Abstract
The active and inactive X chromosomes have distinct epigenetic marks in somatic nuclei, which undergo reprogramming after transplantation into oocytes. We show that, despite the disappearance of Xist RNA coating in 30 min, the epigenetic memory of the inactive X persists with the precocious appearance of histone H3 trimethylation of lysine 27 (H3-3meK27), without the expected colocalization with Eed/Ezh2. Subsequently, Xist re-appears on the original inactive X, and the silent Xist on the active X undergoes re-activation, resulting in unusual biallelic Xist RNA domains. Despite this abnormal Xist expression pattern, colocalization of H3-3meK27 and Eed is thereafter confined to a single Xist domain, which is presumably on the original inactive X. These epigenetic events differ markedly from the kinetics of preferential paternal X inactivation in normal embryos. All the epigenetic marks on the X are apparently erased in the epiblast, suggesting that the oocyte and epiblast may have distinct properties for stepwise programming of the genome.
Keywords: epigenetic reprogramming, histone modification, X inactivation, Xist
Introduction
The reprogramming of a somatic nucleus by oocyte cytoplasm is a prime example of a change in genome function that requires appropriate epigenetic modifications. In mammals, dosage compensation of X-linked genes in females occurs through the inactivation of one of the two X chromosomes (Lyon, 1961). Initially, the maternal X chromosome is imprinted to remain active (Xm* to denote the imprint) whereas the paternal X (Xp) is preferentially inactivated in pre-implantation embryos (Huynh & Lee, 2003; Mak et al, 2004; Okamoto et al, 2004) and this state is maintained in extraembryonic tissues (Takagi & Sasaki, 1975). By contrast, there is random inactivation of either the Xp or the Xm in the embryo proper after re-activation of the inactive paternal X chromosome (Mak et al, 2004; Okamoto et al, 2004). Thus, a female somatic donor nucleus when transplanted into an oocyte contains a randomly selected inactive X (Xi) and an active X (Xa), but no maternal imprint, which differs from the parental X chromosomes, Xp and Xm*, in a normal zygote.
Recent advances have explained in greater detail the earliest molecular events that lead to stepwise conversion of an entire X chromosome from a euchromatic to a heterochromatic conformation during pre-implantation development (Heard, 2004). The process is regulated by the expression and cis-localization of a large noncoding RNA, the X inactive transcript (Xist; Brockdorff et al, 1991; Brown et al, 1991; Panning et al, 1997; Sheardown et al, 1997). Xist RNA, in turn, recruits chromatin-modifying factors, including the PRC2 Polycomb group (PcG) complex Eed/Ezh2 (Enx-1), which results in chromosome-wide H3-3meK27 methylation (Plath et al, 2003; Silva et al, 2003; Kohlmaier et al, 2004; Rougeulle et al, 2004). As shown recently, the PRC1 (Mel18/Ring1B) complex also becomes enriched on the Xi in early development (de Napoles et al, 2004). Given these well-characterized epigenetic marks associated with X inactivation, this process is highly suited for investigations into the events underlying reprogramming of a somatic cell nucleus by oocyte cytoplasm.
In a previous study on cloned mouse embryos (Eggan et al, 2000), a green fluorescent protein transgene carried by a somatic-cell-derived Xi was shown to be re-activated after transfer into an enucleated oocyte, but then became preferentially inactivated in extraembryonic tissues. Conversely, in the fetus random X inactivation was observed, suggesting that the original epigenetic marks on Xi were erased on epiblast formation. However, the early crucial molecular events underlying re-activation and subsequent inactivation at the level of both Xist expression and X-linked chromatin modifications were largely unknown at that time, which precluded detailed examination of the fate of the Xi and Xa in somatic nuclei after transplantation into the oocyte. To gain insight into the early reprogramming events, we have examined the initial epigenetic changes associated with Xi and Xa after transplantation of female somatic nuclei into enucleated mouse oocytes (SCNT embryos). Our study shows that the X chromosome reprogramming initiation involves unusual events starting only 30 min after nuclear transfer. The kinetics of these events differ markedly from those in normal female embryos.
