Abstract
The proapoptotic protein encoded by Par4 (prostate apoptosis response 4) has been implicated in tumour suppression, particularly in the prostate. We report here that Par4-null mice are prone to develop tumours, both spontaneously and on carcinogenic treatment. The endometrium and prostate of Par4-null mice were particularly sensitive to the development of proliferative lesions. Most (80%) Par4-null females presented endometrial hyperplasia by 9 months of age, and a significant proportion (36%) developed endometrial adenocarcinomas after 1 year of age. Similarly, Par4-null males showed a high incidence of prostate hyperplasia and prostatic intraepithelial neoplasias, and were extraordinarily sensitive to testosterone-induced prostate hyperplasia. Finally, the uterus and prostate of young Par4-null mice have increased levels of the apoptosis inhibitor XIAP (X-chromosome-linked inhibitor of apoptosis), supporting the previously proposed function of Par4 as an inhibitor of the ζPKC (atypical protein kinase)–NF-κB (nuclear factor-κB)–XIAP pathway. These data show that Par4 has an important role in tumour suppression, with a particular relevance in the endometrium and prostate.
Keywords: tumour suppression, apoptosis, protein kinase C, Par4, prostate, endometrium
Introduction
The Par4 (PAWR; prostate apoptosis response 4) gene was originally identified in prostate cells that were undergoing apoptosis after androgen withdrawal (Sells et al, 1994). The human Par4 gene is located at the 12q21 chromosomal region and encodes a 38 kDa protein that is highly conserved along vertebrate evolution and without paralogues in the human or mouse genomes (El-Guendy & Rangnekar, 2003). Since its discovery, Par4 has been shown to possess a remarkably potent apoptotic activity in a variety of cellular systems and in response to numerous stimuli, including UV, cytokines, hormone deprivation and cytotoxics (Diaz-Meco et al, 1996; Sells et al, 1997; Mattson et al, 1999; Moscat & Diaz-Meco, 2003; Gurumurthy & Rangnekar, 2004). Of relevance for cancer, oncogenic transformation of in vitro-cultured cells by Ras involves a severe reduction in Par4 protein levels and, reciprocally, restoration of Par4 through ectopic expression efficiently prevents neoplastic transformation (Barradas et al, 1999; Nalca et al, 1999; Qiu et al, 1999). Consistent with this view, Par4 levels are abnormally low in a variety of cancers, including renal cell carcinomas (Cook et al, 1999), neuroblastomas (Kogel et al, 2001) and acute lymphatic leukaemia (Boehrer et al, 2002). Moreover, the antitumoral activity of COX2 inhibitors is mediated in part by the induction of Par4 levels (Zhang & DuBois, 2000; Dempke et al, 2001). Taken together, this evidence strongly suggests that Par4 could have tumoursuppression activity.
A number of nonexclusive mechanisms have been characterized that explain the potent apoptotic activity of Par4 (El-Guendy & Rangnekar, 2003). The best experimentally substantiated function of Par4 is the binding and inhibition of the atypical protein kinases C (aPKCs, namely ζPKC and λ/ιPKC; Diaz-Meco et al, 1996; Moscat & Diaz-Meco, 2003). An important function of atypical PKCs is the positive regulation of nuclear factor-κB (NF-κB; Moscat et al, 2003); consequently, cells and tissues from ζPKC-null mice have lower levels of NF-κB activity and are more susceptible to tumour necrosis factor α-triggered apoptosis (Leitges et al, 2001; Martin et al, 2002). Importantly, these phenotypes are reversed in Par4-null embryo fibroblasts and lymphocytes, which show hyperactivation of NF-κB, increased levels of the NF-κB transcriptional target XIAP (X-chromosome-linked inhibitor of apoptosis) and resistance to apoptosis (Garcia-Cao et al, 2003; Lafuente et al, 2003). Together, these data provide solid functional and genetic evidence in support of Par4 as a negative regulator of the ζPKC–NF-κB–XIAP pathway (Garcia-Cao et al, 2003; Moscat & Diaz-Meco, 2003). In addition to this, Par4 acts in the nucleus as a cofactor of the Wilms' tumoursuppressor protein WT1 (Johnstone et al, 1996; Richard et al, 2001). The Par4–WT1 complex has been implicated in the transcriptional repression of the proapoptotic protein Bcl2 (Cheema et al, 2003), although it cannot be ruled out that Par4 could also act on Bcl2 through NF-κB.
