Abstract
Recent work has highlighted two main levels of global organization of the Escherichia coli chromosome. Macrodomains are large domains inferred from structural data consisting of loci showing the same intracellular positioning. Replichores, defined by base composition skews, coincide with the replication arms in normal cells. We used chromosome inversions to show that the dif site, which resolves chromosome dimers, only functions when located at the junction of the replichores, whatever their size. This is the first evidence that replichore polarization has a role in chromosome segregation. We also show that disruption of the Ter macrodomain provokes a cell-cycle defect independent from dimer resolution. This confirms the existence of the Ter macrodomain and suggests a role in chromosome dynamics.
Keywords: chromosome structure, replichores, macrodomains, segregation, xer
Introduction
The Escherichia coli chromosome shows two main levels of global organization, macrodomains (Niki et al, 2000) and replichores (Blattner et al, 1997). Both levels were discovered recently, whereas the presence of topological domains, long thought to be important players, now seems to be related to local organization of chromosomal DNA and gene expression (Deng et al, 2004; Postow et al, 2004; Moulin et al, 2005). Replication runs from the origin, oriC, to the diametrically opposed terminus region, which is flanked by two series of replication terminators that arrest replication forks in a polar manner, thus defining a 330-kb-long region where termination must occur (Louarn et al, 2005). A number of elements show a biased orientation following the origin–terminus axis. This has led to the definition of replichores as accounting for this global organization (Blattner et al, 1997). Numerous oligonucleotide motifs display a skewed orientation following replichores, which in two cases has functional implications (Salzberg et al, 1998; Lobry & Louarn, 2003). The RecBCD complexes, which process doublestrand breaks during their repair, recognize a skewed octamer termed Chi. This is thought to favour recombinational repair of breaks occurring behind replication forks (Kuzminov, 1999; Gruss & Michel, 2001). A second function of replichore organization has been suggested in the resolution of chromosome dimers (Corre et al, 2000; Pérals et al, 2000; Lobry & Louarn, 2003). Chromosome dimers are produced by homologous recombination and resolved at division by the Xer sitespecific recombination system (reviewed by Lesterlin et al, 2004). In Xer recombination, XerC and XerD recombine pairs of dif sites, a 28-base-pair (bp)-long sequence located in the terminus. Recombination requires an interaction of XerCD/dif with FtsK, a DNA translocase associated with the division septum (Steiner et al, 1999; Aussel et al, 2002; Yates et al, 2003). Dimer resolution also depends on the orientation of the sequences flanking dif (Pérals et al, 2000). The molecular basis of this phenomenon involves sequence polarization, certainly by short, repeated oligomeric motifs thought to orient FtsK translocation towards the dif sites (Pérals et al, 2000; Capiaux et al, 2002; Corre & Louarn, 2002; Lobry & Louarn, 2003; Bigot et al, 2004).
Macrodomains (domains hereafter) were discovered by cytological studies (Niki et al, 2000). They contain loci showing the same intracellular positioning and choreography during the cell cycle. The Ori domain is about 1 Mb and contains oriC and migS, a site involved in its bipolar migration after replication (Yamaichi & Niki, 2004). The Ter domain (Ter hereafter) is also about 1 Mb, roughly centred on dif and flanked by two ‘nondivisible zones' that are regions containing a complex patchwork of segments in which inversions are deleterious (Rebollo et al, 1988; Guijo et al, 2001). Domain organization has recently received further support, as recombination between two sites in the same domain is much more frequent than between two sites in different domains (Valens et al, 2004). Domains thus seem to be structural entities insulated from the rest of the chromosome. In addition to Ori and Ter, Valens and co-workers defined Left and Right, two new domains on either side of Ter (Fig 1).
Figure 1.