Results And Discussion
We isolated tail-tip fibroblasts from adult female mice as the source of somatic nuclei. The characteristics of the Xi in these somatic cells were verified using immunofluorescence (IF) combined with Xist RNA fluorescence in situ hybridization (FISH) analysis. In these somatic nuclei, the Xi was found to be coated with Xist RNA in almost 100% of tail-tip cells. The H3-3meK27 is another putative epigenetic mark on Xi, which is introduced during development (Erhardt et al, 2003; Plath et al, 2003; Silva et al, 2003). Although we did not overtly find all the somatic cells enriched in H3-3meK27 (supplementary Fig S1 online), we detected between 20% and 40% of the cells in which the H3-3meK27 mark colocalized with Xist RNA. This is similar to results reported for adult-ear fibroblasts (Rougeulle et al, 2004), suggesting that levels of H3-3meK27 could be lower, or perhaps less well detected by antibodies, in fully differentiated adult cells than in early embryonic cells. Nevertheless, these observations show that somatic nuclei apparently have appropriate epigenetic modifications associated with Xi.
The tail-tip nuclei were transferred to enucleated oocytes by electro-fusion (Ogura et al, 2000) and were then examined by IF and Xist RNA FISH at various stages after their transfer. Previous studies have shown that during normal pre-implantation development, Xist from Xp is first expressed at the two-cell stage when principal embryonic gene activation occurs, followed by accumulation of the RNA in cis at the four-cell stage (Okamoto et al, 2004). The PcG proteins, Eed and Ezh2, accumulate on the Xp from the 16-cell stage onwards and this is accompanied by the appearance of H3-3meK27 and H3-K9 methylation marks on this chromosome (Okamoto et al, 2004). However, in embryos resulting from transplantation of tail-tip somatic nuclei, the Xist RNA coating that was initially associated with Xi disappeared only 30 min after SCNT to an enucleated oocyte, and it was completely absent at 2 h (Fig 1A–C; supplementary Table S1 online). This suggests that the oocyte cytoplasm contains factors that induce Xist RNA delocalization or destabilization.
Figure 1.
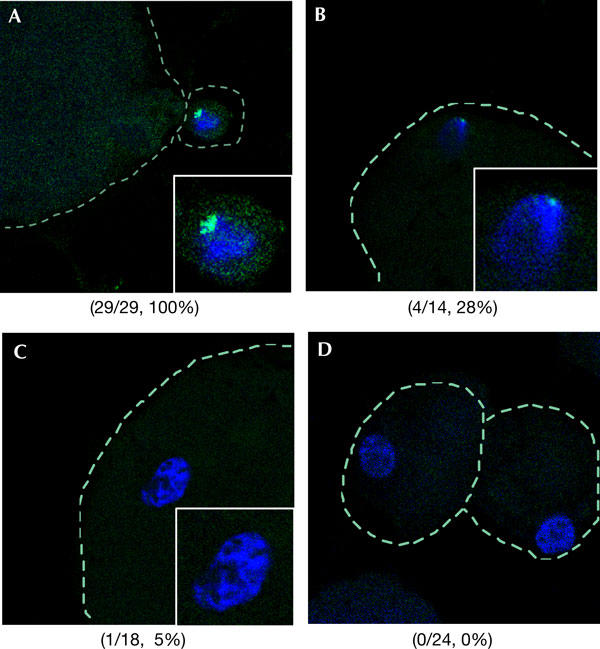
Progressive loss of Xist RNA coating from somatic nuclei in SCNT embryos. Shown are RNA fluorescence in situ hybridization for Xist RNA coating (green) and DNA staining with TOTO3 (blue). (A) An Xist RNA domain was seen in somatic donor nucleus before fusion with enucleated oocyte (Xist RNA domain/total, 29/29). (B) At 30 min after SCNT, the Xist domain disappeared from a significant number of cells (Xist RNA domain/total, 4/14). (C) After 2 h, there was a further reduction in the number of cells with Xist RNA domains in SCNT embryos (Xist RNA domain/total, 1/18). (D) The coating of Xist RNA was completely lost at the two-cell stage (Xist RNA domain/total, 0/24).
