Abstract
As Rift Valley fever (RVF) virus, and probably all members of the family Bunyaviridae, matures in the Golgi apparatus, the targeting of the virus glycoproteins to the Golgi apparatus plays a pivotal role in the virus replication cycle. No consensus Golgi localization motif appears to be shared among the glycoproteins of these viruses. The viruses of the family Bunyaviridae synthesize their glycoproteins, GN and GC, as a polyprotein. The Golgi localization signal of RVF virus has been shown to reside within the GN protein by use of a plasmid-based transient expression system to synthesize individual GN and GC proteins. While the distribution of individually expressed GN significantly overlaps with cellular Golgi proteins such as β-COP and GS-28, GC expressed in the absence of GN localizes to the endoplasmic reticulum. Further analysis of expressed GN truncated proteins and green fluorescent protein/GN chimeric proteins demonstrated that the RVF virus Golgi localization signal mapped to a 48-amino-acid region of GN encompassing the 20-amino-acid transmembrane domain and the adjacent 28 amino acids of the cytosolic tail.
Rift Valley fever (RVF) virus is a member of the genus Phlebovirus within the family Bunyaviridae. RVF virus is endemic to much of sub-Saharan Africa; however, regions affected by this pathogen are expanding as a result of introductions of the virus from sub-Saharan Africa into neighboring regions, such as Egypt and the Arabian Peninsula (3-5, 22). RVF virus causes severe disease in livestock and humans in a cycle that is linked with periods of unusually high rainfall (17). Heavy rainfall creates pools of standing water, or “dambos,” that provide breeding habitat for the mosquito vectors of RVF virus (18). Although the RVF virus natural infectious cycle has been linked to the Aedes sp. mosquito, many mosquito species can be infected and subsequently transmit RVF virus (9, 10, 16, 21). The fact that many mosquito species can act as vectors increases the likelihood of RVF virus becoming endemic to areas outside of its traditional range.
RVF virus, like all viruses of the family Bunyaviridae, has a tripartite negative-stranded RNA genome (28). Most, if not all, members of the family Bunyaviridae mature by budding into the lumen of the Golgi (27). Localization of the envelope glycoproteins is a crucial step in the virus maturation process. The two RVF virus envelope glycoproteins localize to the Golgi apparatus in the absence of other virally encoded proteins (31), and thus one or both of these glycoproteins contains a Golgi localization signal. Following Golgi localization, the remaining structural proteins and the genome are recruited, followed by viral budding. Members of the family Bunyaviridae lack matrix proteins; therefore, the glycoproteins (presumably the cytosolic tail region of one of the glycoproteins) are responsible for the recruitment of the remaining core elements of the virion. Mature virions are then released from the cell through fusion of virion-filled Golgi elements with the plasma membrane (27).
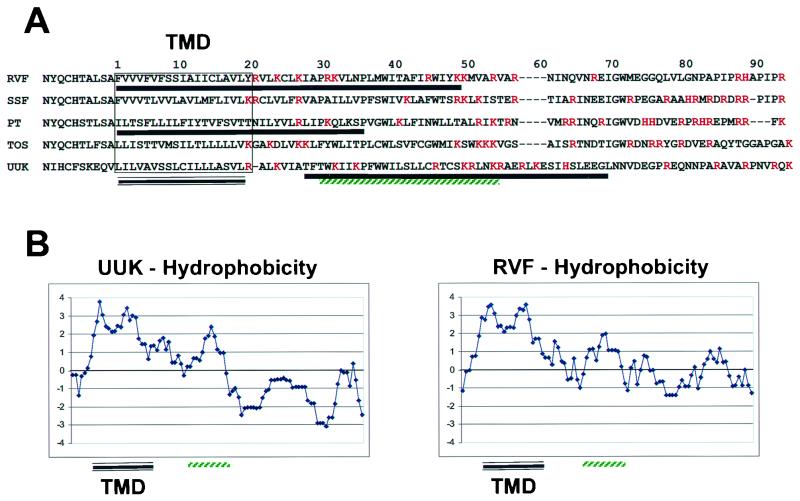
The envelope glycoproteins of Uukuniemi and Punta Toro viruses, both belonging to the genus Phlebovirus, have been characterized with respect to their subcellular localization (1, 19, 20, 23, 29). Both the carboxy-terminal glycoprotein (GC) and the amino-terminal glycoprotein (GN) localize to the Golgi apparatus when expressed together as a polyprotein precursor. However, GC does not localize to the Golgi apparatus when expressed in the absence of GN (6, 23); it instead localizes to the endoplasmic reticulum (ER). The GC of all members of the genus Phlebovirus contain lysine-based ER retrieval signals at their extreme carboxy terminus. Therefore, GC is thought to attain Golgi localization through physical interaction with GN. Both viruses have Golgi localization signals in their GN. However, the location of the signal within the GN was not consistent between Uukuniemi and Punta Toro viruses (1, 2, 19). The Golgi localization signal for the Uukuniemi virus GN is contained exclusively within the cytosolic tail and the signal for the Punta Toro virus GN is contained within a region encompassing the transmembrane domain and 10 amino acids of the cytosolic tail. However, there is little amino acid sequence conservation in the carboxy-terminal regions of the GN of members of the genus Phlebovirus (Fig. 1A). It is therefore plausible that within this genus more than one strategy is employed for envelope glycoprotein localization.
FIG. 1.
(A) ClustalW alignment of the carboxy-terminal region of the GN of viruses belonging to the genus Phlebovirus. The alignment begins 10 amino acids upstream of the transmembrane domain and concludes just prior to the signal sequence for GC. Abbreviations: SSF, Sicilian sandfly fever virus; PT, Punta Toro virus; TOS, Toscana virus; UUK, Uukuniemi virus. The transmembrane domain (TMD) is boxed and underlined, and the regions involved in Golgi localization of GN for RVF, PT and UUK are underlined with a single solid black line. The hydrophobic region of the UUK GN found sufficient for localization of a cytoplasmic protein chimera to the Golgi is underlined in green. Basic amino acids are in red. (B) Kyte-Doolittle hydrophobicity plots of the carboxy-terminal region of the GN for UUK and RVF. Positive scores indicate hydrophobic amino acid content, while negative numbers correspond to hydrophilic amino acid content. The TMD and second hydrophobic peak are indicated as in panel A.