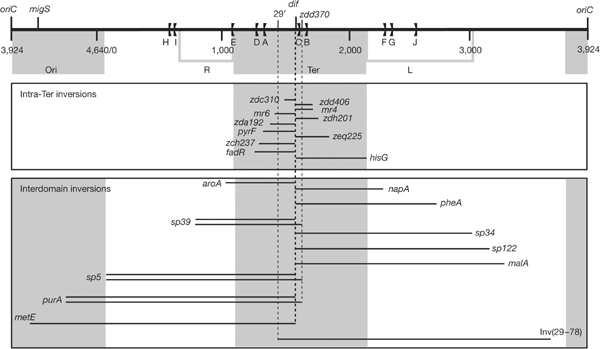
Map of the inversions. Top panel: map of the chromosome linearized at oriC. Coordinates are in kb. Positions of migS, dif, the Ori, Right (R), Left (L) and Ter domains, and the replication terminators (the flags) are shown. Middle and bottom panel: inverted fragments. Each line represents a pair of Plr+ and Plr− inversions from dif (zdd346::lacZ::attB and zdd347::lacZ::attB positions; Fig 2) or a single inversion from zdd370::lacZ::attB (only with attP at sp5, sp39, and purA) and in the case of the Inv(29–78) inversion. The respective positions of the attP-Kn insertions are given. The three shortest inversions (sp17, zdd355 and zdd370) in Fig 3 are not shown.
We report an analysis of the effects of large chromosomal inversions between the dif region and other positions of the chromosome. The data reveal that dif resolves chromosome dimers if it lies at the junction of the replichores, whatever their relative size. This strongly suggests that polarization signals are present all along the replichores and oriented following the oriC–dif axis and proves a role for replichore organization in chromosome segregation. Inversions also confirm the existence of Ter and highlight the importance of its integrity for chromosome segregation and cell division.
Results And Discussion
dif only acts at the replichore junction
Inversions of chromosome segments were constructed by site-specific recombination catalysed by the bacteriophage λ integrase (Int), using a previously described system (Valens et al, 2004; Methods). Briefly, an attP site and a lacZ::attB gene are inserted into the chromosome as the two end points of the inversion (Fig 2). The lacZ::attB gene encodes an active β-galactosidase, and recombination between attP and attB splits lacZ::attB in two inactive parts. This allows the screening of inversion-carrying bacteria on X-gal-containing plates (Methods). The expression of Int is controlled by temperature, which allows a precise control of the level of recombination.
Figure 2.
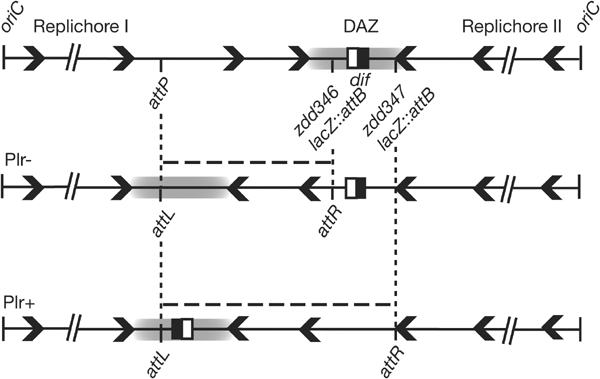
Inversion engineering. The chromosome is represented linearized at oriC with the two replichores polarized from oriC to dif (the black and white square). Arrowheads symbolize polarization. The grey zone is the DAZ, which is the junction of inversely polarized sequences where dif must lie to be active. Inversions occur between lacZ::attB and attP sites. A couple of Plr+ and Plr− inversions are detailed. Only Plr+ inversions keep dif at the junction of the newly formed replichores.
Starting from a strain deleted for the natural attB site, a pair of strains was constructed by insertion of lacZ::attB on either side of dif (positions zdd346 and zdd347, located at −696 and +994 bp, respectively; Fig 2; Pérals et al, 2000). Appropriately oriented attP sites were introduced into these strains (Methods; Fig 1). Every attP insertion was combined with the two lacZ::attB constructs, resulting in two strains that differ only by the position of dif with respect to the inverted fragment (Fig 2). For clarity, inversions are designated Plr+ (polarization+) when the relative orientation of dif and the inverted fragment are conserved (e.g. inversions encompassing dif), and Plr− in the opposite case (Fig 2).
