Abstract
Efficient photosynthesis is of fundamental importance for plant survival and fitness. However, in oxygenic photosynthesis, the complex apparatus responsible for the conversion of light into chemical energy is susceptible to photodamage. Oxygenic photosynthetic organisms have therefore evolved several protective mechanisms to deal with light energy. Rapidly inducible non-photochemical quenching (NPQ) is a short-term response by which plants and eukaryotic algae dissipate excitation energy as heat. This review focuses on recent advances in the elucidation of the molecular mechanisms underlying this protective quenching pathway in higher plants.
Keywords: photosynthesis, non-photochemical quenching, PsbS, zeaxanthin, light-harvesting complex
Introduction
During photosynthesis, photons are absorbed by antenna pigments, such as protein-bound chlorophylls (Chl) and carotenoids. The excitation energy is transferred from the site of absorption, primarily the light-harvesting complexes (LHCs), to the reaction centres (RCs; Nield et al, 2000). Here, excitation is converted into charge separation, which drives the electron flow between photosystem II (PSII) and photosystem I (PSI) through the cytochrome b6f complex. The net result of this process is the oxidation of water molecules, the production of molecular oxygen, the reduction of NADP+ and the generation of a proton gradient (ΔpH). The energy stored as ΔpH is exploited for ATP synthesis. The interplay between light and oxygenic photosynthesis is an enterprise of complex regulation. This short review focuses on the molecular mechanisms that allow sophisticated regulation of the amount of excitation energy transferred to the RC of PSII.
The photosynthetic apparatus is highly dynamic and able to respond to several environmental stimuli, including changes in the quality and quantity of incident light and the availability of carbon dioxide. A short-term response is ensured by non-photochemical quenching (NPQ), a process in which absorbed light energy is dissipated as heat and does not take part in photochemistry. The phenomenon involves quenching of chlorophyll a (Chla) fluorescence, which is induced under steady-state illumination and which can be analysed in terms of three components: state transition (qT), ΔpH-dependent quenching (qE) and photoinhibition (qI). The majority of NPQ is believed to occur through qE in the PSII antenna pigments bound to the light-harvesting proteins (LHCII; Demmig-Adams & Adams, 1992).
Non-photochemical quenching: state transition
Rapid reorganization of the light-harvesting apparatus, termed 'state transition', occurs in response to changes in the availability of carbon dioxide and the reduction state of chloroplasts. A kinase system becomes activated and phosphorylates a fraction of LHCII proteins. As a consequence, lateral redistribution of the phosphorylated LHCII proteins and their association with PSI take place (Wollman, 2001; Allen & Forsberg, 2001). Because the fluorescence yield of PSII diminishes during state transition due to antenna size reduction, this process, also called qT, is considered a component of NPQ. A thylakoid-associated Ser–Thr regulatory kinase, STN7, recently identified in Arabidopsis, has been shown to be required for state transition (Bellafiore et al, 2005) and cytochrome b6f has been recognized as a key partner in kinase activation in Chlamydomonas (Wollman & Lemaire, 1988). In previous models, state transition was considered necessary to maximize photosynthetic efficiency by balancing the excitation of the two photosystems. A recent reinterpretation, however, indicates a different role: in conditions of limiting CO2 and a high reduction level of the chloroplast, the photosynthetic apparatus is switched from the oxygenic type, with two photosystems working in series, to an ATP-generator type, with cyclic electron flow around PSI (for comprehensive reviews, see Wollman, 2001; Aro & Ohad, 2003).
