Summary
The European Union Framework 6 Pharma–Planta Consortium
The first recombinant plant-derived pharmaceutical protein (PDP) was human serum albumin, initially produced in 1990 in transgenic tobacco and potato plants (Sijmons et al, 1990). Fifteen years on, the first technical proteins produced in transgenic plants are on the market, and proof of concept has been established for the production of many therapeutic proteins, including antibodies, blood products, cytokines, growth factors, hormones, recombinant enzymes and human and veterinary vaccines (Twyman et al, 2005). Furthermore, several PDP products for the treatment of human diseases are approaching commercialization (Table 1), including recombinant gastric lipase for the treatment of cystic fibrosis, and antibodies for the prevention of dental caries and the treatment of non-Hodgkin's lymphoma (Ma et al, 2003). There are also several veterinary vaccines in the pipeline; Dow AgroSciences (Indianapolis, IN, USA) announced recently their intention to produce plant-based vaccines for the animal health industry.
Table 1.
Plant-derived pharmaceutical proteins that are closest to commercialization for the treatment of human diseases
| Product | Class | Indication | Company/Organization | Crop | Status |
|---|---|---|---|---|---|
| Various singlechain Fv antibody fragments | Antibody | Non-Hodgkin's lymphoma | Large Scale Biology Corp | Viral vectors in tobacco | Phase I |
| CaroRx | Antibody | Dental caries | Planet Biotechnology Inc. | Transgenic tobacco | Phase II |
| E.coli heatlabile toxin | Vaccine | Diarrhoea | Prodigene Inc. | Transgenic maize | Phase I |
| Arntzen group (Tacket et al, 1998) | Transgenic potato | Phase I | |||
| Gastric lipase | Therapeutic enzyme | Cystic fibrosis, pancreatitis | Meristem Therapeutics | Transgenic maize | Phase II |
| Hepatitis B Virus surface antigen | Vaccine | Hepatitis B | Arntzen group (Richter et al, 2000) | Transgenic potato | Phase I |
| Thomas Jefferson University/Polish Academy of Sciences | Transgenic lettuce | Phase I | |||
| Human intrinsic factor | Dietary | Vitamin B12 deficiency | Cobento Biotech AS | Transgenic Arabidopsis | Phase II |
| Lactoferrin | Dietary | Gastrointestinal infections | Meristem Therapeutics | Transgenic maize | Phase I |
| Norwalk virus capsid protein | Vaccine | Norwalk virus infection | Arntzen group (Tacket et al, 2000) | Transgenic potato | Phase I |
| Rabies glycoprotein | Vaccine | Rabies | Yusibov et al (2002) | Viral vectors in spinach | Phase I |
As molecular farming has come of age, there have been technological developments on many levels, including transformation methods, control of gene expression, protein targeting and accumulation, the use of different crops as production platforms (Twyman et al, 2003), and modifications to alter the structural and functional properties of the product. One of the most important driving factors has been yield improvement, as product yield has a significant impact on economic feasibility. Strategies to improve the recombinant protein yield in plants include the development of novel promoters, the improvement of protein stability and accumulation through the use of signals that target the protein to intracellular compartments, and the improvement of downstream processing technologies (Menkhaus et al, 2004).
Attention is now shifting from basic research towards commercial exploitation, and molecular farming is reaching the stage at which it could challenge established production technologies that use bacteria, yeast and cultured mammalian cells. In this review, we highlight not only recent progress in molecular farming and its potential for commercial drug development and production, but also the regulatory control, biosafety and political impacts of the technology, and its related intellectual property (IP) issues.
Ever since plants were first considered as a production system for recombinant pharmaceutical proteins, many potential benefits have been claimed. But, as the technology has matured, so too has the appreciation of the real advantages that plants provide. There is little doubt that transgenic plants offer an unparalleled potential for scalability. Growing plants in the field provides opportunities for virtually unlimited production, and even if the growing sites were strictly isolated to avoid mixing or cross-pollination with other crops, there are still many areas in the world where large-scale production could take place. Even under containment, it would be possible to grow a large number of pharmaceutical plants; immense greenhouse facilities are already used routinely by the horticultural and food industries. It has been estimated that 250 acres of greenhouse space would be sufficient to grow enough transgenic potato plants to meet South East Asia's annual demand for the hepatitis B virus vaccine (C. Arntzen, personal communication).