For all positions of attP assayed, both Plr+ and Plr− inversions were obtained, the largest pair of inversions inverting half the chromosome (2.27 Mb; metE in Fig 1). Inversions with attP sites located inside Ter (intra-Ter inversions) were frequent and obtained at low levels of Int expression. A temperature shift at 42°C for 8 min was sufficient to yield 10–100% inverted clones. In contrast, inversions with attP sites outside Ter (interdomain inversions) were infrequent and required higher levels of Int (30 min at 42°C yielded less than 1% inversion). This is consistent with data obtained using the same system and fixed att sites at other positions inside Ter and strongly suggests that the Ter domain is a structural unit spatially insulated from the rest of the chromosome (Valens et al, 2004).
The growth rate, plating efficiency and cell morphology of inversion-carrying strains were analysed (Figs 3, 4 and 5; supplementary Tables 1,2 online). Quantification of the growth defect provoked by intra-Ter inversions was performed using a co-culture assay as described previously (Pérals et al, 2000). Inverted strains were co-cultivated with their wild-type (noninverted) counterpart and the ratio of the two strains was measured over 80 generations (Methods). This provides a measure of the relative growth rate of the two strains, which, in the case of dif inactivation, may be expressed as a frequency of abortive division due to unresolved dimers (Fig 3). Plr− inversions induced more severe viability defects than their Plr+ counterpart, which suggests that dimer resolution is at least partially inactivated by Plr− inversions. Consistent with this hypothesis, inactivation of XerC—which inactivates dimer resolution—and of RecA—which prevents dimer formation—in both the Plr+ and Plr− strains abolished their phenotypic difference (not shown). This confirms that dif needs to lie at the junction of inversely polarized sequences to resolve dimers and shows that sequences from different parts of Ter can activate or inhibit dif activity depending on their orientation.
Figure 3.
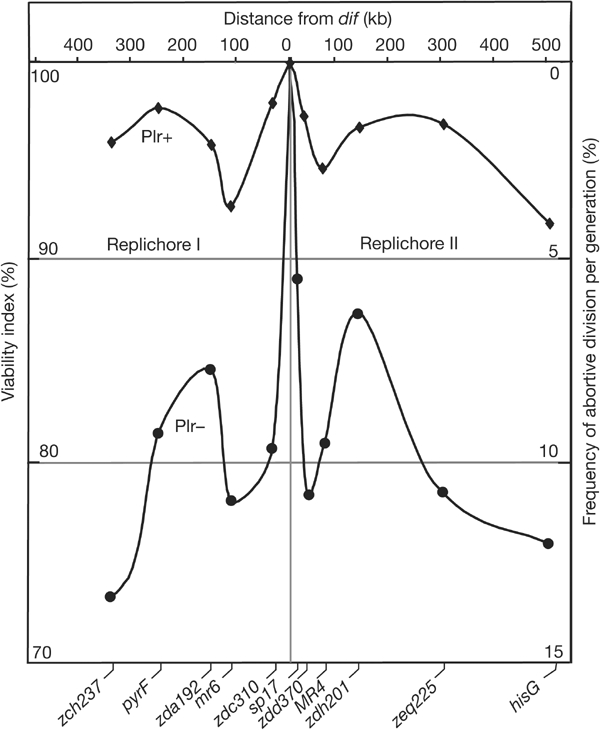
Intra-Ter inversions. The viability of the strain carrying Plr+ and Plr− inversions is plotted as a function of the distance separating attP from dif (top scale). The respective positions of the attP sites are given (circles: Plr−; diamonds: Plr+). The scale on the left is the viability index (measured using the co-culture assay, 70% for a dif-deleted strain, 100% for the wild type). The scale on the right is the inferred frequency of abortive divisions per generation.
Figure 4.