At the two-cell stage—which is when in normal female embryos (control embryos), the paternally inherited Xist allele on Xp usually begins its expression—no Xist RNA was detectable in SCNT embryos (Fig 1D). However, there was a striking and precocious appearance of an H3-3meK27 domain that occurred within 8 h of SCNT, which was detected as a single nuclear domain on one X chromosome (Fig 2; supplementary Table S1 online). This early detection of H3-3meK27 was not associated with the Eed protein, a component of the Polycomb PRC2 complex (Fig 2A–C), as far as could be detected by IF. This is surprising, as the PRC2 complex is responsible for laying down the H3-3meK27 mark in normal embryos (Silva et al, 2003), although at a slightly later stage of normal development. The appearance of Eed at the H3-3meK27 domain was first detected at the two-cell stage in SCNT embryos (Fig 2D) and persisted thereafter (supplementary Fig S3A,B online; supplementary Table S2 online), unlike the situation in normal female fertilized embryos in which Eed first accumulates on the paternal X chromosome at the morula stage (Fig 2E; supplementary Fig S3C,D online). Therefore, we reasoned that the early appearance of the H3-3meK27 mark in SCNT embryos may represent an enduring mark on the original Xi in donor somatic nuclei, which we detected at least in a proportion of the donor cells before transfer to the oocyte. Its precocious appearance may be the result of an ‘unmasking' or exposure of the epitope, resulting from the marked changes that affect the chromatin of the somatic-cell nucleus in the oocyte cytoplasm after activation. Consistent with this, we observed a marked overall loss in the somatic-cell nucleus of histone H3 methylation at the amino-acid residue immediately adjacent to the H3 Lys-27, namely H3-R26, within 30 min of SCNT (supplementary Fig S2 online; supplementary Table S1 online). These dynamic changes in histone modifications are likely to be symptomatic of significant reprogramming of the somatic nucleus.
Figure 2.
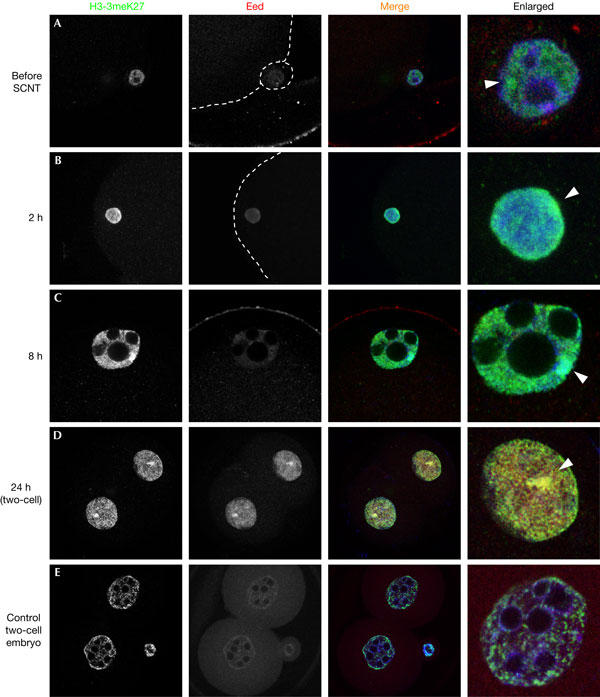
Detection of H3-3meK27 and Eed in one- to two-cell SCNT embryos. Embryos were immunostained at 0, 2, 8 and 24 h after SCNT with H3-3meK27 (green) and Eed (red) antibodies. The white arrowheads indicate a domain of H3-3meK27. (A–C) A weak signal for H3-3meK27 was detected without Eed in somatic nucleus before transfer to oocytes, and also at 8 h after nuclear transfer. (D) At 24 h after SCNT (two-cell stage), both H3-3meK27 and Eed accumulated to a single domain in each blastomere. (E) There was no similar detectable signal or colocalization of H3-3meK27 and Eed in the two-cell stage control embryo.