...molecular farming is reaching the stage at which it could challenge established production technologies that use bacteria, yeast and cultured mammalian cells
This level of production is clearly not necessary for all protein pharmaceuticals, but there are many cases in which large quantities are required. Monoclonal antibodies (mAbs), the largest class of biologics now in development, are often needed in substantial amounts. For example, two licensed antibodies, palivizumab and infliximab, are used systemically at doses of 10–15 mg kg−1 body weight. Many mAbs are also being developed as topical mucosal treatments, in which the dosage requirements and the need for repeated applications are likely to be much greater due to the washing effects of mucosal secretions. For example, the Guy's 13 mAb, which prevents colonization of the oral cavity by Streptococcus mutans, required a dose of 22.5 mg per course of treatment (Ma et al, 1998). If this were administered to the child population of Europe alone, a production capacity of more than 1,000 kg per year would be required.
Other topical agents are likely to be needed at similar levels. HIV protein microbicides could be used to prevent HIV transmission by topical vaginal or rectal application. However, these reagents have not yet been tested in humans because existing manufacturing capacities are not able to produce sufficient amounts for clinical tests. Studies in rhesus macaques suggest that regular doses of at least 5 mg are required to provide vaginal coverage (Shattock & Moore, 2003). Thus, to treat one million women, it would be necessary to produce approximately 260 kg of the protein per year. Current recombinant protein-manufacturing capabilities would be stretched to the limit if they were to produce any of these topical reagents.
Producing therapeutics for the developed world is important, but there is also a moral imperative to provide medicines for developing countries. We will probably see an effective vaccine against HIV in the next few years, but as soon as it is developed, the global demand will far exceed our ability to produce the compound. Beyond HIV, there are many other infectious diseases that require attention. Recombinant hepatitis B vaccine, for instance—produced in genetically modified yeast at present—cannot be made in sufficient quantities and at a low enough cost to meet the demands of developing countries. The scalability of production in transgenic plants could offer one of the few practical solutions to overcome these commercial and moral dilemmas.
Regardless of the availability of a vaccine or protein therapeutic, cost is a major factor for developing countries. In addition, it is likely that new vaccines will become more complex in composition, have longer development times and consequently will cost more when they come to market. Although the cost of production in plants will be low, this may not be the most important economic factor. 'Cost of goods' has relatively little impact on the market price of new pharmaceuticals, as illustrated by the dramatic fall in prices when drugs lose patent protection and generics become available. Several other factors influence the price of new vaccines, including regulatory requirements for drug development and manufacture—which have become more stringent and costly in recent years—the high failure rate of new drugs, and the protection of IP.
Production in transgenic plants could offer a new model for vaccine development. The initial production technology is low-tech and inexpensive, which facilitates entry into a pharmaceutical development programme with relatively low initial investment costs. This could allow the participation of a wider audience beyond the well-established multinational pharmaceutical companies. Hopefully, developing countries would be involved, and the focus would shift to specific regional diseases that do not otherwise feature prominently in current drug development.
Growing plants in the field provides opportunities for virtually unlimited production...
Another fundamental advantage of plants has always been the range and diversity of recombinant molecules that they can potentially produce. As higher eukaryotes, plants are able to synthesize small peptides, polypeptides and complex multimeric proteins, many of which cannot be made in microbial systems (Ma et al, 2003). This versatility is reflected in the increasing number of recombinant proteins that has been reported in the literature. For many molecules, such as antibodies, the presence of plant chaperones that are homologous to those in mammalian cells is an important factor, as these chaperones control the efficiency of protein assembly and the extent of protein degradation. In addition, targeting recombinant proteins to the plant secretory pathway ensures that N-glycosylation and other post-translational modifications take place. The overall benefits are not only in the range of recombinant proteins that can be made in plants, but also in the flexibility that is allowed in the engineering of new pharmaceutical proteins, which can be designed with plant expression in mind (Chargelegue et al, 2005).