Non-photochemical quenching: photoinhibition
The photosynthetic apparatus must deal with marked changes in light intensity. Directional movements of whole leaves and/or chloroplasts, which may allow the plant to optimize light absorption, are relatively slow processes. Rather than regulating light absorption, a fast response is obtained through the regulation of dissipative de-excitation of absorbed photons. In normal conditions, most of the energy in singlet-excited chlorophyll (1Chl*) is used at the RC to drive electron transport; in this case, Chl fluorescence quenching is correlated to charge separation (photochemical quenching). However, when the rate of formation of 1Chla* exceeds the overall rate of its energy conversion at the RC, intersystem crossing leads to an increasing population of 3Chla* (triplet state) in the antenna moiety, which can activate molecular oxygen to its highly reactive singlet state (1O2). Singlet oxygen molecules, as well as the other forms of reactive oxygen species (ROS), are known to induce oxidative damage to pigments, proteins and lipids in the thylakoid membrane, thereby impairing overall photosynthetic efficiency (photoinhibition). The fluorescence quenching associated with this phenomenon, which is slowly reversible or even partially irreversible, represents the portion of NPQ indicated as qI. By scavenging the triplet excited state of Chl and dissipating associated energy by fast thermalization, carotenoids prevent activation of oxygen, thus protecting chlorophyll–protein complexes from photo-oxidation. Without the protection exerted by carotenoids, rapid and complete destruction of the entire photosystems would occur.
Non-photochemical quenching: ΔpH-dependent quenching
When the acceptor quinones are reduced at steady state, charge separation at the PSII RC is followed by recombination, with a high probability of the formation of 3P680 and singlet oxygen. The chlorophylls of the special pair P680 cannot be protected against oxygen activation because the two nearby β-carotene molecules are not close enough (Ferreira et al, 2004). For this reason, regulation of the transmission of excitation to the PSII RC is of the utmost importance.
The portion of NPQ named qE has a central role in this context and is a ΔpH-dependent, rapidly inducible component. qE is also called feedback de-excitation, because thermal dissipation of antenna 1Chla* is stimulated by ΔpH, which builds up across the thylakoid membrane during photosynthetic electron transport and is therefore brought about by the same excitation that qE contributes to dissipation. qE has been shown to be important for plant fitness in variable light conditions rather than for the induction of tolerance to high-intensity light itself (Kulheim et al, 2002), and may easily be measured as the quickly reversible portion of maximal PSII fluorescence quenching, which is not associated with charge separation (Demmig-Adams & Adams, 2000).
During the past decade, our understanding of qE has been greatly advanced, particularly by the selection of NPQ-deficient Arabidopsis mutants by video imaging of Chl fluorescence quenching (Niyogi et al, 1998), the successful application of resonance Raman spectroscopy (Robert et al, 2004), which yielded structural information about specific pigment molecules in thylakoid membrane complexes in vivo and in vitro, and the ability to detect non-fluorescent, optically dark excited states of pigments by femtosecond transient absorption kinetics in intact membranes (Ma et al, 2003; Holt et al, 2005). The resolution of the crystal structure of the isolated antenna LHCII complex (Liu et al, 2004; Standfuss et al, 2005), as well as in vitro reconstitution of antenna proteins (Sandonà et al, 1998) has also significantly contributed to the identification of the key components responsible for qE. They are pigments of the xanthophyll cycle, particularly zeaxanthin (Zea), the PSII S subunit (PsbS) protein, and components of the LHCII light-harvesting apparatus (Fig 1). Despite the attention devoted to the qE component of NPQ, the actual biophysical and biochemical mechanisms for energy dissipation have only recently been proposed. The 'hot topics' in this respect are: How does zeaxanthin contribute to qE, and where is its site of action? How is PsbS involved? Are there parallel and independent mechanisms for qE?
Figure 1.

Organization of photosystem II and light-harvesting complex II in the thylakoid membrane. Cp43, Cp47: internal antenna chlorophyll–protein complexes. D1, D2: main components of reaction centres (RCs) with binding sites for electron acceptor quinones (QB, QA). P680: chlorophyll special pair. Other cofactors associated with D1/D2: pheophytin (Phe), non-haem iron (Fe), Mn-cluster. Accessory chlorophylls and β-carotene are not shown. Chl, chlorophyll; PQH2, plastoquinone pool; cytb6f, cytochrome b6f complex; YZ, D1-Tyr161. For a more detailed description, see Ferreira et al (2004). Scheme adapted from J. Nield (Imperial College London, UK) with permission. See downloads section of www.bio.ic.ac.uk/research/nield/.