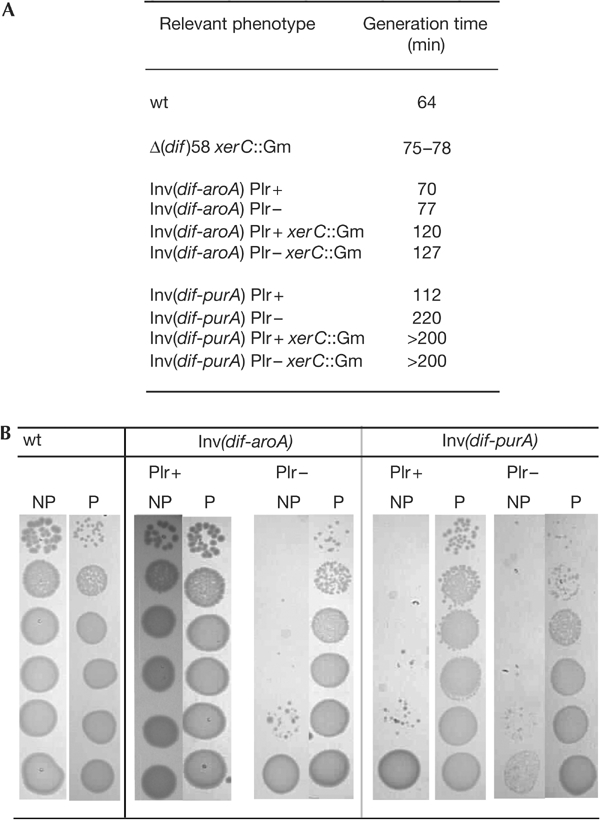
Interdomain inversions. Examples of growth defects provoked by interdomain inversions. (A) Generation times were calculated from growth curves in permissive conditions. (B) Serial dilutions of liquid culture grown overnight in permissive conditions were plated in permissive (P, synthetic medium at 30°C) and nonpermissive (NP, here synthetic medium at 42°C) conditions and incubated for 36 h (wt) or 48 h (inverted strains). Note the similar colony size of Inv(dif-aroA) in P and NP conditions despite the difference in temperature (compare with the wt). Note also the appearance of revertant clones (big colonies) in NP.
Figure 5.
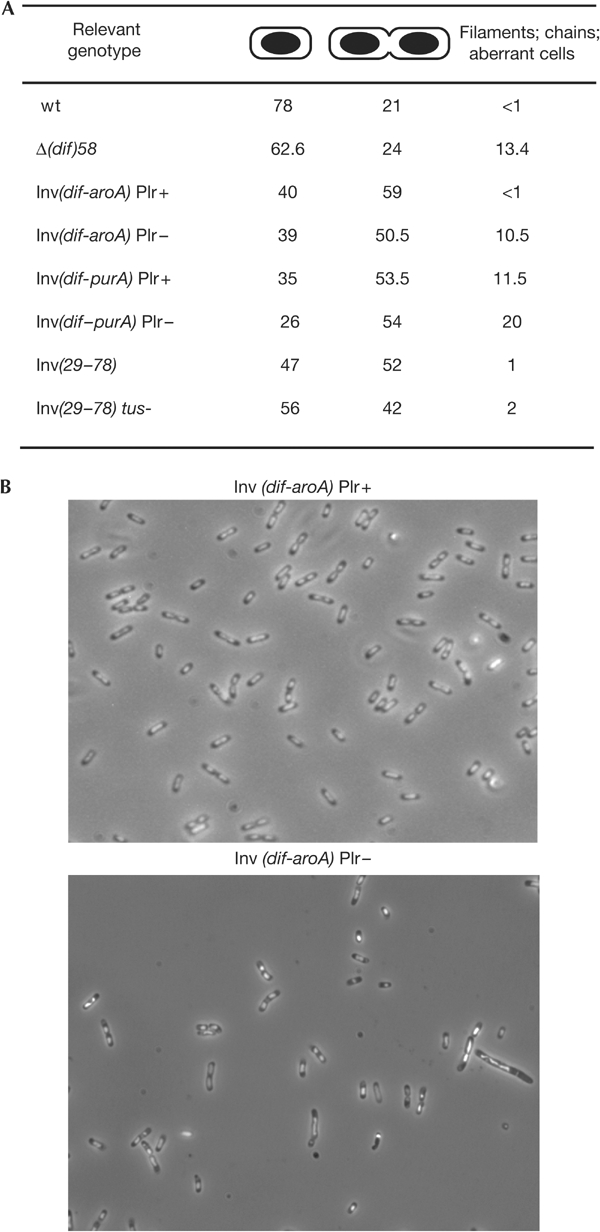
Interdomain inversions provoke an over-representation of division figures. (A) Normal cells with one or two nucleoids and abnormal cells (filaments, chains and other abnormal cells such as wider cells apparently containing several chromosomes) were counted on micrographs of bacteria grown in permissive conditions (400 cells per count). (B) Examples of micrographs of cells grown in permissive conditions.