In the present work, we analyse in detail the ageing-associated pathologies of Par4-null mice, as well as their susceptibility to chemically and hormonally induced neoplasias and proliferative lesions. We provide direct proof of the role of Par4 in tumour suppression.
Results And Discussion
Tumour-prone phenotype of Par4-null mice
The generation of Par4-null mice has been described previously in the context of a study on the apoptotic susceptibility of Par4-null cells ex vivo (Garcia-Cao et al, 2003). Here, we have observed, during 3 years, cohorts of Par4+/+, Par4+/− and Par4−/− mice derived from the same founders and, thus, sharing the same mixed genetic background (CD1/129Sv, 75:25; Fig 1). Par4−/− mice showed a statistically significant decrease in their survival compared with either Par4+/+ or Par4+/− mice (log-rank test P<0.0001). Specifically, the average lifespan of Par4−/− mice was 19 months, whereas the average lifespan of Par4+/+ and Par4+/− mice was ∼24 months (Table 1). When moribund, mice were killed and complete histopathological analyses were performed. Alterations typical of old mice, such as glomerulonephritis, or cysts in the kidney, uterus or ovaries, were observed with similar frequencies in the three genotypes. Importantly, the incidence of spontaneous tumours was much higher in Par4−/− mice (87%) than in Par4+/+ mice (55%), with heterozygous mice showing an intermediate incidence (Table 1). The number of mice carrying more than one tumour, each affecting different organs, was significantly increased in the case of Par4−/− and Par4+/− genotypes compared with Par4+/+ mice (Table 1). Specifically, 43% of the Par4-null mice presented more than one tumour at the time of death. Moreover, in the case of Par4−/− mice, more than half of the tumours (57%) were carcinomas, whereas in the case of Par4+/+ mice, only 15% of the tumours were carcinomas (Table 2). Together, these data show that the proapoptotic protein Par4 has an important role in tumour suppression. A number of interesting features emerged when considering the different types of tissue affected by tumours (Table 2) and, on the basis of this, we have focused our subsequent analyses on three tissues, namely bladder, uterus and prostate, as explained in the following sections.
Figure 1.
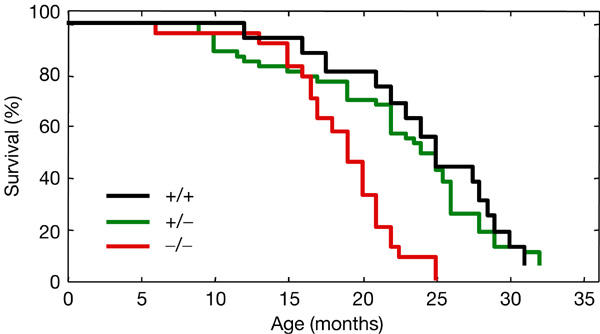
Decreased lifespan of Par4-null mice. Cohorts of mice of the indicated genotype and with the same genetic background (CD1/129Sv, 75:25) were observed for 3 years (Par4+/+: n=16; Par4+/−: n=47; Par4−/−: n=24; each group was composed of almost equal numbers of males and females). The figure indicates the surviving fraction at each age. The differences between Par4−/− and Par4+/+ mice, as well as between Par4−/− and Par4+/− mice, were statistically significant (log-rank test P<0.0001).
Table 1.
Spontaneous tumorigenesis in Par4-deficient mice
| Par4 genotype | Average lifespan (months) | Tumour-free mice |
Tumour-bearing micea |
|
|---|---|---|---|---|
| ⩾1 tumour | ⩾2 tumours | |||
| +/+ |
25 |
45% (9/20) |
55% (11/20) |
5% (1/20) |
| +/− |
24 |
29% (14/48) |
71% (34/48) |
33% (16/48)b |
| −/− | 19 | 13% (3/23) | 87% (20/23)b | 43% (10/23)b |
aThe numbers of malignant and nonmalignant tumours present at different organs were scored for each moribund mouse. Multifocal tumours at the same organ or multicentric lymphomas were considered as a single tumour. Metastases were not observed in any case. Preneoplastic lesions, such as hyperplasias, metaplasias or dysplasias, are not considered for this table.
bThe difference between Par4-deficient and wild-type mice is statistically significant (Fisher's test, P<0.05).
Table 2.