There has been much interest in the oral delivery of vaccines using edible transgenic plant material, but one major hurdle is the need to ensure an appropriate immune response. Despite the advantages of oral delivery, only the live (Sabin) polio vaccine is delivered by this route at present, which reflects important gaps in our knowledge of the mucosal immune system. However, PDPs would undoubtedly assist vaccine programmes in developing countries by simplifying immunization regimens and reducing the costs of vaccine production, purification, storage and administration. Nevertheless, it is important to recognize that edible vaccines will not be delivered as fresh produce, as often suggested. A regulated product requires controlled delivery of standardized doses, so some level of processing of the edible plant material would be required. This need not include complex purification, but is more likely to involve simple and inexpensive food processing techniques that are readily available, such as freeze drying.
Although the potential advantages of plants for PDP production are now becoming clearer, concerns remain about low product yield, modified glycan structures and the impact of pharmaceutical plants on the environment. Thus, the importance of continuing basic research and improving the technology cannot be over-emphasized in the rush to commercialization, and this must be achieved in a manner consistent with evolving health and environmental regulatory frameworks.
One example of an emerging production technology is multiple-transgene direct DNA transfer, which simultaneously introduces all the components required for the expression of complex recombinant macromolecules into the plant genome. Nicholson et al (2005) delivered into rice plants four transgenes that represent the components of a secretory antibody. Approximately 20% of the resulting plants carried all four genes. Even though they were delivered on different plasmids, such multiple transgenes are frequently inherited in a linked fashion (Chen et al, 1998). This is a major advantage over alternative gene-transfer methods that involve the stepwise introduction of individual components followed by successive rounds of crossing to generate plants containing the fully assembled molecule. Direct DNA transfer also allows the introduction into plants of minimal expression cassettes that contain only the promoter, open reading frame and terminator sequences. As no vector backbone sequences are transferred, this substantially increases transgene stability and expression levels by preventing the integration of potentially recombinogenic sequences (Fu et al, 2000; Loc et al, 2002; Christou & Kohli, 2005).
Another fundamental advantage of plants has always been the range and diversity of recombinant molecules that they can potentially produce
The differential glycosylation of proteins produced in in vitro systems or in non-native species has also been a potential concern. In the case of plant glycosylation, it is unlikely that plant glycans will be immunogenic or allergenic, but altered glycans might affect the functionality of some recombinant glycoproteins. Strategies are being devised to 'humanize' the plant glycosylation machinery by inhibiting glycosylation enzymes (Strasser et al, 2004), complemented, if necessary, with mammalian counterparts (Bakker et al, 2001).
Another maturing technology is the synthesis of PDPs in plastids. This confers significant advantages, primarily because of the large number of transgene copies in homoplasmic transformants, which allows the production of extremely high levels of recombinant protein (Bock, 2001; Daniell, 2002). The use of plastids as a vaccine production platform seemed promising when a tetanus toxin fragment (TetC) was produced at 25% of total soluble cellular protein in tobacco chloroplasts (Tregoning et al, 2003). However, there have also been several cases in which there was little or no expression of a target pharmaceutical molecule in plastids. Codon optimization can be an issue, as was shown in the case of TetC, in which transgenes with a high AT content were expressed at the highest levels. Recombinant proteins are also subject to proteolysis in plastids. Birch-Machin et al (2004) expressed high levels of rotavirus VP6 protein in the chloroplasts of young tobacco leaves, but they diminished rapidly as the leaves matured. The choice of target antigen must therefore reflect an understanding of the microenvironment in the chloroplast and its functionality, to ensure efficient and stable protein accumulation without adversely affecting chloroplast function.
Whilst transformation and transgene expression are important technological goals, it is also necessary to consider the wider impact of pharmaceutical plants on human and environmental health. Two safety issues are often raised in this context: the possible transgene escape through pollen or seed dispersal, and the potential for recombinant molecules to enter the food chain. Transformation of the plastid genome is one of several strategies that have been put forward to minimize transgene flow through pollen (Daniell, 2002), as the plastid DNA is maternally inherited in most crop plants (Fig 1). An alternative is the use of male sterile plant lines, in which no pollen is produced. Seed dispersal could be prevented by making seed viability dependent on an exogenous stimulus, such as the application of a chemical inducer (Daniell, 2002).
...most regulatory processes do not readily accommodate pharmaceutical crops because the current regulations have been developed for food and feed crops...
Figure 1.