Golgi localization of cellular proteins is thought to involve retrieval and/or retention processes (12, 24, 26). Retrieval of resident Golgi proteins from distal compartments is thought to rely in large part on signals contained within the sequence of the cytosolic tail of the escaped Golgi protein (24, 26). These sequences within the cytosolic tail of resident trans-Golgi network (TGN) proteins, such as those found in furin and TGN-38, recruit adapter proteins that in turn allow for packaging into clathrin-coated vesicles destined for the Golgi apparatus (11). Retention refers to the process by which resident Golgi proteins are prevented from moving into distal compartments. Retention signals are more diverse and can be contained within transmembrane domains, lumenal domains and multimerization domains (24). Unlike the retrieval motifs present in cytosolic tails of TGN proteins, there is no sequence homology among retention signals; these signals may instead be conformational in character. Viruses, such as RVF, which mature in the Golgi apparatus, presumably take advantage of the existing cellular machinery in order to localize their envelope glycoproteins properly. Thus, it is likely that retrieval and/or retention signals present in the RVF virus glycoproteins show similarity, either structural or amino acid sequence, to cellular resident Golgi proteins.
Primary to the understanding of RVF virus maturation is a characterization of the mechanism by which the structural elements of the virion are localized to the Golgi apparatus. We have begun a characterization of RVF virus maturation by identifying GN as containing a Golgi localization signal. Furthermore, we have mapped the Golgi localization signal to a 47-amino-acid region that includes the transmembrane domain and 28 amino acids of the cytosolic tail. This region of GN is sufficient to localize a GFP chimera to the Golgi apparatus. Contrary to expectations, deletion of either hydrophobic region within the Golgi localization signal does not lead to secretion to the plasma membrane, but rather to retention in the ER.
MATERIALS AND METHODS
Immunofluorescence.
BHK-T7 cells were grown on glass coverslips in 24-well plates to 80% confluency and then transfected with the various glycoprotein expression constructs by using Lipofectamine Plus (Invitrogen Life Technologies) under conditions described by the manufacturer. For experiments involving expression of GC alone, cells were cotransfected with pC-T7Pol, a plasmid that expresses T7 polymerase (25). All immunofluorescence experiments utilized cells that were fixed at 24 h posttransfection. For experiments involving internal staining, cells were fixed with 2% paraformaldehyde for 30 min at 4°C. The fixative was then removed and replaced with phosphate-buffered saline and 1% bovine serum albumin followed by permeabilization with 0.2% saponin for 5 min at room temperature. For cell surface staining, plates were chilled on ice for 10 min, at which point the medium was removed and prechilled fresh medium containing the appropriate antibody was added and allowed to incubate on ice for 30 min. Following incubation with the antibody, cells were washed three times with chilled medium. Following the washes, cells were fixed and permeabilized as for internal staining. Samples were examined on a Zeiss Axioplan microscope utilizing a 63× oil immersion objective and photographed with a Nikon CoolPix 9500 digital camera.
Cell lines and culture.
BHK cells that express T7 polymerase (BHK-T7) were obtained from Klaus K. Conzelmann. BHK-T7 cells were grown in Glasgow modified Eagle medium supplemented with 10% fetal calf serum, 1% nonessential amino acids, 5% tryptose phosphate broth, and Geneticin (1 mg/ml). Cells were maintained in 37°C incubators in the presence of water saturated with 5% CO2-95% air.
Materials.
Cell culture medium, pcDNA1.1, TOPO-TA pCR4, and Lipofectamine Plus were purchased from Invitrogen Life Technologies (Carlsbad, Calif.). A QuikChange mutagenesis kit was obtained from Stratagene (La Jolla, Calif.). pCMV8, saponin, and mouse anti-FLAG were purchased from Sigma (St. Louis, Mo.). Alexa A488-conjugated anti-mouse IgG, Alexa A594-conjugated anti-human IgG, and Alexa A594-conjugated anti-rabbit IgG secondary antibodies were purchased from Molecular Probes (Eugene, Oreg.). Monoclonal antibodies to GS-28 were obtained from BD Transduction Laboratories (Lexington, Ky.). Rabbit anti-β-COP antibodies were obtained from Affinity Bioreagents (Golden, Colo.) ECL Plus Western blotting detection system and horseradish peroxidase-conjugated goat anti-mouse and goat anti-rabbit antibodies were obtained from Amersham Pharmacia Biotech (Piscataway, N.J.). Ready-Gel precast sodium dodecyl sulfate-polyacrylamide gels and polyvinylidene difluoride membranes were obtained from Bio-Rad Laboratories (Hercules, Calif.). Monoclonal antibodies to GC (R4-6G4-1-1) and GN (R5-3G2-1A) were a generous gift from Jonathan Smith at the United States Army Medical Research Institute of Infectious Diseases (USAMRIID). Human anti-RVF virus serum was obtained from a convalescent individual who had been naturally infected with RVF virus. The rabbit anti-GN antibody was generated at Resgen (Huntsville, Ala.) against the peptide EDPHLRNRPGKGHNYID conjugated to keyhole limpet hemocyanin.
Plasmid construction.