Spontaneous tumours and prostatic lesions in Par4-deficient mice
|
Par4 genotype |
|||
|---|---|---|---|
| +/+ (n=20) (8 F, 12 M) | +/− (n=48) (22 F, 26 M) | −/− (n=23) (14 F, 9 M) | |
| Carcinomas (incidence) |
3/20 (15%) |
13/48 (27%) |
13/23a (57%) |
| Lung |
0 |
5 |
1 |
| Liver |
1 |
4 |
2 |
| Urinary bladder |
0 |
0 |
2 |
| Endometrium |
0 |
1 |
5 (36% of F) |
| Otherb |
2 |
3 |
3 |
| Other malignant tumours |
7 |
11 |
7 |
| Haemangiosarcomas |
4 |
3 |
2 |
| Lymphomas |
2 |
4 |
4 |
| Otherc |
1 |
4 |
1 |
| Total malignant tumours (tumours/mouse) |
10/20 (0.5) |
24/48 (0.5) |
20/23 (0.9) |
| Total tumoursd (tumours/mouse) |
12/20 (0.6) |
53/48 (1.1) |
32/23 (1.4) |
| Prostate hyperplasia |
1 |
2 |
5 (56% of M) |
| Prostate intraepithelial neoplasia (PIN) |
0 |
2 (8% of M) |
2 (22% of M) |
| Total prostatic lesions | 1/12 (8% of M) | 4/26 (15% of M) | 7/9a (77% of M) |
aThe difference between Par4−/− and wild-type mice is statistically significant (Fisher's test, P<0.005).
bThe types of carcinomas were: for Par4(+/+), salivary glands and thyroid; for Par4(+/−), kidney, intestine and mammary gland; and for Par4(−/−), kidney, intestine and ovary.
cThe types of malignant tumours were: for Par4(+/+), histiocytic sarcoma; for Par4(+/−), fibrosarcomas (3 ×) and osteosarcoma; and for Par4(−/−), fibrosarcoma.
d Includes nonmalignant tumours, most of which were adenomas in the lung, liver and testis (Leydig cells), as well as haemangiomas. Preneoplastic lesions, such as hyperplasias, metaplasias or dysplasias, were not considered for this section of the table.
F, female; M, male.
Urinary bladder carcinomas in Par4-null mice
To further assess the role of Par4 as a tumour suppressor, we decided to perform chemically-induced carcinogenesis. Previous to this, we noted that according to two public repositories of gene expression data (GNF, http://expression.gnf.org; and SOURCE, http://genome-www5.stanford.edu; Su et al, 2002), Par4 shows maximal mRNA levels in the urinary bladder. Indeed, we considered it of relevance that some Par4−/− mice developed spontaneous urinary bladder carcinomas (Table 2), a tumour type that very rarely appears in aged wild-type mice (Mouse Tumor Biology Database, http://tumor.informatics.jax.org/straingrid.html). Thus, Par4+/+ and Par4−/− littermates were treated with a well-established carcinogenic regimen (N-butyl-N-(4-hydroxybutyl)nitrosamine, BBN) that efficiently produces bladder carcinomas. Interestingly, Par4-null mice were significantly more sensitive to the induction of bladder carcinomas than wild-type controls (log-rank test P<0.05; Fig 2A). Specifically, Par4+/+ mice survived up to 55 weeks since initiation of the treatment, whereas the complete Par4−/− cohort succumbed before 40 weeks, which represents a reduction of 38% in latency time. A representative example of a bladder carcinoma is shown in Fig 2B. These data corroborate the relevance of Par4 in tumour suppression and, particularly, in the context of the urinary bladder.
Figure 2.