Zeaxanthin and ΔpH-dependent quenching. The importance of the xanthophyll cycle (Yamamoto, 1979) in high light conditions became clear more than a decade ago (Demmig-Adams & Adams, 1996). The most abundant xanthophyll pigment in the thylakoid membrane is lutein, but β-carotene-derived violaxanthin (Vio) is also present. Vio is synthesized from Zea via antheraxanthin in low light conditions by the stromal activity of zeaxanthin epoxidase. Under intense light, lumenal pH decreases and, at a critical threshold, Vio de-epoxidase converts Vio back to Zea (Fig 2). The latter is absent in the thylakoid membrane in normal light conditions, but it is required for qE to occur, as shown by the fact that npq1 mutants lacking Vio de-epoxidase have greatly reduced NPQ (Niyogi et al, 1998).
Figure 2.
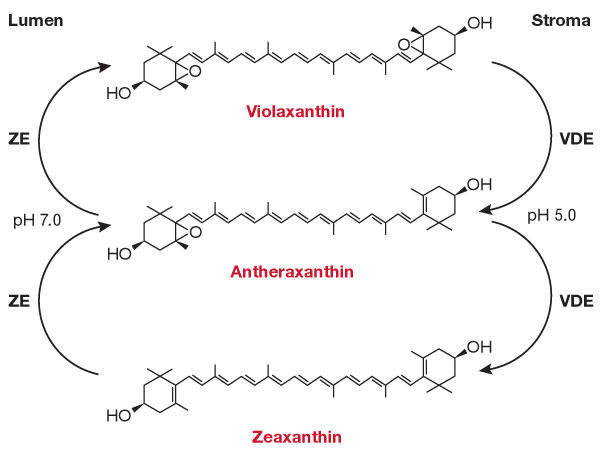
The xanthophyll cycle. VDE, violaxanthin de-epoxidase; ZE, zeaxanthin epoxidase.
A long-standing debate has arisen over what feature of Zea might render this particular pigment essential for qE. All carotenoids are able to dissipate excitation energy by rapid internal conversion, but it has been calculated that only those with ten or more conjugated carbon–carbon double bonds have an excited singlet state (S1) at an energy level low enough to accept energy from 1Chla*. Although the S1 state of carotenoids is dipole-forbidden for direct one-photon excitation, it can be detected after rapid internal conversion of the S2 state. However, direct determination of the in vitro energy levels of the S1 state of Zea (11 double bonds) and Vio (9 double bonds) led to the discovery that both pigments have an S1 state that enables direct quenching of 1Chl* through singlet–singlet energy transfer (Polivka et al, 1999). The S1 state of Zea has a particularly short lifetime (10 ps), which allows for rapid thermal dissipation of excitation energy. Accordingly, an 11 ps lifetime has been found for Zea S1 in reconstituted LHCII (Polivka et al, 2002) and also, as measured by femtosecond transient absorption (TA) kinetics, in intact thylakoids under maximal qE (Ma et al, 2003). In the latter case, excitation of the S1 state of Zea was observed after selective excitation of the first excited singlet state of Chla (Qy band).