A plasmid (pSP76-6) containing the RVF virus GN and GC genes (but lacking NSM) was obtained from Michael D. Parker at USAMRIID (7). A BamHI/EcoRI fragment which contains the entire GN and GC coding region was removed from pSP76-6 and subcloned into the BamHI/EcoRI sites of pcDNA1.1 to create GN/GC. A stop codon and an EcoRI site immediately downstream of the stop codon were introduced by QuikChange mutagenesis into the GN/GC plasmid using oligonucleotides STOPRI-M (CATATGCATCAGCATGATCAGAACGAATTCAGGCAAGCTCC) and STOPRI-MRC (GGAGCTTGCCTGAATTCGTTCTGATCATGCTGATGCATATG). The resulting plasmid was digested with EcoRI, the fragment containing the GC sequences was removed, and the plasmid was religated, thus generating GN. The GN plasmid was mutagenized with QuikChange to introduce a K→stop mutation at position 48 of the cytosolic tail (numbering as in Fig. 1) using oligonucleotides T2KSTOP (CTTCATCAGATGGATATATTAGAAGATGGTTGCCAG) and T2KSTOPRC (CTGGCAACCATCTTCTAATATATCCATCTGATGAAG), thus creating the GNK48Stop plasmid. The GN plasmid was mutagenized with QuikChange to introduce a V→stop mutation at position 32 (numbering as in Fig. 1) using oligonucleotides G2VSTOP (GAAGATTGCCCCAAGGTAAGTTCTGAATCCAC) and G2VSTOPRC (GTGGATTCAGAACTTACCTTGGGGCAATCTTC), thus creating the GNV32Stop plasmid. Green fluorescent protein (GFP) with HindIII ends was generated by amplifying the open reading frame of GFP with oligonucleotides H3GFP5 (AAGCTTATGGTGAGCAAGGGCGAGGAG) and H3GFP3B (AAGCTTCTTGTACAGCTCGTCCATGCC) and the PCR product was then cloned into pCR4. The HindIII GFP fragment was then subcloned into pCMV8 to yield a plasmid that expresses GFP with an N-terminal signal peptide. The region of GN encoding the transmembrane domain and the cytosolic tail (amino acids 1 to 93 as numbered in Fig. 1) was amplified with oligonucleotides G2GRECOL (GAATTCCTTTGTTGTTGTGTTTGTATTC) and G2GRSAL (GTCGACTTAACGTGGGATTGGGGCATGACG) and the PCR product was then cloned into pCR4. The region of GN encoding amino acids 32 to 93 of the cytosolic tail was amplified with oligonucleotides G2GRECOS (GAATTCCGTTCTGAATCCACTAATGTGG) and G2GRSAL (GTCGACTTAACGTGGGATTGGGGCATGACG) and the PCR product was then cloned into pCR4. The EcoRI/SalI fragments of GN were then subcloned into the EcoRI/SalI sites of the GFP/signal peptide plasmid to yield GFP/GN1-93 and GFP/GN32-93. The GFP/GN1-93 plasmid was mutagenized with QuikChange to introduce a K→stop mutation at position 48 of the cytosolic tail (numbering as in Fig. 1) using oligonucleotides T2KSTOP and T2KSTOPRC to create the GFP/GN1-48 plasmid. The GFP/GN1-93 plasmid was mutagenized with QuikChange to introduce a V→stop mutation at position 32 (numbering as in Fig. 1) using oligonucleotides G2VSTOP and G2VSTOPRC to create the GFP/GN1-32 plasmid. The pC-T7Pol plasmid was obtained from Yoshihiro Kawaoka (25).
RESULTS
Amino acid alignment of the GN of viruses belonging to the genus Phlebovirus.
A ClustalW alignment of amino acid sequences of the GN belonging to viruses of the genus Phlebovirus was performed to determine if there is any consensus between the known Golgi localization signals of Uukuniemi and Punta Toro virus GN and the remaining uncharacterized virus GN (Fig. 1A). While the sequence identity among the GN is low, we did find that there are two conserved features within the C-terminal region. The first is that all GN have two hydrophobic domains near the C terminus, one of which is the transmembrane domain (Fig. 1B). The second hydrophobic domain (amino acids 29 to 53; Fig. 1) from Uukuniemi virus GN was found to be sufficient to localize a cytosolic protein to the outer leaflet of Golgi membranes (2). This ∼30-amino-acid region is hydrophobic in character; however, due to the presence of several positively charged amino acids in the region it is unlikely to be a transmembrane domain. The second feature shared by the GN is an extremely basic region that begins around amino acid 20 and continues until the leader peptide for the GC (Fig. 1A). Further analysis of the other genera within the family Bunyaviridae showed that the hydrophobic domain following the transmembrane domain is a conserved feature of the GN of all Bunyaviridae family members (data not shown).
The regions determined to be involved in Golgi localization of GN belonging to Uukuniemi and Punta Toro viruses do not overlap except for a small window between amino acid 27 and 35 (Fig. 1A). Furthermore, the genus Phlebovirus GN proteins do not contain known Golgi retrieval signals, such as the tyrosine-based retrieval signals found in cellular TGN proteins.
The GN of RVF virus contains a Golgi localization signal.