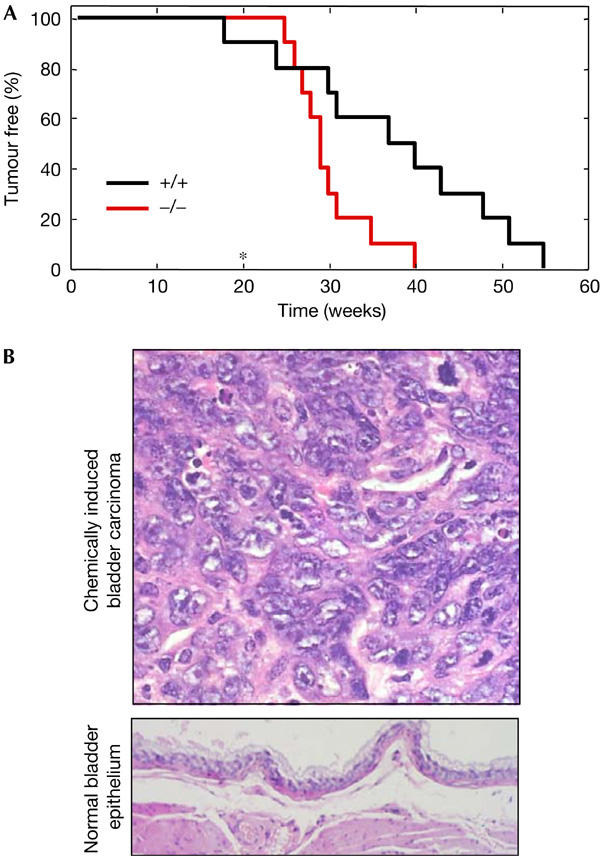
Increased susceptibility of Par4-null mice to chemically induced urinary bladder carcinomas. (A) Kinetics of tumour incidence in cohorts of mice of the indicated genotype (genetic background CD1/129Sv, 75:25). Urinary bladder carcinomas were induced with N-butyl-N-(4-hydroxybutyl)nitrosamine (BBN) in the drinking water for the first 20 weeks (end of treatment is indicated with an asterisk). Each group was composed of ten animals. The differences between Par4−/− and Par4+/+ mice were statistically significant (log-rank test P<0.05). (B) (Top) Representative example of a BBN-induced transitional cell carcinoma at high magnification. These carcinomas were composed of solid sheets or nests of transitional cells separated by a delicate stroma and invading the adjacent muscle walls. There are frequent atypical depolarized cells with evident nuclei and mitotic figures. Solid sheets often show areas of metaplastic squamous cells. (Bottom) Normal bladder epithelium at low magnification.
Endometrial carcinomas in Par4-null mice
Among the various types of carcinoma arising in Par4-null mice, endometrial adenocarcinomas were the most prominent, being observed in 36% of the Par4-null females (Table 2 and Fig 3A). To identify the precursor lesions and their prevalence, we analysed uteri from Par4+/+ and Par4−/− females at different ages. As shown in Fig 3B, at 3 months of age, there were no detectable lesions. Interestingly, at 9 months of age, 80% of the Par4−/− females presented endometrial hyperplasia, whereas none of the Par4+/+ females showed alterations (Fig 3B; see examples in Fig 3C,D). To explore the mechanistic basis of the proliferative defects observed in the uterus, we looked at the levels of the anti-apoptotic protein XIAP as a surrogate marker for the predicted activation of the ζPKC–NF-κB pathway in Par4-null tissues (Garcia-Cao et al, 2003). Importantly, we observed significantly increased levels of XIAP in the uteri of Par4-null females compared with their Par4-wt littermates (Fig 3E). This observation validates the function of Par4, in the uterus, as an inhibitor of the ζPKC–NF-κB–XIAP pathway, which could be at the basis of the deregulated proliferation and cancer susceptibility observed in the endometrium of Par4-null females. To further examine the implication of Par4 in the biology of the uterus, we tested whether oestrogens could regulate Par4 protein levels. Interestingly, Par4 levels in the uterus were markedly reduced on injection of oestradiol (Fig 3F). Since oestradiol is a potent inducer of proliferation of endometrial cells, this result further suggests an association between endometrial proliferation and downregulation of Par4. Together, our data implicate Par4 as a key negative regulator of proliferation in the endometrium. We propose that, in the absence of Par4, endometrial proliferation is unbalanced, probably because of increased cell survival mediated by NF-κB targets such as XIAP. This deregulation of proliferation could explain the development of endometrial hyperplasias and, eventually, the appearance of endometrial adenocarcinomas.
Figure 3.