An additional unexpected observation in SCNT embryos was the appearance of a single domain of dimethylation of histone H3 at lysine 9 (H3-2meK9) after 2 h, which colocalized with histone methyltransferase, G9a, as well as with H3-3meK27 (supplementary Figs S4,S5 online; supplementary Table S1 online). Both H3-2meK9 and G9a were not initially detectable in donor somatic nuclei (supplementary Fig S4A online). However, the accumulation of both H3-2meK9 and G9a rapidly disappeared at the two-cell stage (supplementary Fig S4D online; supplementary Table S1 online), which occurred coincidentally with the accumulation of Eed on the X chromosome (Fig 2D). Similar sequences of early epigenetic modifications are not observed in control embryos (not shown), although H3-2meK9 modification on the X chromosome is observed at the late morula stage (Heard et al, 2001; Rougeulle et al, 2004). The G9a enzyme has been reported to have a predominantly H3-K9 methylation activity although biochemical analyses show a weak H3-K27 methylation activity (Peters et al, 2003). The biological significance of this observation is unknown at present, but it serves to illustrate the epigenetic perturbations that occur during the early stages of development of SCNT embryos.
Xist RNA expression and histone modifications of the X chromosome were also investigated at subsequent stages of development. Here, we made another unexpected finding, as two domains of Xist RNA could be found in most of the cells from the eight-cell stage onwards in SCNT embryos (Fig 3B,C; supplementary Table S2 online). This suggests that both the Xi that originally expressed Xist and the Xa on which Xist gene was silent are capable of activating Xist expression at this time. This type of Xist expression pattern is never observed in control female embryos generated by in vitro fertilization of oocytes (Fig 3D–F), where a single Xist domain is seen, in pre-implantation embryos, to be associated with Xp (see below).
Figure 3.
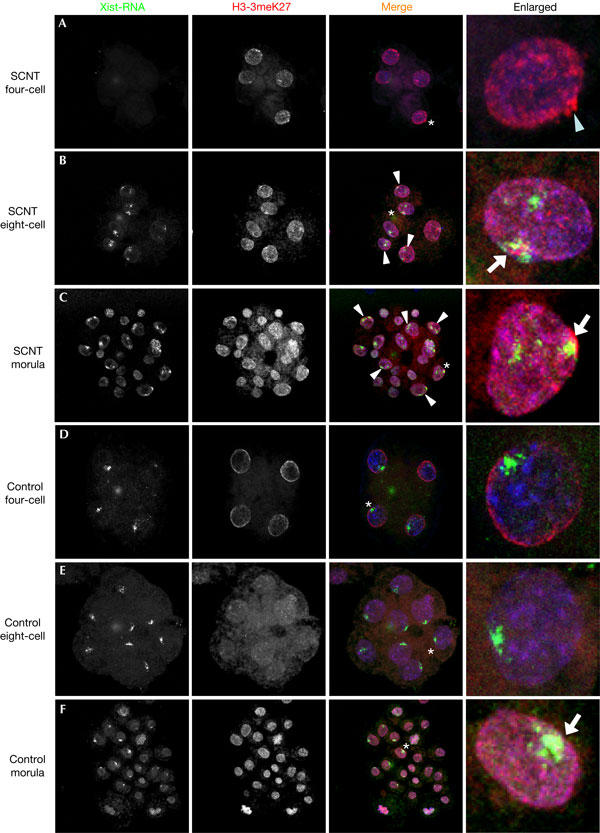
Xist RNA coating in relation to H3-3meK27 from four-cell stages to morula stages. (A–C) SCNT embryos and (D–F) control embryos were immunostained with H3-3meK27 (red) combined with Xist RNA fluorescence in situ hybridization (green). DNA was stained with TOTO3 (shown in blue). White arrowheads indicate the blastomeres that showed two domains of Xist RNA coating. The magnified field is indicated in the figure with asterisks and white arrows indicate colocalization of Xist RNA and H3-3meK27. (A) Xist RNA was not detectable in most of the SCNT embryos at the four-cell stage. The light-blue arrowhead indicates a domain of H3-3meK27 on the X chromosome. (B) Two domains of Xist RNA coating were observed from six- to eight-cell embryos, but only one of the two domains colocalized with H3-3meK27 in more than half the embryos examined. (C) The two domains of Xist RNA coating continued in morula, and in early blastocyst (not shown). (D) Control in vitro-fertilized embryos show a single domain of Xist RNA coating at the four-cell stages, which continued to (E) eight-cell stages and (F) morula stages, which do not colocalize with H3-3meK27 except in some blastomeres of morula (white arrow).