Interdomain inversions were obtained only in certain conditions and reverted frequently (see below). This complicated the viability analysis and made the use of the co-culture assay unreliable. Nonetheless, it was always clear that strains carrying Plr+ inversion show lower frequencies of abortive division and grow faster than their Plr− counterparts (Fig 4; supplementary Table 2 online). In each case, the Plr+/Plr− difference was abolished when both strains were rendered xerC−. Thus, whatever the viability defect provoked by the Plr+ inversions, it was always enhanced in its Plr− counterpart due to less efficient dimer resolution. Interestingly, for both intra-Ter and interdomain inversions, the Plr+/Plr− difference varied for the different attP positions (Figs 3, 4; supplementary Tables 1 and 2 online). This suggests that the different parts of the chromosome show different density and/or quality of polarization. We conclude that DNA sequences located at every position tested around the chromosome have the capacity to support or to inhibit dif activity depending on their orientation with respect to dif. This strongly suggests that the polarization signals at work in the dif region are present all around the chromosome and oriented following the oriC–dif axis. From this, it seems that replichores run precisely from oriC to dif and have an important role in chromosome organization and segregation.
Inversions reveal the domain organization
Inverted strains show different phenotypes that highlight the domain organization of the chromosome. Intra-Ter inversions are obtained in rich medium at 37°C. They provoke little or no growth defect and filamentation, which is largely due to dif inactivation in Plr− inversions (Fig 3). In contrast, interdomain inversions are only obtained in synthetic medium and at a low temperature (30°C). At a high temperature (37 or 42°C), or in rich medium, they provoke growth defects ranging from a significant increase in generation time to an inability to form colonies, and they revert frequently (Fig 4B). No simple correlation between the size of the inverted fragment and the growth defect provoked was found. However, it is interesting to note that the two inversions with attP sites inside Ori (purA and metE; Fig 1) provoked noticeably strong phenotypes (Fig 4).
To further separate the phenotypes due to unresolved dimers from other effects, a new lacZ::attB construct was inserted at position zdd370, 23.2 kb from dif. Inversions from this position should have no effect because this leaves enough polarized DNA around dif to support its activity (Pérals et al, 2000). This construct was combined with three attP insertions outside Ter (Fig 1). Consistent with our prediction, strains with inversions from zdd370 do not form filaments, which strongly suggests a full efficiency in dimer resolution. However, like inversions from the dif position, they are severely impaired for growth in rich medium at high temperature, showing that this phenotype is not primarily due to dif inactivation (not shown). Notably, a strain carrying a large interdomain inversion from Ter, the Inv(29–78) inversion (Fig 1), was previously reported to show a similar rich medium sensitivity, and chromosome dimer resolution was shown to be unaffected by this inversion (Louarn et al, 1985; Cornet et al, 1996). The growth defect provoked by interdomain inversions thus seems to be due to disruption of the integrity of Ter or to the abutting of Ter and non-Ter sequences. The borders of Ter as defined here agree with those proposed in previous studies (Niki et al, 2000; Valens et al, 2004).