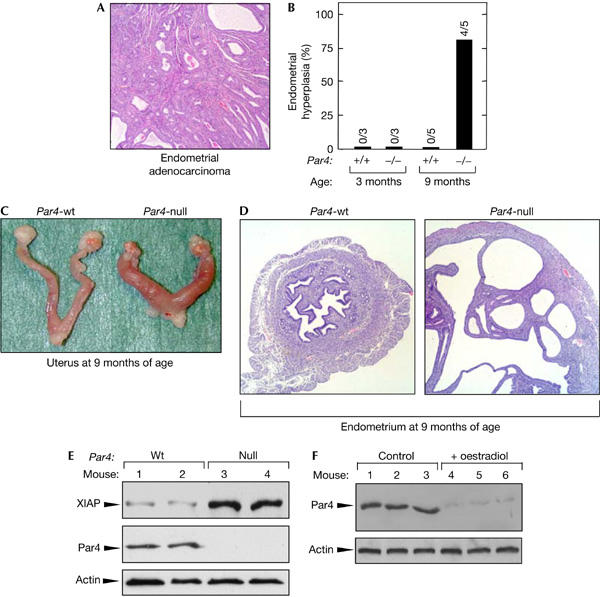
Par4-null females are prone to develop spontaneous endometrial carcinomas. (A) Representative example of a spontaneous endometrial carcinoma in a Par4-null female. Endometrial adenocarcinomas were observed in 36% of moribund Par4-null females (typically aged between 18 and 24 months; see Fig 1 and Table 2). Endometrial carcinomas presented diffuse proliferation of irregular glands lined by eosinophilic or amphiphilic columnar cells arranged in acini or nests. Tumoral cells possess hyperchromatic nuclei and can invade the muscular layer and serosa. Areas of haemorrhage and necrosis were observed, but distant metastases were not observed. (B) Incidence of endometrial hyperplasia in Par4-null females. To identify the precursor lesion of the adenocarcinomas, females of the indicated age and genotype (genetic background C57BL6 >90%) were killed and their uteri were examined. Endometrial hyperplasia was consistently found in Par4-null females, but was not found in any of the wild-type females analysed. (C) Representative macroscopic views of wild-type and Par4-null uteri, as indicated. Note the enlargement of the external diameter of the Par4-null uterus. (D) Representative example of endometrial hyperplasia in Par4-null females at 9 months of age. A normal uterus from a Par4-wt female is shown at the same magnification for comparison. Endometrial hyperplasias were characterized by a marked increase in the number of glands that became tortuous, dilated and usually cystic. Lesions show a diffuse pattern and are not accompanied by nuclear atypias. (E) Increased XIAP levels in the uteri of Par4-null females. Protein extracts were prepared from two 6-week-old females of each genotype and immunoblotted for detection of the indicated proteins. (F) Par4 protein levels in the uterus are regulated by oestradiol. Wild-type females were injected daily with 17β-oestradiol (8 μg/kg) for 3 days and killed 24 h after the end of the hormonal treatment. Protein extracts from the uteri of three females, either nontreated or treated, were analysed by immunoblot with anti-Par4 antibodies.
Prostatic lesions in Par4-null mice
Considering the amount of published circumstantial evidence pointing to a critical role of Par4 in the regulation of prostate apoptosis and survival (Gurumurthy & Rangnekar, 2004), we found the high incidence of lesions observed in dorsolateral prostate in Par4-null mice to be of relevance (affecting up to 77% of the males; Table 2). Examples of prostatic intraepithelial neoplasia (PIN) are shown in Fig 4A. To further corroborate the susceptibility of Par4-null mice to develop prostatic lesions, 4-week-old mice were treated for 4 weeks with a mixture of testosterone and oestradiol (provided continuously through subcutaneous pellets), a treatment that is carcinogenic in mice with genetic alterations in tumour suppressors (Wang et al, 2000). Interestingly, all Par4-null mice showed focal hyperplasias in the dorsolateral prostate and one of them even showed PIN, whereas the prostates of wild-type mice were normal under these conditions (Fig 4B,C). As we did before in the case of the uterus, we sought functional evidence in the prostate for the proposed role of Par4 as a negative regulator of the ζPKC–NF-κB–XIAP pathway (Garcia-Cao et al, 2003; Moscat & Diaz-Meco, 2003). For this, we examined the levels of XIAP in the prostate of young Par4-wt and Par4-null males (Fig 4D). Importantly, XIAP levels were reproducibly increased in the Par4-null prostates, providing strong evidence for the deregulation of the ζPKC–NF-κB–XIAP pathway. In summary, Par-null males are highly susceptible to both spontaneous and hormonally induced prostatic lesions, and this could be caused by the upregulation of the ζPKC–NF-κB–XIAP pathway.
Figure 4.