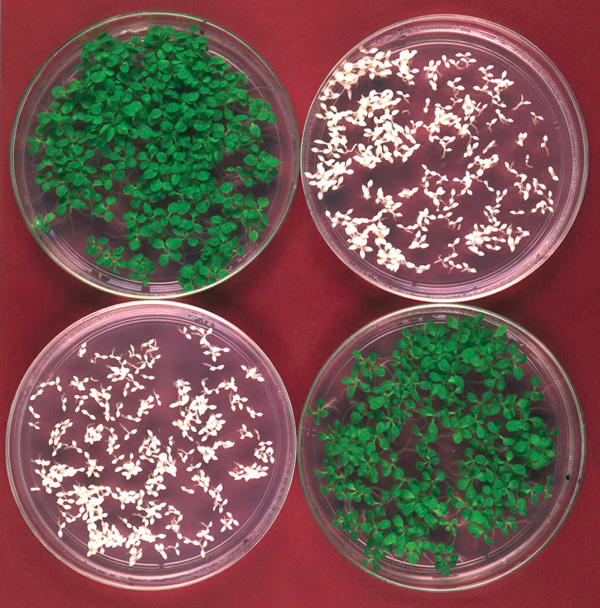
Reciprocal crosses showing maternal inheritance of a transgene integrated into the chloroplast genome. Surface-sterilized seeds are germinated on antibiotic-containing medium and analysed for inheritance of the chloroplast-encoded antibiotic resistance gene. Selfed plastid transformants ('transplastomics') and crosses with a plastid transformant as the maternal parent gives rise to uniformly resistant (green) progeny, whereas seeds collected from wild-type plants yield antibiotic-sensitive (white) seedlings. Most importantly, pollen from transplastomic plants does not transmit the transgene (cross-labelled in red), thus greatly reducing transgene escape.
To avoid inadvertent entry into the food chain, the use of non-food crops such as tobacco is one option, but it is not prudent to exclude a priori major crop plants as hosts, particularly if the target molecule poses little or no risk to environmental or human health, such as recombinant antibodies. In cases where direct oral administration of the pharmaceutical is desirable, edible plants are clearly preferred. Additional safety measures that can be taken include the use of contained production facilities, such as greenhouses, the development of phenotypic markers for identity preservation—for example, green or purple fruit colour in tomato as a label for transgenic lines expressing pharmaceuticals—and the use of inducible expression systems that require the application of a chemical inducer to switch on transgene expression. Fluorescent marker proteins such as GFP or DsRed could be used not only as visual selection agents, but also as traceable markers for macroscopic detection of transgene activity. In this way, the expression of a linked transgene could be monitored visually (Harper et al, 1999), and transgene movement could be traced without molecular techniques in agronomic and ecological studies (Fig 2). Tethering the transgene to an inducible promoter makes expression dependent on an externally applied stimulus such as ethanol vapour, a method that works efficiently on potato tubers after harvest (Sweetman et al, 2002). Control of expression is also an issue in the case of plastid transgenes, particularly in view of the high yields that can be achieved. One promising approach is to use a nuclear-expressed, plastid-targeted phage T7 RNA polymerase to transcribe the plastid transgene selectively (Magee et al, 2004).
Figure 2.
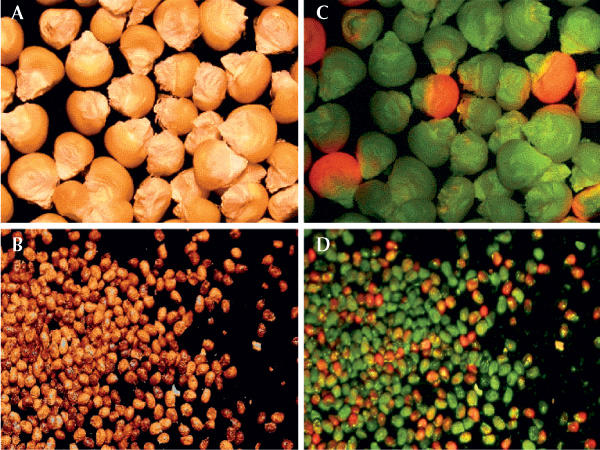
Detection of transgenic seeds using red fluorescent protein (DsRed) as a visible marker. Maize and tobacco seeds (A and B) observed under green light (C and D). Courtesy of T. Rademacher (RWTH, Aachen, Germany).