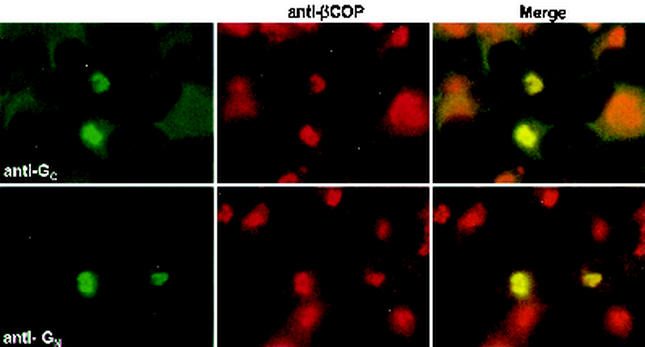
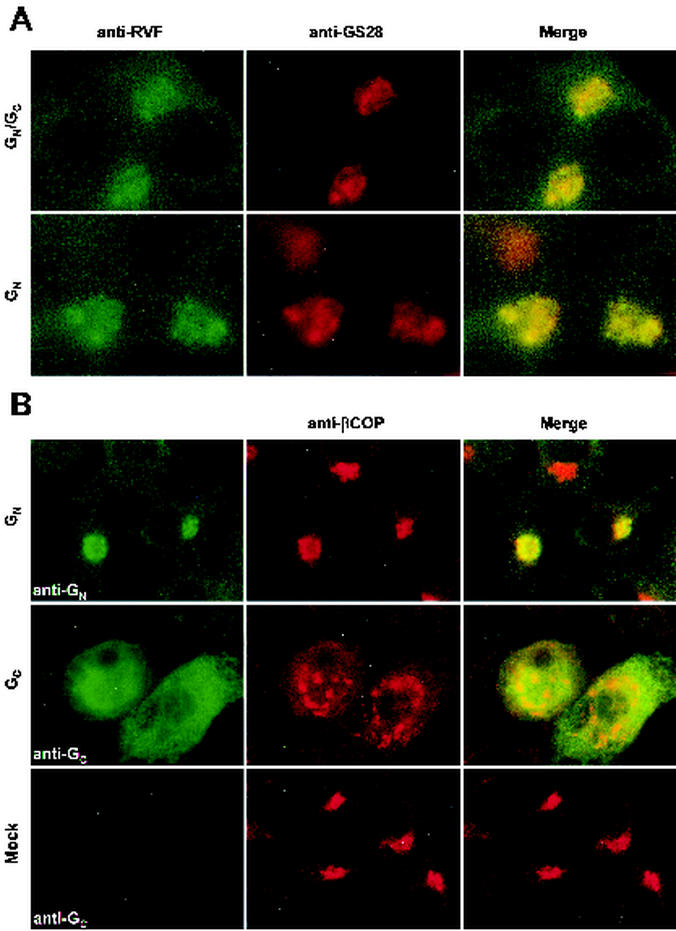
The envelope glycoproteins, GN and GC, of RVF virus have been found to localize to the Golgi apparatus both in RVF virus-infected cells and when expressed as a polyprotein from a vaccinia virus recombinant (31). To eliminate potential effects on the Golgi mediated by vaccinia virus, we utilized a T7 expression system in which BHK-T7 cells (that are stably transformed with a plasmid that expresses T7 polymerase) are transfected with the glycoprotein expression plasmids outlined in Fig. 2. In agreement with previous studies, we found that both GN and GC expressed by our GN/GC polyprotein construct colocalize with β-COP, a Golgi peripheral membrane protein (Fig. 3) (8).
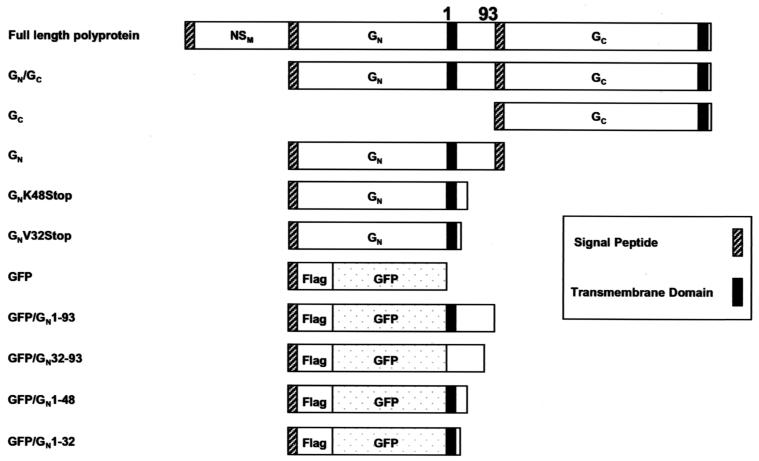
FIG. 2.
Names and diagrams of glycoprotein and chimeric expression constructs used in this study. The numbering of the amino acids in the carboxy-terminal tail of RVF virus GN is identical to that shown in Fig. 1.
FIG. 3.
GC and GN localize to Golgi membranes. BHK-T7 cells expressing GN/GC were fixed and permeabilized as described in Materials and Methods. Cells were then incubated with rabbit anti-βCOP (red channel) and either mouse anti-GC or mouse anti-GN (green channel).
To determine which glycoprotein contained a Golgi localization motif, we then went on to characterize two additional constructs, one that expresses GN alone and the other that expresses only GC. Very low levels of expression were obtained from our GC expression construct in BHK-T7 cells. In order to increase the expression of GC, we increased the amount of T7 polymerase in the cells by cotransfecting a plasmid that expresses T7 polymerase from a cytomegalovirus promoter. The immunofluorescent staining pattern of GC is consistent with localization to the ER; however, the morphology of the Golgi complex was altered compared to that of mock-infected cells (Fig. 4B). Disruption of the Golgi apparatus is likely a nonspecific effect of expressing the GC glycoprotein at a high level. Localization of GC to the ER was the expected result, since GC contains a KKXX-type (K = lysine; X = any amino acid) ER retrieval motif at the carboxy terminus of the protein. However, GN expressed from our GN construct colocalizes with both GS-28 (Fig. 4A), an integral membrane protein of the cis/medial Golgi apparatus (30), and β-COP (Fig. 4B) in a manner indistinguishable from our GN/GC polyprotein construct (Fig. 4A). Therefore, GN contains a Golgi localization motif.
FIG. 4.
GN localizes to Golgi membranes in the absence of GC. (A) BHK-T7 cells expressing GN/GC or GN were fixed and permeabilized as described in Materials and Methods. Cells were then incubated with mouse anti-GS-28 (red channel) and human anti-RVF virus (green channel). (B) BHK-T7 cells transfected with GN, GC, or empty vector. Cells transfected with GC also received a plasmid that expresses additional T7 polymerase. Cells were incubated with rabbit anti-βCOP (red channel) and either mouse anti-GN or mouse anti-GC (green channel).
The C-terminal basic region of GN is dispensable for Golgi localization.