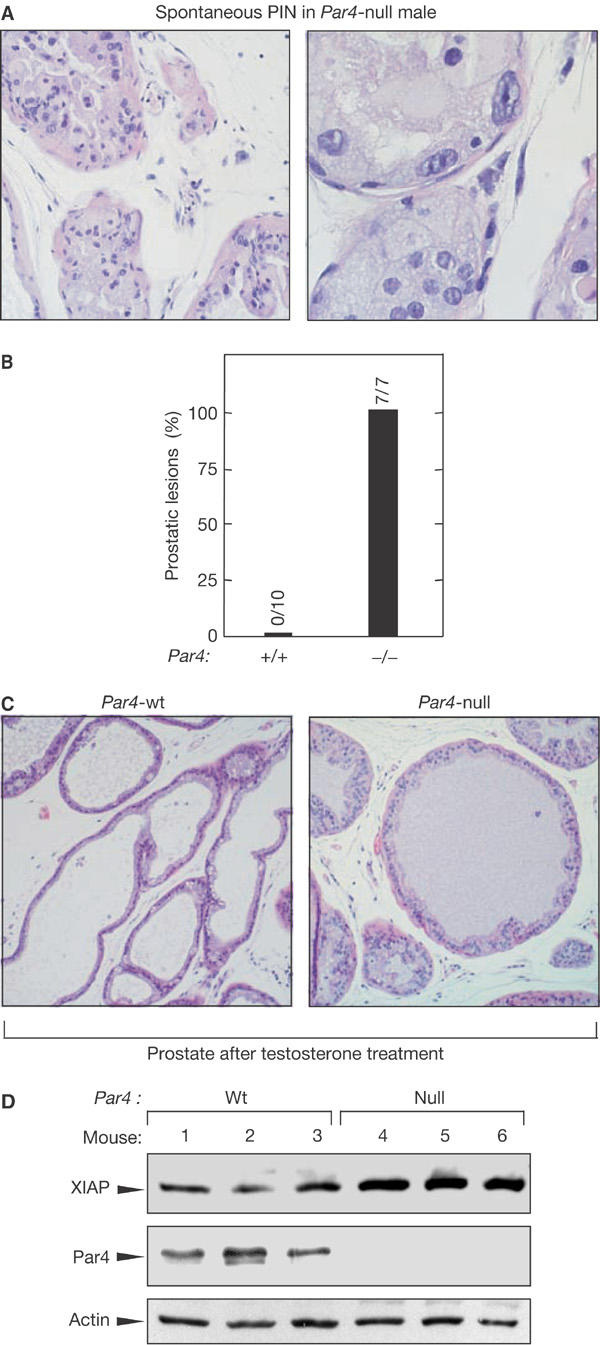
Par4-null males are prone to develop prostatic lesions. (A) Representative examples of prostatic intraepithelial neoplasia (PIN) in Par4-null males (Table 2). PIN consists of intraluminal focal proliferation of several glands (see example at low magnification in the left panel), composed by atypical stratified epithelial cells with hyperchromatic nuclei, prominent nucleoli or karyomegalia and loss of cellular polarity (see example at high magnification in the right panel). All PINs and hyperplasias observed in Par4-null mice were located in the dorsolateral prostate. (B) Incidence of hormonally induced prostate hyperplasia in Par4-null males. Mice (4-week-old) of the indicated gentoype (genetic background C57BL6 >90%) were treated for 4 weeks with subcutaneous pellets containing testosterone (25 mg) and oestradiol (2.5 mg). At the end of this period, mice were killed and their prostates were analysed for the presence of hyperplasia and PIN. Prostate hyperplasia was consistently found in Par4-null males, but was not found in any of the wild-type males analysed. One of the seven Par4-null males presented PIN. (C) Representative example of hormonally induced prostate hyperplasia (see (B)). Prostate hyperplasias were characterized by focal areas of multilayered epithelial cells with occasional patterns of cribiform proliferation. Cytologic atypias (hyperchromatic nuclei and loss of polarity) were not observed. Hyperplasias were located in the dorsolateral prostate. In the case of wild-type males, their prostates had a normal morphology after the same hormonal treatment. (D) Increased XIAP levels in the prostates of Par4-null males. Protein extracts were prepared from three 8-week-old males of each genotype and immunoblotted for detection of the indicated proteins.
Conclusions
In this work, we provide unambiguous evidence for the tumour-suppression activity of Par4 in the context of a mammal. The impact of Par4 is more prevalent in the endometrium and prostate, which reinforces the connection between Par4 and the biology of hormone-dependent tissues. Importantly, the endometrium and prostate of Par4-null mice show abnormally increased levels of XIAP, which is consistent with the proposed role of Par4 as a negative regulator of the ζPKC–NF-κB–XIAP pathway. Deregulation of this pathway could be at the basis of the increased tumour susceptibility of Par4-null mice. Collectively, our data prove that Par4 is a tumour suppressor of prostate and endometrial cancer and also other types of cancer, such as bladder carcinomas.