Interdomain inversions delay cell division
Strikingly, strains with interdomain inversions all seem to show the same cell-cycle defect. Exponentially growing cultures are enriched in cells harbouring two partially segregated nucleoids, generally separated by a constricting division septum (Fig 5). These represent more than half of the cells carrying interdomain inversions, whereas only 21% of wild-type cells are at this stage in the same growth conditions. In permissive conditions, the vast majority of cells carrying interdomain inversions eventually divide and produce viable cells. In nonpermissive conditions, blocked cells increase to 80%, division is more delayed and frequently produces dead cells (not shown). The percentage of double cells does not increase in Plr− conditions, which shows that the phenotype is not due to unresolved dimers (Fig 5). Interdomain inversions from zdd370 also provoked this phenotype (not shown) as well as the Inv(29–78) inversion (Fig 5; Louarn et al, 1985; Cornet et al, 1996). Importantly, inactivation of Tus in the Inv(29–78)-carrying strain only moderately lowered the over-representation of cells in division (Fig 5). This phenotype is thus not primarily due to the inversion of replication terminators.
The phenotype of division delay provoked by interdomain inversions is unusual. Previously reported division blockage usually occurs at the early steps of septum constriction: for instance, during nucleoid occlusion or SOS induction (Wu & Errington, 2004). Conversely, septum constriction on incompletely segregated nucleoids leads to chromosome breaks: for instance, in the case of unresolved dimers (Hendricks et al, 2000). We suggest that a factor in chromosome segregation/cell division needs more time to complete its role due to chromosome rearrangement and acts as a checkpoint. Preliminary data show that this checkpoint does not depend on FtsK (not shown).
Speculation
We have provided two important insights: (i) The functional polarization detected around dif concerns the whole chromosome and may therefore have roles in addition to dimer resolution: for instance, in replication or rebuilding of structured chromosomes after replication. It may also control chromosome plasticity, for instance, by imposing a correct orientation of the incoming DNA during horizontal transfer. (ii) Disruption of Ter disrupts the coordination of late steps of the cell cycle, providing the first example of the importance of domain organization in the cell cycle. Our favourite view is that Ter, or a central subdomain, is the preferred zone for final chromosome separation, decatenation and dimer resolution. This could be controlled by sister chromosome cohesion specific to this region. Delocalization or disruption of the cohesive zone could slow chromosome separation and disrupt its coordination with cell division.
Methods
Strains and plasmids. Strains carrying Inv(28–78) were previously described (Cornet et al, 1996). Other strains were derived from XL13, a spontaneous StR derivative of MG1657 (MG1655 ΔlacMluI, ΔattB::Sp/Sm; Valens et al, 2004). lacZ::attB was cloned from pOM5-attB (Valens et al, 2004) into the zdd346, zdd347 and zdd370 positions carried by plasmids pLN156, pFC40 and pFC89 (Cornet et al, 1996; Pérals et al, 2000), to yield plasmids pCL5, pCL6 and pCL17, respectively. attP-Kn was cloned from pNKBOR-attP (Valens et al, 2004) into the TcR determinant of pFC68 (Cornet et al, 1996) to yield pCL14. These constructs were introduced into the chromosome by the method described for this family of plasmid (Cornet et al, 1994). attP-Kn was introduced into the pre-existing insertion of Tn10 transposons or Tc fragments (Rebollo et al, 1988; Guijo et al, 2001), using pCL14 or by P1 transduction from strains from the collection of Valens et al (2004). The xerC::Gm allele (Aussel et al, 2002) was introduced into strains by P1 transduction. Plasmid pTSA29-AK (Valens et al, 2004) carries a cI857-PR-int construct.
General procedures. Strains were grown in Luria broth or M9 broth (0.2% casamino acids; 0.4% glucose; Miller, 1992) and, when required, ampicillin (25 μg/ml), kanamycin (50 μg/ml), streptomycin (200 μg/ml), gentamycin (7.5 μg/ml), chloramphenicol (20 μg/ml) and 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-gal; 80 μg/ml). For microscopic observations, bacteria were grown to optical density (OD)600=0.6, incubated for 30 mn at 30°C in the presence of 5 μg/ml of 4,6-diamino-2-phenylindol (DAPI), recovered in M9 broth and observed using a Leica DMRB microscope.