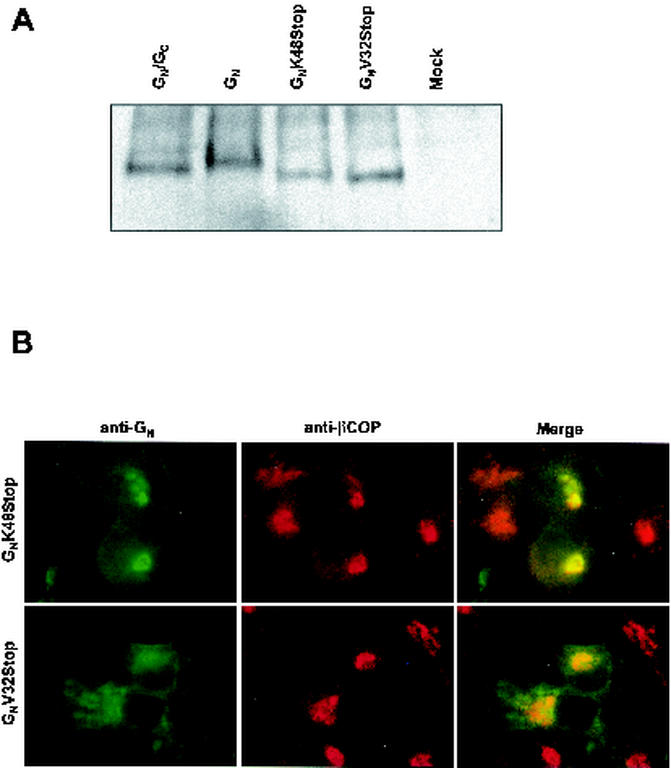
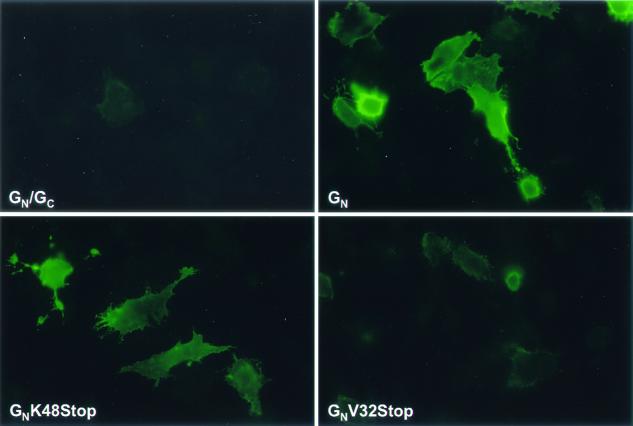
To map the Golgi localization signal of GN, we made two C-terminal truncation mutants of GN as depicted in Fig. 2. The first, GNK48Stop, lacks the leader sequence of GC and 42 amino acids at the C terminus of GN. The second, GNV32Stop, lacks another 16 amino acids of the cytosolic tail, including the equivalent region found to be important for Golgi localization of Uukuniemi virus GN. By Western blot analysis, we found that the GNV32Stop and GNV32Stop proteins run slightly faster than GN produced from either our GN/GC or GN construct, consistent with the deletion of 58 and 74 amino acids, respectively, from the C terminus of the protein (Fig. 5A). We then compared the localization of the proteins produced by these constructs with that of our full-length GN construct. The GNK48Stop protein colocalizes with β-COP in a manner indistinguishable from that of GN (compare Fig. 5B with Fig. 4B). These data suggest the amino acids 48 to 93 of the cytosolic tail are not required for Golgi localization. Further delineation of the region of GN involved in Golgi localization was attempted utilizing the GNV32Stop protein. By contrast, the GNV32Stop protein localized to the ER (Fig. 5B); this result was unexpected, as deletion of the corresponding region in Uukuniemi or Punta Toro virus GN results in either plasma membrane localization or no effect on localization of the protein (1, 19). It is likely that the ER localization that we observed with the GNV32Stop protein is a result of retention caused by misfolding of the protein.
FIG. 5.
The basic carboxy terminus of GN is dispensable for Golgi localization. (A) Western blot comparing the relative sizes and expression levels of the various GN proteins. (B) Immunofluorescence staining pattern of GNK48Stop and GNV32Stop. Cells were incubated with mouse anti-GN (green channel) and rabbit anti-βCOP (red channel).
A fraction of GN is found on the plasma membrane.
Many cellular proteins attain steady-state Golgi localization through a process that involves retrieval from distal compartments, including the plasma membrane (12, 24, 26). Protein expression constructs for GN/GC, GN, GNK48Stop, and GNV32Stop were evaluated for the amount of GN they display on the surface of transfected BHK-T7 cells. Our GN/GC polyprotein construct has a minimal amount of GN protein on the cell surface compared with GN expressed in the absence of GC (Fig. 6). This result suggests that GC plays a role in either retention of GN in the Golgi apparatus or retrieval from the plasma membrane. Similar to the GN expression construct, the GNK48Stop construct displays significant amounts of protein on the cell surface (Fig. 6). By contrast, the GNV32Stop construct displays very small amounts of protein on the cell surface (Fig. 6), consistent with our localization of the protein to the ER in permeabilized cells (Fig. 5B).
FIG. 6.
GN expression on the plasma membrane is increased when GN is expressed in the absence of GC. Cells were surface labeled with mouse anti-GN as described in Materials and Methods.
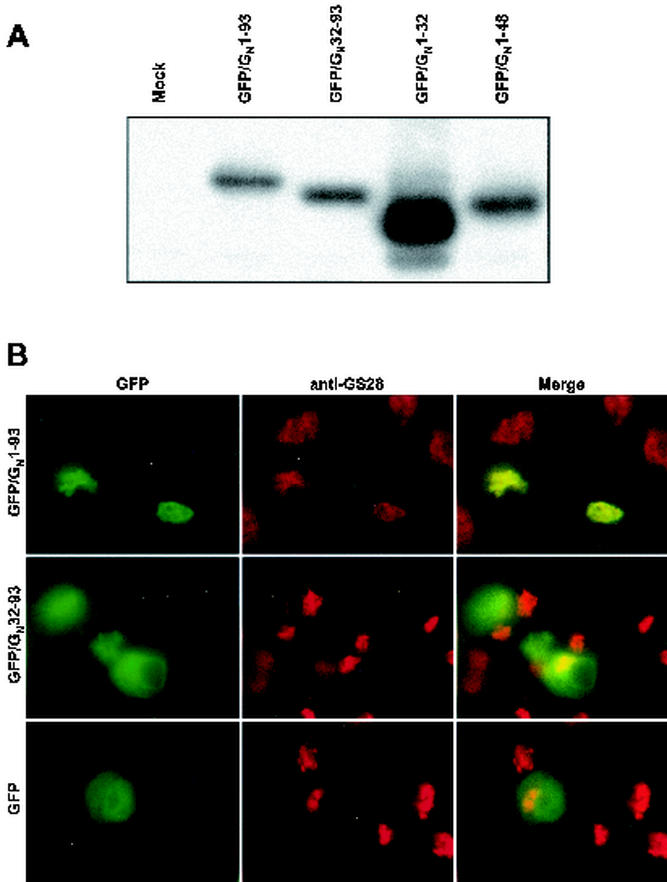
A 47-amino-acid region, which includes the transmembrane domain and a portion of the cytosolic tail of GN, is sufficient to localize a GFP chimera to the Golgi apparatus.