Another distinctive feature of Zea with respect to other xanthophylls is its presumed low ionization potential (Dreuw et al, 2003; Holt et al, 2004). The kinetics of the process have been investigated by femtosecond TA measurements in the range 900–1080 nm, characteristic of carotenoid cation radical absorption. Subtraction of quenched from unquenched TA data reveals a rise in the signal, with a time constant of 11 ps, followed by a decay with a time constant of 150 ps (Holt et al, 2005). The same experiments performed on thylakoids from various Arabidopsis mutants with different pigment composition allowed the authors to conclude that Zea is necessary in causing this phenomenon. In the model proposed by Holt et al (2005), the 11 ps component was assigned to energy transfer from excited bulk 1Chl* to a Chla–Zea heterodimer. This step was followed by a non-resolved 0.1–1 ps component, corresponding to an electron transfer, with the formation of a chargeseparated Chl−/Zea+ pair. Charge recombination accounts for the 150 ps component of the observed kinetics. This mechanism is proposed to be responsible for excess energy dissipation during qE. These findings seem to resolve the long-standing issue of whether the role of Zea is that of a direct quencher or not, and indicate direct quenching. The effective site of these events and the contribution of other factors known to be critical for qE, such as that of PsbS, were not discussed in this framework.
One interesting observation concerning the optical properties of the thylakoid membrane during qE is the presence of a 535 nm band in the qE difference spectrum (ΔA535). This band has been ascribed to a red shift to 525 nm of the absorption maximum of the Zea spectrum (Ruban et al, 2002), which is 503 nm in vivo. Apparently, two redshifted Zea molecules (about 15% of the Zea pool) can account for the observed band. It has been proposed that a severe change in the environment of Zea—most probably due to its binding to highly hydrophobic antenna proteins—specifically alters the configuration of this carotenoid, resulting in a red shift of its absorption spectrum (Ruban et al, 2002). In this respect, it is important to note that the in vitro association of Zea with PsbS protein, another key player of qE, results in a red shift of Zea, whereas Vio does not bind to the protein (Aspinall-O'Dea et al, 2002). The activation of Zea by red shift of its excited S2 state presumably reflects a shift towards a lower energy level of the S1 state as well. Whether this adjustment is a consequence of the formation of the Chl–Zea heterodimer is still unclear, but direct excitation of Zea, as well as heterodimer formation, are PsbS-dependent (Ma et al, 2003; Holt et al, 2005).
In addition to its role in direct quenching, Zea has been proposed to have a role in the regulation of the organization of LHCII–PSII complexes. In the Arabidopsis double mutant lut2/npq2, in which Zea is the only xanthophyll present, it has been shown to function in vivo as a light-harvesting pigment, to decrease LHCII size and stability and to induce LHCII monomerization, which suggests a role for Zea in long-term photoadaptation (Havaux et al, 2004).
Light-harvesting complex II and ΔpH-dependent quenching. An alternative to direct quenching by Zea involves carotenoid-mediated changes in the organization of antenna complexes, resulting in Chl–Chl quenching. A role in qE has been proposed for both minor LHCII proteins (Lhcb4, 5 and 6, also named CP29, CP26 and CP24, respectively) and the peripheral LHCII trimers (Lhcb1, 2 and 3; Horton & Ruban, 1992, 2005; Wentworth et al, 2004). CP29 and CP26 bind dicyclohexylcarbodiimide (DCCD; a qE inhibitor that interacts with proton-active residues in a hydrophobic environment), and all the LHCII proteins bind Zea in a pH-dependent manner and to varying extents. Moreover, aggregation-induced in vitro quenching kinetics in LHCII, CP26 and CP29 resemble qE kinetics in intact chloroplasts. The role of LHCII proteins in qE has been questioned, as repression of individual LHCII genes does not induce phenotypic qE changes (Andersson et al, 2001, 2003). However, in field conditions, each LHC protein seems to be important for plant fitness (Ganeteg et al, 2004) and recent data indicate that, in the Chl-binding protein CP26, a Zea-induced conformational change may be responsible for at least part of qE (Dall'Osto et al, 2005).
In a different, structure-based model (Liu et al, 2004), aggregation of LHCII trimers mediated by digalactosyl diacylglycerol, together with a proton-induced conformational change of LHCII, position the linker chlorophylls (Chla 614 and Chlb 605, according to the chlorophyll nomenclature used by the authors) at the trimer–trimer interface in the best orientation for promoting energy transfer to the closely located xanthophyll-cycle carotenoids. Thus, the actual quenching site proposed by these authors is the Chla 613/614 pair, together with xanthophyll-cycle carotenoids.