The regulation of pharmaceutical crops is still a developing field, with the majority of experience coming from North America. In the USA, most field trials for genetically modified organisms (GMOs) rely on risk mitigation in the form of strict confinement and regular inspections to limit any environmental exposure. These measures have become stricter for pharmaceutical crops during the past few years. Environmental assessments are only required when a regulated article is being petitioned for deregulated status, such as before widespread environmental release. Until then, field trials are designed to confine the regulated article, and in this way reduce the risk by limiting exposure. There are no plans at present to deregulate any pharmaceutical crops in the USA, and as such they are likely to remain under experimental permit for the foreseeable future.
In the EU, regulators strive to consider pharmaceutical crops on a case-by-case basis. However, most regulatory processes do not readily accommodate pharmaceutical crops because the current regulations have been developed for food and feed crops and their potential impact on the environment. There have been limited attempts so far to adapt these regulations for pharmaceutical crops, but at present there is no 'natural home' for assessing pharmaceutical crops in the EU regulatory process. A similar situation exists in South Africa, where applications for the contained release of a pharmaceutical crop and subsequent field trial must follow the same steps as an application for a normal GMO, and should include the necessary risk-assessment information.
Many of the plants being developed for the production of PDPs are food and feed crops. A first step in developing a regulatory framework is to consider whether PDP products and by-products can be adequately segregated from other commodities, particularly those intended for human and animal consumption. A case in 2002, involving Prodigene Inc. (College Station, TX, USA), showed that, although safeguards are in place to prevent adulterated food products entering the food chain, the biology of the production crop and subsequent rotation crops must be taken into account in regulation and containment strategies.
Most of the steps that prevent pharmaceutical crops becoming mixed with food crops involve relatively low-tech measures, such as meticulous planning and execution of each step in the production process. The crop must be grown in isolation from breeding materials to avoid genetic and mechanical mixing. Field trials need to be carried out in isolation, away from conventional agricultural crop trials. Parent seed for commercial production and the commercial crops themselves must be isolated from other plants of the same species or wild relatives, to avoid stray pollination. In practice, such mixing might be difficult to detect, so effective handling and labelling protocols are also essential, in addition to the use of labelling technology, such as fluorescent markers, as described earlier.
Many of these considerations are equally relevant to existing conventional crops where genetic admixtures can occur, and successful co-existence strategies are already in widespread practice. One example is the production of 'high-erucic acid rape' (HEAR), which yields specialized oils for industrial processing. Erucic acid is highly toxic, and HEAR seeds can become mixed with seeds from food and feed oilseed rape; therefore production protocols are tightly defined and controlled. In North America, producers are required to grow HEAR under contract registration, and the Canadian Food Inspection Agency (CFIA) mandate this requirement at the time of varietal registration (Smyth & Phillips, 2002). Similarly, measures are taken to ensure that opium poppy seeds used for food are completely separated from the other plant parts from which opiates are extracted. The production of opium poppies is also carried out in complete physical isolation (Mascia & Flavell, 2004).
As well as adhering to the same strict regulations as conventional GM food and feed crops, PDPs also need to satisfy the regulations set out by the agencies that oversee the production of pharmaceuticals. Both the US Food and Drug Administration (FDA) and the European Agency for the Evaluation of Medicinal Products (EMEA) published draft documents in 2002 that addressed quality aspects in the processing of medicinal products made from GM plants (FDA 2002; EMEA 2002). These guidelines address the potential scientific and technological hurdles that must be overcome before plants can be considered as a viable alternative for existing biopharmaceutical production systems (Drossard, 2004).
One of the most important goals in molecular farming is the development of new drugs and vaccines targeting important diseases in both developed and developing countries. Here, government and foundation funding of PDP production represents a new generation of public-sector initiatives that seek to rectify a widely acknowledged imbalance: the lack of investment in research and development focusing on health technologies for the poor (CMH, 2001). The private sector cannot address this imbalance adequately because it needs to make a return on its investment, which the market for the poor does not provide. The public sector is therefore making substantially increased investments in health technology innovation through public–private partnerships. These product-development partnerships face a common problem: how to manage IP. This is no small challenge. IP management is a complex and empirical field that embraces many issues including patenting, the protection of confidential information and the formation of cooperative research and development programmes (Mahoney, 2004). In an area such as molecular farming, in which there are many organizational actors, there are three overriding challenges to IP management: determining freedom to operate, securing new IP as it is developed, and deploying IP to enable product development in partnership with private companies.