The data presented in Fig. 4 and 5 suggest that the Golgi localization signal of GN is contained within either the hydrophobic domains or the lumenal domain or both. We constructed the set of GFP chimeras diagrammed in Fig. 2 to further map the Golgi localization signal of GN. A signal peptide was fused to the N terminus of GFP to allow entry into the secretory pathway. The signal peptide-GFP fusion protein does not contain a transmembrane domain or a Golgi localization signal; thus, it showed diffuse staining consistent with distribution of the protein throughout the secretory system (Fig. 7B). Our first two constructs, GFP/GN1-93 and GFP/GN32-93, are chimeras representing the fusion of the transmembrane domain plus the cytosolic tail or just the cytosolic tail, respectively, to the C terminus of GFP (Fig. 2). Expression from these chimeric constructs was first verified by Western blotting (Fig. 7A). All constructs expressed proteins of the proper size and were therefore used in subsequent colocalization experiments. The GFP/GN1-93 protein colocalized with GS-28, demonstrating that a Golgi localization signal is contained within the transmembrane domain and the cytosolic tail (Fig. 7B). Furthermore, this result demonstrates that the lumenal domain of GN is dispensable for Golgi localization. By contrast, the GFP/GN32-93 protein did not colocalize with GS-28 and instead displayed a localization pattern consistent with ER localization (Fig. 7B).
FIG. 7.
The transmembrane domain and cytosolic tail region of GN is sufficient to localize a GFP/GN chimera to the Golgi. (A) Western blot comparing the relative sizes and expression levels of the various GFP/GN proteins. (B) Fluorescence staining pattern of GFP/GN1-93 and GFP/GN32-93. Cells were fixed and permeabilized as described in Materials and Methods and were incubated with mouse anti-GS-28 (red channel).
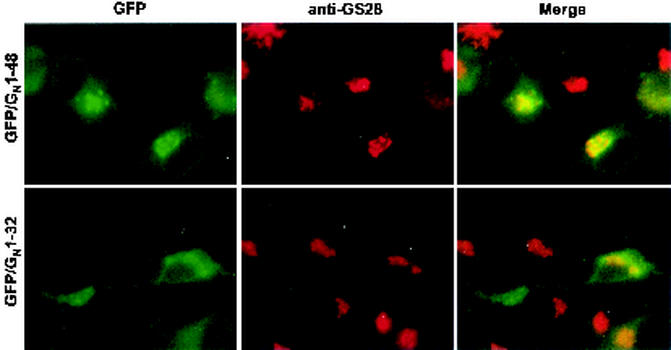
The Golgi localization signal was further mapped with two more chimeric proteins based on our GFP/GN construct (Fig. 2). GFP/GN1-48 is truncated at a position analogous to the GNK48Stop construct, while GFP/GN1-32 is truncated at a position analogous to the GNV32Stop construct. The GFP/GN1-48 protein colocalizes with GS-28 in a manner indistinguishable from GFP/GN1-93 and GN (Fig. 8), demonstrating that the 47-amino-acid region that includes the transmembrane domain and 28 amino acids of the cytosolic tail contains a Golgi localization signal. By contrast, the GFP/GN1-32 protein appears to localize to the ER (Fig. 8) similarly to the analogous GNV32Stop construct (Fig. 5B) and is likely retained in the ER as a result of misfolding.
FIG. 8.
The transmembrane domain (20 amino acids) plus 28 amino acids of the cytosolic tail of GN is sufficient to localize a GFP/GN chimera to the Golgi. Cells were fixed and permeabilized as described in Materials and Methods and were incubated with mouse anti-GS-28 (red channel).
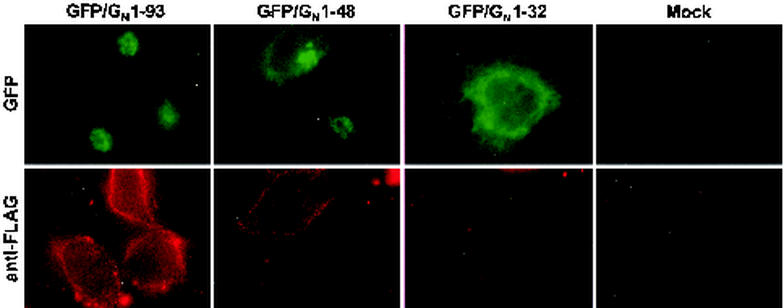
The GFP/GN chimeric proteins behaved like the analogous GN constructs with respect to Golgi localization. In order to determine if the chimeric proteins were indeed behaving similarly to the GN proteins, we evaluated them with respect to cell surface expression. Protein expression constructs for GFP/GN1-93, GFP/GN1-48, and GFP/GN1-32 were evaluated for the amount of chimeric protein they display on the surface of transfected BHK-T7 cells. As indicated in Fig. 2, all GFP/GN proteins are epitope-tagged with FLAG at the amino terminus. This epitope allows us to determine if the GFP/GN chimeras are expressed on the cell surface. GFP/GN1-93 and GFP/GN1-48 proteins could be visualized on the cell surface (Fig. 9), similar to the analogous GN proteins (Fig. 6). By contrast, the GFP/GN1-32 construct displays very small amounts of protein on the cell surface (Fig. 9), consistent with our localization of the protein to the ER (Fig. 8). Therefore, the GFP/GN chimeras appear to model the localization dynamics of GN accurately.
FIG. 9.