On the basis of a three-dimensional structure of pea LHCII that was recently determined by Kühlbrandt and collaborators at 2.5 Å resolution (Standfuss et al, 2005), a mechanism for qE at the level of the main antenna has been proposed, in partial agreement with the hypothesis of Liu et al (2004). The quenching site is similarly located in each LHCII monomer, where the two most redshifted Chla are in close contact with a bound Vio. On acidification of the lumen and production of Zea, Vio is substituted by Zea, with stronger binding due to its higher hydrophobicity. The excitation energy collected in the monomer is funnelled to the neighbouring red-shifted Chlas and is finally dissipated after transfer to Zea. By contrast to the model proposed by Liu et al (2004), this mechanism does not imply any conformational rearrangement of the LHCII moiety, and is supported by the fact that the two structures were obtained from crystals grown at different pH: 7–7.5 in the structure from spinach resolved by the Chinese group, and 5–5.5 in that from pea by the German group. The former condition is one in which no qE quenching is expected, whereas the latter probably yields a quenched state. Despite this, the two structures overlap perfectly.
PsbS and ΔpH-dependent quenching. PsbS has been identified as an essential participant in qE by isolation of npq4 Arabidopsis mutants, which are defective in qE and either do not express PsbS proteins, or express mutated versions (Li et al, 2000). npq4 mutants also lack light-induced ΔA535, but show no alteration in other PSII and LHCII proteins or in the xanthophyll cycle (Li et al, 2000; Peterson & Havir, 2000). Several properties of PsbS seem to be important for qE. First, PsbS contains eight conserved acidic amino-acid residues on the lumenal side, two of which (E122 and E226) have been shown to be essential for qE, ΔA535 (Li et al, 2002, 2004) and for binding of the qE inhibitor DCCD (Li et al, 2004). The two acidic residues are thought to enable PsbS to sense lumenal acidification and trigger qE. A similar role had also been hypothesized for other antenna proteins (Horton & Ruban, 1992). Second, the ability of PsbS to bind Zea has been shown in vitro (Aspinall-O'Dea et al, 2002). The fact that ΔA535, which arises from activation of Zea molecules, and the direct excitation of Zea and formation of the Chl–Zea heterodimer (see above) are missing in the absence of PsbS, strongly suggest that Zea can bind to PsbS in vivo as well. Moreover, neither phenomenon can be observed if PsbS is mutated at the E122 and E226 residues (Ma et al, 2003; Li et al, 2004), which reinforces the viewpoint that protonation of PsbS is the first step in the quenching process. A remaining question is whether protonation of PsbS is a prerequisite for Zea binding to the protein, or whether protonation of the PsbS–Zea complex induces a conformational rearrangement and allows Zea to quench Chl excitation. In a model proposed by Li et al (2004), low lumenal pH is required for exposure of the two critical glutamate residues that, on protonation, are directly involved in generating two xanthophyll binding sites. This proposal is in good agreement with the light- and pH-dependent reversible dimerization and/or monomerization of PsbS observed in vivo (Bergantino et al, 2003).
In light of the above data and considerations, binding of Zea to PsbS in vivo is highly probable. However, for the PsbS-bound Zea to be one of the Chl quenching centres, there must be a Chl molecule close enough to form the heterodimer observed by Holt et al (2005; see above). This Chl, in turn, must be at a distance suitable for energy transfer from other chlorophylls connected to the bulk antenna. This suggests the presence of at least one Chl on PsbS. Given the contrasting results obtained by various groups (Funk et al, 1995; Dominici et al, 2002), Chl binding to PsbS cannot be ruled out, but it does need further confirmation.
Last, a PsbS-dependent, Zea-independent qE has been shown to occur in the PSII RC as a transient process (Finazzi et al, 2004). Although the authors do not propose a mechanism for this quenching at the RC, they describe an interesting theory, based on previous publications, in which the various forms of NPQ (qT, qI, antenna qE, and RC qE) take place depending on the balance between the dissipation ability of the carbon fixation apparatus and light flux.