For public-sector support of PDPs to achieve its goals, it will be necessary to carry out an in-depth freedom-to-operate (FTO) assessment, which provides a clearer picture of the patents that do, may, and do not stand in the way of developing products. An FTO assessment provides a good sense of the IP issues of concern for any project and helps to minimize the chances of running into costly problems. A valuable adjunct to the FTO analysis would be the creation of a computer database of all the relevant patents in molecular farming.
Public-sector groups involved in PDP production are often dedicated to achieving a social goal. To accomplish this, they should use humanitarian licensing practices. For example, if a group helps to develop a new therapeutic monoclonal antibody, it could license the technology to companies in Europe, but it could also reserve the right to license companies in developing countries that would make the product available to the poor at prices near the cost of production. Whereas most scientists and their institutes will cheerfully support these ideals, in practice most IP is still not developed with humanitarian licensing practices in mind, and efforts to achieve this must be undertaken in a proactive manner. One innovative approach has been adopted by the Pharma–Planta Consortium, an EU-funded project to develop recombinant pharmaceuticals in plants (www.pharma-planta.org), in which 76 scientists have signed a Statement of Intent for Humanitarian Use for all knowledge that is generated during the project.
IP management is a complex process, but there are clear steps that public-sector groups concerned with health can take to achieve the goal of making safe and effective health products available to all. These steps will help to improve the socio-political status of molecular farming, a subject that is discussed in the following section.
The media-led furore over GM food in Europe illustrated very clearly that although research and development success and social acceptance are both necessary for the success of new biotechnology, social rejection alone is sufficient to derail the endeavour. There is frequently the perception—whether scientifically justified or not—that the mixing of pharmaceutical crops with food and feed crops may be harmful to human health, and hence there can be added pressures from the farming, food and retail industries. The regulatory systems in most parts of the world deal principally with scientific risk assessment and are not usually set up to consider socio-political questions. These need to be dealt with in a broader societal context. The regulatory system in South Africa, for instance, has a provision stating that the Council may consider the socio-economic impact of the release on a community living in the vicinity of such an introduction.
Non-governmental organizations (NGOs) such as the Union of Concerned Scientists have described PDP crops as biological factories producing chemical products that present important new policy challenges requiring public debate. Others, including Friends of the Earth and GeneWatch, have stated that the potential for inadvertent consumption of a drug-producing crop and the potential for gene flow to other crops mean that food crops should not be used. Food processors, such as the Grocery Manufacturers of America Inc., are nervous about the risk of mixing food and pharmaceutical crops, and have suggested that the US Department of Agriculture “should adopt...a presumption against the use of food or feed crops for drug or industrial compound manufacturing” (GMA, 2003). They further recommend that these types of non-food crop should not be grown where crops are commonly grown for food or feed.
...industry needs to learn from past mistakes and should be seen to regulate the technology tightly to gain public trust
However, as discussed earlier, the merits of using food crops should not be ignored. In particular, dry seed crops, such as maize, rice, wheat, barley, soybean and pea, offer huge benefits in terms of scalability as well as protein stability (Stoger et al, 2002). Seeds are natural storage organs, with the optimal biochemical environment for the accumulation of large amounts of protein. In this form, proteins can be transported from farm to production factories without the loss of stability. Downstream protein extraction from seeds is also easier than from vegetable or leaf tissue, which will inevitably reduce production costs and benefit the consumer. Food crops as production hosts should therefore not be dismissed, particularly if the target molecule poses little or no environmental risk. Whatever the outcome of the debates and regulation, it will be essential to manage all of the related perceptions, technical issues and risks on a case-by-case basis.
As society learns more about transgenic crops and how to control any potentially harmful effects, the need for regulation may decrease. Until that happens, regulations reassure the public that the new technology is not harmful to them or the environment. PDPs offer a real opportunity for the public to see a distinct, advantageous end use for this technology, with the ability to produce therapeutic drugs more efficiently and with less expense. This has not escaped public attention: when activists destroyed a transgenic maize crop, which was being grown by the French biotechnology company Meristem Therapeutics to produce recombinant gastric lipase for the treatment of cystic fibrosis or pancreatitis, the high-profile NGOs were quick to distance themselves and deny involvement after the Cystic Fibrosis Patients Organization defended the technology. However, industry needs to learn from past mistakes and should be seen to regulate the technology tightly to gain public trust. Any mishandling is likely to foster negative attitudes and could delay the PDP agenda—the provision of inexpensive drugs to all those who need them—by many years.