Methods
Mice and histology. This work was performed with two Par4-deficient strains that differ in their genetic backgrounds. The parental strain was of mixed genetic background (CD1/129Sv, 75:25), and this Par4(CD1/129Sv) strain was backcrossed into C57BL6 at least four times, yielding a Par4(C57BL6) strain with a C57BL6 background higher than 90%. The two basic phenotypes of Par4-null mice, namely endometrial and prostatic proliferative lesions, were observed in both the Par4(CD1/129Sv) and the Par4(C57BL6) strains. The strains used in each experiment are indicated in the figure legends. Animals were housed at the National Center of Biotechnology (Madrid, Spain) and at the Spanish National Cancer Center (Madrid, Spain), inside their corresponding pathogen-free barrier areas. Moribund mice were killed humanely in accordance with the Guidelines for Humane End Points for Animals Used in Biomedical Research. Tumours from non-autolysed tissues were recovered from moribund or recently deceased mice. Tissue samples were fixed in 10% buffered formalin, embedded in paraffin wax, sectioned at 4 μm and stained with haematoxylin and eosin (H&E).
Chemical carcinogenesis and hormonal treatments. For the induction of urinary bladder carcinomas, 3- to 5-month-old mice were exposed to BBN (TCI, Japan) permanently present in the drinking water at a concentration of 0.025% for 20 weeks, as described previously (Ozaki et al, 1998; Garcia-Cao et al, 2002). Mice were observed on a daily basis and they were humanely killed when they manifested signs of morbidity. On necropsy, tumours were evident in the urinary bladder, and the entire urinary system, including kidneys, was extracted for further analysis.
For the analysis of the short-term effect of oestradiol on Par4 levels in the uterus, young female mice were injected daily with 17β-oestradiol (8 μg/kg) for 3 days and killed 24 h after the end of the hormonal treatment. Uteri were collected and protein extracts were prepared in RIPA buffer for western blot analysis.
For the analysis of the long-term effect of testosterone on the prostate (Wang et al, 2000), 1-month-old males were subcutaneously implanted with two pellets of Silastic, one containing 25 mg of testosterone (Sigma, Tres Cantos, Spain) and the other containing 2.5 mg of oestradiol (Sigma). Mice were killed after 4 weeks and prostates were collected for histopathological analyses.
Chemical carcinogenesis and hormonal treatments. Protein extracts from the prostate and uteri were prepared in RIPA buffer. Immunoblots were performed using XIAP antibodies from Becton Dickinson (Franklin Lakes, NJ, USA) and Par4 antibodies (Ab-1) from Oncogene Sciences (Cambridge, MA, USA).
Acknowledgments
We thank M. Muñoz for excellent animal management and A. Efeyan for help with hormonal treatments. This work was supported by grants SAF2003-02613 (to M.T.D.-M.), SAF2002-0187 (to J.M.) and SAF2002-03402 (to M.S.) from the Spanish Ministry of Education and Science, by INTACT (to M.S.) from the European Union and by an institutional grant from Fundación Ramón Areces to the CBMSO. J.M. is the recipient of the Ayuda Investigación Juan March 2001.
References
- Barradas M, Monjas A, Diaz-Meco MT, Serrano M, Moscat J (1999) The downregulation of the pro-apoptotic protein Par-4 is critical for Ras-induced survival and tumor progression. EMBO J 18: 6362–6369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehrer S et al. (2002) In lymphatic cells par-4 sensitizes to apoptosis by down-regulating bcl-2 and promoting disruption of mitochondrial membrane potential and caspase activation. Cancer Res 62: 1768–1775 [PubMed] [Google Scholar]
- Cheema SK, Mishra SK, Rangnekar VM, Tari AM, Kumar R, Lopez-Berestein G (2003) Par-4 transcriptionally regulates Bcl-2 through a WT1-binding site on the bcl-2 promoter. J Biol Chem 278: 19995–20005 [DOI] [PubMed] [Google Scholar]
- Cook J, Krishnan S, Ananth S, Sells SF, Shi Y, Walther MM, Linehan WM, Sukhatme VP, Weinstein MH, Rangnekar VM (1999) Decreased expression of the pro-apoptotic protein Par-4 in renal cell carcinoma. Oncogene 18: 1205–1208 [DOI] [PubMed] [Google Scholar]
- Dempke W, Rie C, Grothey A, Schmoll HJ (2001) Cyclooxygenase-2: a novel target for cancer chemotherapy? J Cancer Res Clin Oncol 127: 411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Meco MT, Municio MM, Frutos S, Sanchez P, Lozano J, Sanz L, Moscat J (1996) The product of par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell 86: 777–786 [DOI] [PubMed] [Google Scholar]