Concluding remarks
With reference to the three 'hot topics' mentioned above, some conclusions may be drawn. Zea is certainly deeply involved in determining qE, and its ability to quench chlorophyll excitation directly has been clearly demonstrated. There is good evidence that this occurs in at least two different sites in the PSII–LHCII supercomplex: in the hydrophobic pocket of LHCII monomers, and in connection with the involvement of PsbS in producing qE.
On the basis of the literature, various mechanisms can be envisaged for qE. A Zea-dependent qE may occur, together with a conformational change in antenna proteins, either on aggregation of LHCII trimers with each other (Liu et al, 2004) or by aggregation of minor antennae CP29 and CP26 with LHCII (Horton & Ruban, 2005).
A variant of this mechanism introduces the formation of a quenching site at the level of each LHCII monomer as the result of binding of Zea, which displaces Vio, with no need for conformational rearrangement of the LHCII trimers (Standfuss et al, 2005). In this view, conformational rearrangements may still be important in relation to the Zea-dependent contribution to qE of the minor antennae CP29 and CP26, which lie in a strategic location inside the PSII–LHCII supercomplex, at the interface between the peripheral LHCII and the internal antennae CP43 and CP47.
Other qE mechanisms depend on PsbS. Some experimental evidence also positions this protein at the interface between LHCII and the PSII core. The quenching mechanism associated with PsbS is also dependent on Zea, but the detailed mechanism is still hypothetical. In one model, PsbS has a critical role in bringing activated Zea into close proximity with a Chl, thus promoting the formation of a Chl–Zea heterodimer that is responsible for the quenching process (Fig 3). Chl may be bound to PsbS itself (Funk et al, 1995) or, more probably, may be located on a neighbouring minor or major antenna protein. PsbS has been proposed to bind Zea on protonation and consequent conformational change (Aspinall O'Dea et al, 2002; Li et al, 2004), which may consist of proton-induced monomerization, followed by its association with an LHCII component (Bergantino et al, 2003).
Figure 3.

Possible mechanism for PsbS-dependent non-photochemical quenching. See text for a detailed description. Green circles: either major or minor antenna proteins. For the sake of clarity, only one chlorophyll (Chl) and one zeaxanthin (Zea, red zigzag) per protein are indicated. Yellow circles: quenching sites. Chl: antenna Chl interacting with Zea.
These mechanisms are all well supported by experimental data and their coexistence is highly probable: some qE persists in PsbS-less Arabidopsis (Li et al, 2000), and PsbS-mediated quenching seems to be essential only in rapidly fluctuating light conditions (Kulheim et al, 2002). Instead, inhibition of single antenna protein expression does not significantly affect feedback de-excitation in field conditions, but it does affect overall plant fitness (Ganeteg et al, 2004). In the emerging scenario, the interplay of these proposed mechanisms ensures the best photoprotective performance in each different and variable light condition.

Ildikò Szabò, Giorgio Mario Giacometti & Elisabetta Bergantino. I.S. is the recipient of an EMBO Young Investigator Award.
Acknowledgments
The authors thank R. Bassi and D. Carbonera for useful discussions. I.S. is grateful to the EMBO Young Investigator Programme for financial support. Thanks are due to G. Walton and C. Friso for revision of the text and figures, respectively.