Acknowledgments
The Pharma–Planta Consortium is funded by the European Union through the Framework 6 research programme.
References
- Bakker H et al. (2001) Galactose-extended glycans of antibodies produced by transgenic plants. Proc Natl Acad Sci USA 98: 2899–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch-Machin I, Newell CA, Hibberd JM, Gray JC (2004) Accumulation of rotavirus VP6 protein in chloroplasts of transplastomic tobacco is limited by protein stability. Plant Biotechnol J 2: 261–270 [DOI] [PubMed] [Google Scholar]
- Bock R (2001) Transgenic chloroplasts in basic research and plant biotechnology. J Mol Biol 312: 425–438 [DOI] [PubMed] [Google Scholar]
- Chargelegue D, Drake PM, Obregon P, Prada A, Fairweather N, Ma JK (2005) A highly immunogenic and protective recombinant vaccine candidate expressed in transgenic plants. Infect Immun (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L et al. (1998) Expression and inheritance of multiple transgenes in rice plants. Nat Biotechnol 16: 1060–1064 [DOI] [PubMed] [Google Scholar]
- Christou P, Kohli A (2005) Transformation method and transgenic plants produced thereby. US Patent No 6846970. 25 Jan, www.uspto.gov
- CMH (2001) Macroeconomics and Health: Investing in Health for Economic Development. Geneva, Switzerland: World Health Organization [Google Scholar]
- Daniell H (2002) Molecular strategies for gene containment in transgenic crops. Nat Biotechnol 20: 581–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossard J (2004) Downstream processing of plant-derived recombinant therapeutic proteins. In Fischer R, Schillberg S (eds), Molecular Farming: Plant-made Pharmaceuticals and Technical Proteins, pp 217–232. Weinheim, Germany: Wiley VCH [Google Scholar]
- EMEA (2002) Points to Consider on Quality Aspects of Medicinal Products Containing Active Substances Produced by Stable Transgene Expression in Higher Plants (CPMP/BWP/764/02) [Draft]. London, UK: European Agency for the Evaluation of Medicinal Products
- FDA (2002) Guidance for Industry: Drugs, Biologics, and Medical Devices Derived from Bioengineered Plants for Use in Humans and Animals [Draft Guidance]. Rockville, MD, USA: United States Food and Drug Administration
- Fu X, Duc LT, Fontana S, Bong BB, Tinjuangjun P, Sudhakar D, Twyman RM, Christou P, Kohli A (2000) Linear transgene constructs lacking vector backbone sequences generate low-copy-number transgenic plants with simple integration patterns. Transgenic Res 9: 11–19 [DOI] [PubMed] [Google Scholar]
- GMA (2003) GMA Comments on USDA Bio-Pharma Permit Regulations. Field Testing of Plants Engineered to Produce Pharmaceutical and Industrial Compounds. Docket 03-031-1. 3 Oct, www.gmabrands.com
- Harper BK, Mabon SA, Leffel SM, Halfhill MD, Richards HA, Moyer KA, Stewart CN Jr (1999) Green fluorescent protein as a marker for expression of a second gene in transgenic plants. Nat Biotechnol 17: 1125–1129 [DOI] [PubMed] [Google Scholar]
- Loc NT, Tinjuangjun P, Gatehouse AM, Christou P, Gatehouse JA (2002) Linear transgene constructs lacking vector backbone sequences generate transgenic rice plants which accumulate higher levels of proteins conferring insect resistance. Mol Breeding 9: 231–244 [Google Scholar]
- Ma JK, Hikmat BY, Wycoff K, Vine ND, Chargelegue D, Yu L, Hein MB, Lehner T (1998) Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat Med 4: 601–606 [DOI] [PubMed] [Google Scholar]
- Ma JK, Drake PM, Christou P (2003) The production of recombinant pharmaceutical proteins in plants. Nat Rev Genet 4: 794–805 [DOI] [PubMed] [Google Scholar]