- El-Guendy N, Rangnekar VM (2003) Apoptosis by Par-4 in cancer and neurodegenerative diseases. Exp Cell Res 283: 51–66 [DOI] [PubMed] [Google Scholar]
- Garcia-Cao I, Garcia-Cao M, Martin-Caballero J, Criado LM, Klatt P, Flores JM, Weill JC, Blasco MA, Serrano M (2002) ‘Super p53' mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J 21: 6225–6235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cao I, Lafuente MJ, Criado LM, Diaz-Meco MT, Serrano M, Moscat J (2003) Genetic inactivation of Par4 results in hyperactivation of NF-κB and impairment of JNK and p38. EMBO Rep 4: 307–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurumurthy S, Rangnekar VM (2004) Par-4 inducible apoptosis in prostate cancer cells. J Cell Biochem 91: 504–512 [DOI] [PubMed] [Google Scholar]
- Johnstone RW et al. (1996) A novel repressor, par-4, modulates transcription and growth suppression functions of the Wilms' tumor suppressor WT1. Mol Cell Biol 16: 6945–6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogel D, Reimertz C, Mech P, Poppe M, Fruhwald MC, Engemann H, Scheidtmann KH, Prehn JH (2001) Dlk/ZIP kinase-induced apoptosis in human medulloblastoma cells: requirement of the mitochondrial apoptosis pathway. Br J Cancer 85: 1801–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuente MJ, Martin P, Garcia-Cao I, Diaz-Meco MT, Serrano M, Moscat J (2003) Regulation of mature T lymphocyte proliferation and differentiation by Par-4. EMBO J 22: 4689–4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitges M, Sanz L, Martin P, Duran A, Braun U, Garcia JF, Camacho F, Diaz-Meco MT, Rennert PD, Moscat J (2001) Targeted disruption of the ζPKC gene results in the impairment of the NF-κB pathway. Mol Cell 8: 771–780 [DOI] [PubMed] [Google Scholar]
- Martin P, Duran A, Minguet S, Gaspar ML, Diaz-Meco MT, Rennert P, Leitges M, Moscat J (2002) Role of ςPKC in B-cell signaling and function. EMBO J 21: 4049–4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Chan SL, Camandola S (1999) Par-4: an emerging pivotal player in neuronal apoptosis and neurodegenerative disorders. J Mol Neurosci 13: 17–30 [DOI] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT (2003) Par-4 keeps the atypical PKCs at bay. Cell Cycle 2: 71–72 [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT, Rennert P (2003) NF-κB activation by protein kinase C isoforms and B-cell function. EMBO Rep 4: 31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalca A, Qiu SG, El-Guendy N, Krishnan S, Rangnekar VM (1999) Oncogenic Ras sensitizes cells to apoptosis by Par-4. J Biol Chem 274: 29976–29983 [DOI] [PubMed] [Google Scholar]
- Ozaki K et al. (1998) High susceptibility of p53(+/−) knockout mice in N-butyl-N-(4-hydroxybutyl)nitrosamine urinary bladder carcinogenesis and lack of frequent mutation in residual allele. Cancer Res 58: 3806–3811 [PubMed] [Google Scholar]
- Qiu SG, Krishnan S, el-Guendy N, Rangnekar VM (1999) Negative regulation of Par-4 by oncogenic Ras is essential for cellular transformation. Oncogene 18: 7115–7123 [DOI] [PubMed] [Google Scholar]
- Richard DJ, Schumacher V, Royer-Pokora B, Roberts SG (2001) Par4 is a coactivator for a splice isoformspecific transcriptional activation domain in WT1. Genes Dev 15: 328–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells SF et al. (1997) Expression and function of the leucine zipper protein Par-4 in apoptosis. Mol Cell Biol 17: 3823–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells SF, Wood DP Jr, Joshi-Barve SS, Muthukumar S, Jacob RJ, Crist SA, Humphreys S, Rangnekar VM (1994) Commonality of the gene programs induced by effectors of apoptosis in androgen-dependent and -independent prostate cells. Cell Growth Differ 5: 457–466 [PubMed] [Google Scholar]
- Su AI et al. (2002) Largescale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci USA 99: 4465–4470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hayward SW, Donjacour AA, Young P, Jacks T, Sage J, Dahiya R, Cardiff RD, Day ML, Cunha GR (2000) Sex hormone-induced carcinogenesis in Rb-deficient prostate tissue. Cancer Res 60: 6008–6017 [PubMed] [Google Scholar]
- Zhang Z, DuBois RN (2000) Par-4, a proapoptotic gene, is regulated by NSAIDs in human colon carcinoma cells. Gastroenterology 118: 1012–1017 [DOI] [PubMed] [Google Scholar]