References
- Allen JF, Forsberg J (2001) Molecular recognition in thylakoid structure and function. Trends Plant Sci 6: 317–326 [DOI] [PubMed] [Google Scholar]
- Andersson J, Walters RG, Horton P, Jansson S (2001) Antisense inhibition of the photosynthetic antenna proteins CP29 and CP26: implications for the mechanism of protective energy dissipation. Plant Cell 13: 1193–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J, Wentworth M, Walters RG, Howard CA, Ruban AV, Horton P, Jansson S (2003) Absence of Lhcb1 and Lhcb2 proteins of the light-harvesting complex of photosystem II—effects on photosynthesis, grana stacking and fitness. Plant J 35: 350–361 [DOI] [PubMed] [Google Scholar]
- Aro EM, Ohad I (2003) Redox regulation of thylakoid protein phosphorylation. Antioxid Redox Signal 5: 55–67 [DOI] [PubMed] [Google Scholar]
- Aspinall-O'Dea M, Wentworth M, Pascal A, Robert B, Ruban A, Horton P (2002) In vitro reconstitution of the activated zeaxanthin state associated with energy dissipation in plants. Proc Natl Acad Sci USA 99: 16331–16335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellafiore S, Barneche F, Peltier G, Rochaix JD (2005) State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433: 892–895 [DOI] [PubMed] [Google Scholar]
- Bergantino E, Segalla A, Brunetta A, Teardo E, Rigoni F, Giacometti GM, Szabò I (2003) Light and pH-dependent structural changes in the PsbS subunit of photosystem II. Proc Natl Acad Sci USA 100: 15265–15270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Osto L, Caffarri S, Bassi R (2005) A mechanism of nonphotochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP26. Plant Cell 17:1217–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW 3rd (1992) Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol 43: 599–626 [Google Scholar]
- Demmig-Adams B, Adams WW 3rd (1996) The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci 1: 21–26 [Google Scholar]
- Demmig-Adams B, Adams WW 3rd (2000) Harvesting sunlight safely. Nature 403: 371–374 [DOI] [PubMed] [Google Scholar]
- Dominici P, Caffarri S, Armenante F, Ceoldo S, Crimi M, Bassi R (2002) Biochemical properties of the PsbS subunit of photosystem II either purified from chloroplast or recombinant. J Biol Chem 277: 22750–22758 [DOI] [PubMed] [Google Scholar]
- Dreuw A, Fleming GR, Head-Gordon M (2003) Charge-transfer state as a possible signature of a zeaxanthin–chlorophyll dimer in the non-photochemical quenching process in green plants. J Phys Chem B 107: 6500–6503 [Google Scholar]
- Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S (2004) Architecture of the photosynthetic oxygen-evolving center. Science 303: 1831–1838 [DOI] [PubMed] [Google Scholar]
- Finazzi G, Johnson GN, Dall'Osto L, Joliot P, Wollmann FA, Bassi R (2004) A zeaxanthin-independent nonphotochemical quenching mechanism localized in the photosystem II core complex. Proc Natl Acad Sci USA 101: 12375–12380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C, Schroeder WP, Napiwotczki A, Tjus SE, Renger G, Andersson B (1995) The PSIIs protein of higher plants: a new type of pigment-binding protein. Biochemistry 34: 11133–11141 [DOI] [PubMed] [Google Scholar]
- Ganeteg U, Kulheim C, Andersson J, Jansson S (2004) Is each light-harvesting complex protein important for plant fitness? Plant Physiol 134: 502–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M, Dall'Osto L, Cuinè S, Giuliano G, Bassi R (2004) The effect of zeaxanthin as the only xanthophyll on the structure and function of the photosynthetic apparatus in Arabidopsis thaliana. J Biol Chem 279: 13878–13888 [DOI] [PubMed] [Google Scholar]
- Holt NE, Fleming GR, Niyogi KK (2004) Toward an understanding of the mechanism of nonphotochemical quenching in green plants. Biochemistry 43: 8281–8289 [DOI] [PubMed] [Google Scholar]
- Holt NE, Zigmantas D, Valkunas L, Li XP, Niyogi KK, Fleming GR (2005) Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307: 433–436 [DOI] [PubMed] [Google Scholar]