Inversion procedure. Strains carrying the lacZ::attB and attP-Kn cassettes (Lac+) were transformed with pTSA29-CXI-AK. Transformants were grown overnight in liquid broth at 30°C, diluted 100-fold, grown at 30°C to OD600=0.3, incubated at 42°C to induce Int expression, returned at 30°C for 3 h to allow segregation of recombinant chromosomes and plated on Xgal-containing medium. After growth, white clones (Lac−) were checked for the absence of attP and attB and the presence of attL and attR by PCR.
Co-culture assay, generation time and plating efficiency. Generation times were deduced from growth curves OD600=f(t) in permissive growth conditions. Plating efficiencies were estimated by growth at different temperatures on L- or M9-containing plates of 10 μl spots of serial dilution of liquid overnight cultures (Fig 4). For co-culture assay, Lac− inverted strains were co-cultivated with XL13 (wt, Lac+), following the general procedure described previously (Pérals et al, 2000). The viability index was deduced from the slope of Lac+/Lac−=f(generation) curves (Fig 3). Frequencies of abortive division were calculated from viability indexes.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Tables
Acknowledgments
We thank A.G. Carpousis, M. Chandler, K. Cam, S Maisnier-Patin and P. Lebourgeois for critical reading and J.M. Louarn for helpful discussions. C.L. was funded by the Fondation pour la Recherche Médicale during part of this work. The research by F.C. and F.X.B. is funded by the CNRS, the French Ministry of Research and the Paul Sabatier University.
References
- Aussel L, Barre FX, Aroyo M, Stasiak A, Stasiak AZ, Sherratt D (2002) FtsK is a DNA motor protein that activates chromosome dimer resolution by switching the catalytic state of the XerC and XerD recombinases. Cell 108: 195–205 [DOI] [PubMed] [Google Scholar]
- Bigot S, Corre J, Louarn JM, Cornet F, Barre FX (2004) FtsK activities in Xer recombination, DNA mobilisation and cell division involve overlapping and separate domains of the protein. Mol Microbiol 54: 876–886 [DOI] [PubMed] [Google Scholar]
- Blattner FR et al. (1997) The complete genome sequence of Escherichia coli K-12. Science 277: 1453–1474 [DOI] [PubMed] [Google Scholar]
- Capiaux H, Lesterlin C, Perals K, Louarn JM, Cornet F (2002) A dual role for the FtsK protein in Escherichia coli chromosome segregation. EMBO Rep 3: 532–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet F, Mortier I, Patte J, Louarn JM (1994) Plasmid pSC101 harbors a recombination site, psi, which is able to resolve plasmid multimers and to substitute for the analogous chromosomal Escherichia coli site dif. J Bacteriol 176: 3188–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet F, Louarn J, Patte J, Louarn JM (1996) Restriction of the activity of the recombination site dif to a small zone of the Escherichia coli chromosome. Genes Dev 10: 1152–1161 [DOI] [PubMed] [Google Scholar]
- Corre J, Louarn JM (2002) Evidence from terminal recombination gradients that FtsK uses replichore polarity to control chromosome terminus positioning at division in Escherichia coli. J Bacteriol 184: 3801–3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corre J, Patte J, Louarn JM (2000) Prophage λ induces terminal recombination in Escherichia coli by inhibiting chromosome dimer resolution. An orientation-dependent cis-effect lending support to bipolarization of the terminus. Genetics 154: 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S, Stein RA, Higgins NP (2004) Transcription-induced barriers to supercoil diffusion in the Salmonella typhimurium chromosome. Proc Natl Acad Sci USA 101: 3398–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss A, Michel B (2001) The replication–recombination connection: insights from genomics. Curr Opin Microbiol 4: 595–601 [DOI] [PubMed] [Google Scholar]