The GFP/GN chimeras behave similarly to the GN mutants with respect to Golgi localization and cell surface expression. Cells were surface labeled with mouse anti-FLAG (red channel) as described in Materials and Methods.
DISCUSSION
A key to the understanding of viral maturation is a characterization of the mechanisms by which the various structural components of the virion localize to the site of maturation. Most, if not all, of the viruses in the family Bunyaviridae mature in the Golgi complex (27). To date, there is no consensus with respect to the type of Golgi localization motif utilized, both cytosolic tail and transmembrane-based localization motifs having been identified previously. This lack of consensus leaves open the possibility that various mechanisms have evolved throughout the virus family for the localization of their envelope glycoproteins. While the study of the envelope glycoproteins does not in itself tell us how viruses of the family Bunyaviridae mature in the Golgi complex, it does tell us how the first step of maturation is initiated and is therefore an important piece in the puzzle. We have shown that the GN of RVF virus contains a Golgi localization signal. Furthermore, we have mapped this signal to a 47-amino-acid region that includes the transmembrane domain and a second hydrophobic domain in the cytosolic tail.
Cellular resident Golgi proteins utilize retention and/or retrieval mechanisms to achieve steady-state Golgi localization (24). Retrieval mechanisms typically utilize sequences within the cytosolic portion of the protein, whereas many retention motifs localize to transmembrane or lumenal regions. Our results show conclusively that a Golgi localization signal is contained within the region containing both the transmembrane domain and the cytosolic tail of GN.
There are two prominent models that describe how proteins may be retained in the Golgi complex. The first model is based on segregation of proteins based on lipid bilayer thickness. The membranes of the Golgi cisternae are rich in phospholipids, while the membranes of distal compartments are increasingly rich in sterols and sphingolipids, reaching a maximum at the plasma membrane (12, 24, 26). Comparison of transmembrane domains of Golgi proteins versus plasma membrane proteins shows that Golgi proteins tend to have shorter transmembrane domains relative to plasma membrane proteins (12, 24, 26). The segregation model proposes that these shorter transmembrane domains form discrete domains that allow them to be excluded from more sterol- or sphingolipid-rich transport vesicles destined for distal compartments. The other model is based on protein oligomerization leading to segregation. This model states that Golgi proteins oligomerize to form complexes that are too large to be packaged into secretory vesicles (12, 24, 26). It is possible that these models are not mutually exclusive, and indeed there are data that lend support to both of these models. In the case of the RVF virus glycoproteins, either model could be employed to explain our data. We have found that the envelope glycoproteins of RVF virus oligomerize (unpublished data). While we have not yet characterized the size of the oligomers formed by the RVF virus glycoproteins, experiments are under way to address this issue. Consistent with the oligomerization hypothesis, we found that GC has an impact on the localization of GN. We also found that more GN was present on the plasma membrane when GN was expressed in the absence of GC. While the amount of GN seen on the cell surface likely represents a small fraction of the total GN, it is nonetheless clear that GC is affecting localization of GN. This result suggests that GC either prevents GN from exiting the Golgi complex or facilitates the retrieval of GN from distal compartments, such as the plasma membrane.
GC localizes to the ER when expressed in the absence of GN. This was the expected result since GC has an ER retention motif of the KKXX type at the C terminus of its short (∼9-amino-acid) cytosolic tail. It is interesting that an ER retrieval motif is present in the GC proteins of all members of genus Phlebovirus. Presumably, binding of GC to GN masks the ER retrieval motif of GC and allows both proteins to be transported to the Golgi apparatus. This type of arrangement is not unique to RVF virus, as the rubella virus glycoproteins, E1 and E2, also carry conflicting sorting determinants (13-15). In this case, E1 contains an ER retention signal in its transmembrane domain (13) yet localizes to the Golgi complex by virtue of its binding to E2, which contains a Golgi retention signal in its transmembrane domain (15). Further experiments will be needed to demonstrate whether a similar situation exists for RVF virus envelope glycoproteins.
In summary, we have characterized a critical early step in the virus maturation process that begins with the localization of the viral glycoproteins to the Golgi apparatus. The system described herein should allow further fine mapping of the signal motifs and elements required for recruitment of viral core components. Identification of such elements may provide useful targets for the development of antiviral agents and the design of hemorrhagic fever disease control strategies.
Acknowledgments
We thank Michael Parker, Connie Schmaljohn, and Jonathan Smith of USAMRIID for their gift of RVF virus glycoprotein clones and antibodies, Klaus Conzelmann for his gift of the BHK-T7 cell line, and Yoshihiro Kawaoka for the pC-T7Pol plasmid. We also thank Thomas Ksiazek and Pierre Rollin for helpful discussions throughout the course of this study.
REFERENCES
- 1.Andersson, A. M., L. Melin, A. Bean, and R. F. Pettersson. 1997. A retention signal necessary and sufficient for Golgi localization maps to the cytoplasmic tail of a Bunyaviridae (Uukuniemi virus) membrane glycoprotein. J. Virol. 71:4717-4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson, A. M., and R. F. Pettersson. 1998. Targeting of a short peptide derived from the cytoplasmic tail of the G1 membrane glycoprotein of Uukuniemi virus (Bunyaviridae) to the Golgi complex. J. Virol. 72:9585-9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1994. Rift Valley fever—Egypt, 1993. Morb. Mortal. Wkly. Rep. 43:693, 699-700. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2000. Outbreak of Rift Valley fever—Saudi Arabia, August-October 2000. Morb. Mortal. Wkly. Rep. 49:905-908. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2000. Outbreak of Rift Valley fever-Yemen, August-October 2000. Morb. Mortal. Wkly. Rep. 49:1065-1066. [PubMed] [Google Scholar]
- 6.Chen, S. Y., Y. Matsuoka, and R. W. Compans. 1991. Golgi complex localization of the Punta Toro virus G2 protein requires its association with the G1 protein. Virology 183:351-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collett, M. S., A. F. Purchio, K. Keegan, S. Frazier, W. Hays, D. K. Anderson, M. D. Parker, C. Schmaljohn, J. Schmidt, and J. M. Dalrymple. 1985. Complete nucleotide sequence of the M RNA segment of Rift Valley fever virus. Virology 144:228-245. [DOI] [PubMed] [Google Scholar]
- 8.Donaldson, J. G., R. A. Kahn, J. Lippincott-Schwartz, and R. D. Klausner. 1991. Binding of ARF and beta-COP to Golgi membranes: possible regulation by a trimeric G protein. Science 254:1197-1199. [DOI] [PubMed] [Google Scholar]
- 9.Gad, A. M., M. M. Hassan, S. el Said, M. I. Moussa, and O. L. Wood. 1987. Rift Valley fever virus transmission by different Egyptian mosquito species. Trans. R. Soc. Trop. Med. Hyg. 81:694-698. [DOI] [PubMed] [Google Scholar]
- 10.Gargan, T. P., II, G. G. Clark, D. J. Dohm, M. J. Turell, and C. L. Bailey. 1988. Vector potential of selected North American mosquito species for Rift Valley fever virus. Am. J. Trop. Med. Hyg. 38:440-446. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh, R. N., W. G. Mallet, T. T. Soe, T. E. McGraw, and F. R. Maxfield. 1998. An endocytosed TGN38 chimeric protein is delivered to the TGN after trafficking through the endocytic recycling compartment in CHO cells. J. Cell Biol. 142:923-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gleeson, P. A. 1998. Targeting of proteins to the Golgi apparatus. Histochem. Cell Biol. 109:517-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hobman, T. C., H. F. Lemon, and K. Jewell. 1997. Characterization of an endoplasmic reticulum retention signal in the rubella virus E1 glycoprotein. J. Virol. 71:7670-7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hobman, T. C., L. Woodward, and M. G. Farquhar. 1993. The rubella virus E2 and E1 spike glycoproteins are targeted to the Golgi complex. J. Cell Biol. 121:269-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobman, T. C., L. Woodward, and M. G. Farquhar. 1995. Targeting of a heterodimeric membrane protein complex to the Golgi: rubella virus E2 glycoprotein contains a transmembrane Golgi retention signal. Mol. Biol. Cell 6:7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jupp, P. G., and A. J. Cornel. 1988. Vector competence tests with Rift Valley fever virus and five South African species of mosquito. J. Am. Mosq. Control Assoc. 4:4-8. [PubMed] [Google Scholar]
- 17.Linthicum, K. J., A. Anyamba, C. J. Tucker, P. W. Kelley, M. F. Myers, and C. J. Peters. 1999. Climate and satellite indicators to forecast Rift Valley fever epidemics in Kenya. Science 285:397-400. [DOI] [PubMed] [Google Scholar]
- 18.Logan, T. M., K. J. Linthicum, P. C. Thande, J. N. Wagateh, G. O. Nelson, and C. R. Roberts. 1991. Egg hatching of Aedes mosquitoes during successive floodings in a Rift Valley fever endemic area in Kenya. J. Am. Mosq. Control Assoc. 7:109-112. [PubMed] [Google Scholar]
- 19.Matsuoka, Y., S. Y. Chen, and R. W. Compans. 1994. A signal for Golgi retention in the bunyavirus G1 glycoprotein. J. Biol. Chem. 269:22565-22573. [PubMed] [Google Scholar]
- 20.Matsuoka, Y., S. Y. Chen, C. E. Holland, and R. W. Compans. 1996. Molecular determinants of Golgi retention in the Punta Toro virus G1 protein. Arch. Biochem. Biophys. 336:184-189. [DOI] [PubMed] [Google Scholar]
- 21.McIntosh, B. M., and P. G. Jupp. 1981. Epidemiological aspects of Rift Valley fever in South Africa with reference to vectors. Contrib. Epidemiol. Biostat. 3:92-99. [Google Scholar]
- 22.Meegan, J. M. 1979. The Rift Valley fever epizootic in Egypt 1977-78. 1. Description of the epizootic and virological studies. Trans. R. Soc. Trop. Med. Hyg. 73:618-623. [DOI] [PubMed] [Google Scholar]
- 23.Melin, L., R. Persson, A. Andersson, A. Bergstrom, R. Ronnholm, and R. F. Pettersson. 1995. The membrane glycoprotein G1 of Uukuniemi virus contains a signal for localization to the Golgi complex. Virus Res. 36:49-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munro, S. 1998. Localization of proteins to the Golgi apparatus. Trends Cell Biol. 8:11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neumann, G., H. Feldmann, S. Watanabe, I. Lukashevich, and Y. Kawaoka. 2002. Reverse genetics demonstrates that proteolytic processing of the Ebola virus glycoprotein is not essential for replication in cell culture. J. Virol. 76:406-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Opat, A. S., C. van Vliet, and P. A. Gleeson. 2001. Trafficking and localisation of resident Golgi glycosylation enzymes. Biochimie 83:763-773. [DOI] [PubMed] [Google Scholar]
- 27.Pettersson, R. F., and L. Melin. 1996. Synthesis, assembly and intracellular transport of Bunyaviridae membrane proteins, p. 159-188. In R. M. Elliott (ed.), The Bunyaviridae. Springer-Verlag, Berlin, Germany.
- 28.Pringle, C. R. 1996. Genetics and genome segment reassortment, p. 189-226. In R. M. Elliot (ed.), The Bunyaviridae. Springer-Verlag, Berlin, Germany.
- 29.Ronnholm, R. 1992. Localization to the Golgi complex of Uukuniemi virus glycoproteins G1 and G2 expressed from cloned cDNAs. J. Virol. 66:4525-4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramaniam, V. N., F. Peter, R. Philp, S. H. Wong, and W. Hong. 1996. GS28, a 28-kilodalton Golgi SNARE that participates in ER-Golgi transport. Science 272:1161-1163. [DOI] [PubMed] [Google Scholar]
- 31.Wasmoen, T. L., L. T. Kakach, and M. S. Collett. 1988. Rift Valley fever virus M segment: cellular localization of M segment-encoded proteins. Virology 166:275-280. [DOI] [PubMed] [Google Scholar]