- Horton P, Ruban AV (1992) Regulation of photosystem II. Photosynth Res 34: 375–385 [DOI] [PubMed] [Google Scholar]
- Horton P, Ruban A (2005) Molecular design of the photosystem II light-harvesting antenna: photosynthesis and photoprotection. J Exp Bot 56: 365–373 [DOI] [PubMed] [Google Scholar]
- Kulheim C, Agren J, Jansson S (2002) Rapid regulation of light harvesting and plant fitness in the field. Science 297: 91–93 [DOI] [PubMed] [Google Scholar]
- Li XP, Bjorkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403: 391–395 [DOI] [PubMed] [Google Scholar]
- Li XP, Phippard A, Pasari J, Niyogi KK (2002) Structure–function analysis of photosystem II subunit S (PsbS) in vivo. Funct Plant Biol 29: 1131–1139 [DOI] [PubMed] [Google Scholar]
- Li XP, Gilmore AM, Caffarri S, Bassi R, Golan T, Kramer D, Niyogi KK (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279: 22866–22874 [DOI] [PubMed] [Google Scholar]
- Liu Z, Yan H, Wang K, Kuang T, Zhang J, Gui L, An X, Chang W (2004) Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428: 287–292 [DOI] [PubMed] [Google Scholar]
- Ma YZ, Holt NE, Li XP, Niyogi KK, Fleming GR (2003) Evidence for direct carotenoid involvement in the regulation of photosynthetic light harvesting. Proc Natl Acad Sci USA 100: 4377–4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nield J, Orlova EV, Morris EP, Gowen B, van Heel M, Barber J (2000) 3D map of the plant photosystem II supercomplex obtained by cryoelectron microscopy and single particle analysis. Nat Struct Biol 7: 44–47 [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Grossman AR, Bjorkman O (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10: 1121–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RB, Havir EA (2000) A nonphotochemical-quenching-deficient mutant of Arabidopsis thaliana possessing normal pigment composition and xanthophyll-cycle activity. Planta 210: 205–214 [DOI] [PubMed] [Google Scholar]
- Polivka T, Herek JL, Zigmantas D, Akerlund HE, Sundstrom V (1999) Direct observation of the (forbidden) S1 state in carotenoids. Proc Natl Acad Sci USA 96: 4914–4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polivka T, Zigmantas D, Sundstrom V, Formaggio E, Cinque G, Bassi R (2002) Carotenoid S(1) state in a recombinant light-harvesting complex of photosystem II. Biochemistry 41: 439–450 [DOI] [PubMed] [Google Scholar]
- Robert B, Horton P, Pascal AA, Ruban AV (2004) Insights into the molecular dynamics of plant light-harvesting proteins in vivo. Trends Plant Sci 9: 385–390 [DOI] [PubMed] [Google Scholar]
- Ruban AV, Pascal AA, Robert B, Horton P (2002) Activation of zeaxanthin is an obligatory event in the regulation of photosynthetic light harvesting. J Biol Chem 277: 7785–7789 [DOI] [PubMed] [Google Scholar]
- Sandonà D, Croce R, Pagano A, Crimi M, Bassi R (1998) Higher plants light harvesting proteins. Structure and function as revealed by mutation analysis of either protein or chromophore moieties. Biochim Biophys Acta 1365: 207–214 [DOI] [PubMed] [Google Scholar]
- Standfuss J, Terwisscha van Scheltinga AC, Lamborghini M, Kuhlbrandt W (2005) Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution. EMBO J 24: 919–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth M, Ruban AV, Horton P (2004) The functional significance of the monomeric and trimeric states of the photosystem II light harvesting complexes. Biochemistry 43: 501–509 [DOI] [PubMed] [Google Scholar]
- Wollman FA (2001) State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J 20: 3623–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman FA, Lemaire C (1988) Studies on kinase-controlled state transitions in photosystem II and b6f mutants from Chlamydomonas reinhardtii which lack quinone-binding proteins. Biochim Biophys Acta 85: 85–94 [Google Scholar]
- Yamamoto HY (1979) Biochemistry of the violaxanthin cycle in higher plants. Pure Appl Chem 51: 639–548 [Google Scholar]