- Guijo MI, Patte J, del Mar Campos M, Louarn JM, Rebollo JE (2001) Localized remodeling of the Escherichia coli chromosome: the patchwork of segments refractory and tolerant to inversion near the replication terminus. Genetics 157: 1413–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks EC, Szerlong H, Hill T, Kuempel P (2000) Cell division, guillotining of dimer chromosomes and SOS induction in resolution mutants (dif, xerC and xerD) of Escherichia coli. Mol Microbiol 36: 973–981 [DOI] [PubMed] [Google Scholar]
- Kuzminov A (1999) Recombinational repair of DNA damage in Escherichia coli and bacteriophage λ. Microbiol Mol Biol Rev 63: 751–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesterlin C, Barre FX, Cornet F (2004) Genetic recombination and the cell cycle: what we have learned from chromosome dimers. Mol Microbiol 54: 1151–1160 [DOI] [PubMed] [Google Scholar]
- Lobry JR, Louarn JM (2003) Polarisation of prokaryotic chromosomes. Curr Opin Microbiol 6: 101–108 [DOI] [PubMed] [Google Scholar]
- Louarn JM, Bouche JP, Legendre F, Louarn J, Patte J (1985) Characterization and properties of very large inversions of the E. coli chromosome along the origin-to-terminus axis. Mol Gen Genet 201: 467–476 [DOI] [PubMed] [Google Scholar]
- Louarn JM, Kuempel P, Cornet F (2005) The terminus region of the Escherichia coli chromosome, or, all's well that ends well. In The Bacterial Chromosome, Higgins NP (ed) pp 251–273. Washington DC, USA: ASM press [Google Scholar]
- Miller JH (1992) A Short Course in Bacterial Genetics. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press [Google Scholar]
- Moulin L, Rahmouni RA, Boccard F (2005) Topological insulators inhibit diffusion of transcription-induced positive supercoils in the chromosome of E. coli. Mol Microbiol 55: 601–610 [DOI] [PubMed] [Google Scholar]
- Niki H, Yamaichi Y, Hiraga S (2000) Dynamic organization of chromosomal DNA in Escherichia coli. Genes Dev 14: 212–223 [PMC free article] [PubMed] [Google Scholar]
- Pérals K, Cornet F, Merlet Y, Delon I, Louarn JM (2000) Functional polarization of the Escherichia coli chromosome terminus: the dif site acts in chromosome dimer resolution only when located between long stretches of opposite polarity. Mol Microbiol 36: 33–43 [DOI] [PubMed] [Google Scholar]
- Postow L, Hardy CD, Arsuaga J, Cozzarelli NR (2004) Topological domain structure of the Escherichia coli chromosome. Genes Dev 18: 1766–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollo J, Francois V, Louarn J (1988) Detection and possible role of two large nondivisible zones on the Escherichia coli chromosome. Proc Natl Acad Sci USA 85: 9391–9395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg SL, Salzberg AJ, Kerlavage AR, Tomb JF (1998) Skewed oligomers and origins of replication. Gene 217: 57–67 [DOI] [PubMed] [Google Scholar]
- Steiner W, Liu G, Donachie WD, Kuempel P (1999) The cytoplasmic domain of FtsK protein is required for resolution of chromosome dimers. Mol Microbiol 31: 579–583 [DOI] [PubMed] [Google Scholar]
- Valens M, Penaud S, Rossignol M, Cornet F, Boccard F (2004) Macrodomain organization of the Escherichia coli chromosome. EMBO J 23: 4330–4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Errington J (2004) Coordination of cell division and chromosome segregation by a nucleoid occlusion protein in Bacillus subtilis. Cell 117: 915–925 [DOI] [PubMed] [Google Scholar]
- Yamaichi Y, Niki H (2004) migS, a cis-acting site that affects bipolar positioning of oriC on the Escherichia coli chromosome. EMBO J 23: 221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J, Aroyo M, Sherratt DJ, Barre FX (2003) Species specificity in the activation of Xer recombination at dif by FtsK. Mol Microbiol 49: 241–249 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables


