-35. [DOI] [PubMed] [Google Scholar]
- 30.Munch, J., N. Stolte, D. Fuchs, C. Stahl-Hennig, and F. Kirchhoff. 2001. Efficient class I major histocompatibility complex down-regulation by simian immunodeficiency virus Nef is associated with a strong selective advantage in infected rhesus macaques. J. Virol. 75:10532-10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pentcheva, T., and M. Edidin. 2001. Clustering of peptide-loaded MHC class i molecules for endoplasmic reticulum export imaged by fluorescence resonance energy transfer. J. Immunol. 166:6625-6632. [DOI] [PubMed] [Google Scholar]
- 33.Piguet, V., L. Wan, C. Borel, A. Mangasarian, N. Demaurex, G. Thomas, and D. Trono. 2000. HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat. Cell Biol. 2:163-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robichaud, G. A., and L. Poulin. 2000. HIV type 1 nef gene inhibits tumor necrosis factor alpha-induced apoptosis and promotes cell proliferation through the action of MAPK and JNK in human glial cells AIDS Res. Hum. Retrovir. 16:1959-1965. [DOI] [PubMed] [Google Scholar]
- 35.Rossi, F., A. Gallina, and G. Milanesi. 1996. Nef-CD4 physical interaction sensed with the yeast two-hybrid system. Virology 217:397-403. [DOI] [PubMed] [Google Scholar]
- 36.Saksela, K. 1997. HIV-1 Nef and host cell protein kinases. Front. Biosci. 2:d606-d618. [DOI] [PubMed] [Google Scholar]
- 37.Schaefer, T. M., I. Bell, B. A. Fallert, and T. A. Reinhart. 2000. The T-cell receptor zeta chain contains two homologous domains with which simian immunodeficiency virus Nef interacts and mediates down-modulation. J. Virol. 74:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 39.Shugars, D. C., M. S. Smith, D. H. Glueck, P. V. Nantermet, F. Seillier-Moiseiwitsch, and R. Swanstrom. 1993. Analysis of human immunodeficiency virus type 1 nef gene sequences present in vivo. J. Virol. 67:4639-4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sol-Foulon, N., A. Moris, C. Nobile, C. Boccaccio, A. Engering, J. P. Abastado, J. M. Heard, Y. van Kooyk, and O. Schwartz. 2002. HIV-1 Nef-induced upregulation of DC-SIGN in dendritic cells promotes lymphocyte clustering and viral spread. Immunity 16:145-155. [DOI] [PubMed] [Google Scholar]
- 41.Story, C. M., M. H. Furman, and H. L. Ploegh. 1999. The cytosolic tail of class I MHC heavy chain is required for its dislocation by the human cytomegalovirus US2 and US11 gene products. Proc. Natl. Acad. Sci. USA 96:8516-8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stumptner-Cuvelette, P., S. Morchoisne, M. Dugast, S. Le Gall, G. Raposo, O. Schwartz, and P. Benaroch. 2001. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. USA 98:12144-12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sugita, M., and M. B. Brenner. 1995. Association of the invariant chain with major histocompatibility complex class I molecules directs trafficking to endocytic compartments. J. Biol. Chem. 270:1443-1448. [DOI] [PubMed] [Google Scholar]
- 44.Swann, S. A., M. Williams, C. M. Story, K. R. Bobbitt, R. Fleis, and K. L. Collins. 2001. HIV-1 Nef blocks transport of MHC class I molecules to the cell surface via a PI 3-kinase-dependent pathway. Virology 282:267-277. [DOI] [PubMed] [Google Scholar]
- 45.Swigut, T., N. Shohdy, and J. Skowronski. 2001. Mechanism for down-regulation of CD28 by Nef. EMBO J. 20:1593-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tornatore, C., K. Meyers, W. Atwood, K. Conant, and E. Major. 1994. Temporal patterns of human immunodeficiency virus type 1 transcripts in human fetal astrocytes. J. Virol. 68:93-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walk, S. F., M. Alexander, B. Maier, M. L. Hammarskjold, D. M. Rekosh, and K. S. Ravichandran. 2001. Design and use of an inducibly activated human immunodeficiency virus type 1 Nef to study immune modulation. J. Virol. 75:834-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wan, L., S. S. Molloy, L. Thomas, G. Liu, Y. Xiang, S. L. Rybak, and G. Thomas. 1998. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 94:205-216. [DOI] [PubMed] [Google Scholar]
- 49.Wolf, D., V. Witte, B. Laffert, K. Blume, E. Stromer, S. Trapp, P. d'Aloja, A. Schurmann, and A. S. Baur. 2001. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat. Med. 7:1217-1224. [DOI] [PubMed] [Google Scholar]
- 50.Yoon, K., J. G. Jeong, and S. Kim. 2001. Stable expression of human immunodeficiency virus type 1 Nef confers resistance against Fas-mediated apoptosis AIDS Res. Hum. Retrovir. 17:99-104. [DOI] [PubMed] [Google Scholar]