- Magee AM, Coyne S, Murphy D, Horvath EM, Medgyesy P, Kavanagh TA (2004) T7 RNA polymerase-directed expression of an antibody fragment transgene in plastids causes a semi-lethal pale-green seedling phenotype. Transgenic Res 13: 325–337 [DOI] [PubMed] [Google Scholar]
- Mahoney RT (ed) (2004) Handbook of Best Practices for Management of Intellectual Property in Health Research and Development. Oxford, UK: Centre for the Management of Intellectual Property in Health Research and Development (MIHR) [Google Scholar]
- Mascia PN, Flavell RB (2004) Safe and acceptable strategies for producing foreign molecules in plants. Curr Opin Plant Biol 7: 189–195 [DOI] [PubMed] [Google Scholar]
- Menkhaus TJ, Bai Y, Zhang C, Nikolov ZL, Glatz CE (2004) Considerations for the recovery of recombinant proteins from plants. Biotechnol Prog 20: 1001–1014 [DOI] [PubMed] [Google Scholar]
- Nicholson L, Gonzalez-Melendi P, van Dolleweerd C, Tuck H, Perrin Y, Ma JK, Fischer R, Christou P, Stoger E (2005) A recombinant multimeric immunoglobulin expressed in rice shows assembly-dependent subcellular localization in endosperm cells. Plant Biotechnol J 3: 115–127 [DOI] [PubMed] [Google Scholar]
- Richter LJ, Thanavala Y, Arntzen CJ, Mason HS (2000) Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat Biotechnol 18: 1167–1171 [DOI] [PubMed] [Google Scholar]
- Shattock RJ, Moore JP (2003) Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol 1: 25–34 [DOI] [PubMed] [Google Scholar]
- Sijmons PC, Dekker BM, Schrammeijer B, Verwoerd TC, van den Elzen PJ, Hoekema A (1990) Production of correctly processed human serum albumin in transgenic plants. Biotechnology (NY) 8: 217–221 [DOI] [PubMed] [Google Scholar]
- Smyth S, Phillips PWB (2002) Production differentiation alternatives: identity preservation, segregation, and traceability. AgBioForum 5: 30–42 [Google Scholar]
- Stoger E, Sack M, Perrin Y, Vaquero C, Torres E, Twyman RM, Christou P, Fischer R (2002) Practical considerations for pharmaceutical antibody production in different crop systems. Mol Breeding 9: 149–158 [Google Scholar]
- Strasser R, Altmann F, Mach L, Glossl J, Steinkellner H (2004) Generation of Arabidopsis thaliana plants with complex N-glycans lacking β1,2-linked xylose and core α1,3-linked fucose. FEBS Lett 561: 132–136 [DOI] [PubMed] [Google Scholar]
- Sweetman JP, Chu C, Qu N, Greenland AJ, Sonnewald U, Jepson I (2002) Ethanol vapor is an efficient inducer of the alc gene expression system in model and crop plant species. Plant Physiol 129: 943–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacket CO, Mason HS, Losonsky G, Clements JD, Levine MM, Arntzen CJ (1998) Immunogenicity in humans of a recombinant bacterial-antigen delivered in transgenic potato. Nature Med 4: 607–609 [DOI] [PubMed] [Google Scholar]
- Tacket CO, Mason HS, Losonsky G, Estes MK, Levine MM, Arntzen CJ (2000) Human immune responses to a novel norwalk virus vaccine delivered in transgenic potatoes. J Infect Dis 182: 302–305 [DOI] [PubMed] [Google Scholar]
- Tregoning R et al. (2003) Expression of tetanus toxin Fragment C in tobacco chloroplasts. Nucleic Acids Res 31: 1174–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman RM, Stoger E, Schillberg S, Christou P, Fischer R (2003) Molecular farming in plants: host systems and expression technology. Trends Biotechnol 21: 570–578 [DOI] [PubMed] [Google Scholar]
- Twyman RM, Schillberg S, Fischer R (2005) Transgenic plants in the biopharmaceutical market. Expert Opin Emerg Drugs 10: 185–218 [DOI] [PubMed] [Google Scholar]
- Yusibov V et al. (2002) Expression in plants and immunogenicity of plant virus-based experimental rabies vaccine. Vaccine 20: 3155–3164 [DOI] [PubMed] [Google Scholar]