To confirm biallelic Xist expression in SCNT embryos, we exploited the presence of polymorphisms between the two Xist alleles in the donor nucleus from an interspecific cross between C57BL/6 and Mus spretus mice (Kay et al, 1993). In the resulting SCNT embryos, allelespecific reverse transcription–PCR (RT—PCR)/restriction fragment length polymorphism (RFLP) was performed at different embryonic stages. Biallelic Xist expression was detectable in most of the SCNT embryos at the eight-cell stage, which confirms the appearance of two Xist RNA domains that were detected by IF (Fig 4). In control in vitro-fertilized (IVF) embryos, there was exclusive monoallelic expression of the paternally derived Xist locus, consistent with the preferential inactivation of the paternal X chromosome in pre-implantation embryos. Given that the SCNT embryos are derived from a single somatic cell nucleus in which either one or the other Xist allele was expressed, this result shows that both the previously active and inactive Xist alleles are expressed at this stage.
Figure 4.
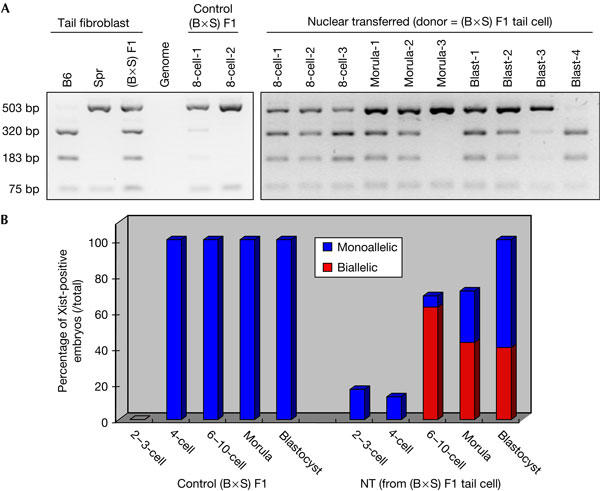
Verification of allelic expression of Xist transcript in SCNT embryos. (A) Complementary DNA from individual SCNT embryo was PCR amplified using Xist transcriptspecific primer sets, and the PCR products were digested with HindIII. Tail fibroblast cDNAs from C57BL/6 (B6), Mus spretus (Spr) and (B6 × Spr) F1 and cDNA from (B6 × Spr) F1 in vitro-fertilized (IVF) embryos were used as positive controls. (B) Summary of allelic expression of Xist transcript. In the control IVF embryos ((B6 × Spr) F1), monoallelic Xist expression (blue bar) was detected from the four-cell stage onwards. In contrast, Xist expression in the SCNT embryo was delayed and mainly detected from the eight-cell stage onwards (68% of total analysed embryos), and the proportion of cells with Xist increased progressively at the morula (71%) and the blastocyst stage (100%). Among the cells showing Xist RNA domains, 91% at the eight-cell stage, 60% at the morula stage and 40% at the blastocyst stage showed biallelic Xist RNA domains in SCNT embryos (denoted by red bars).
Although biallelic Xist expression was observed from the eight-cell stage, H3-3meK27 remained restricted to only one X chromosome (Fig 3B,C; supplementary Table S2 online). This is presumably the previously inactive X chromosome, as the histone modification mark is present before and after SCNT of female donor cell nuclei. Thus, H3-3meK27 does not seem to be solely dependent on Xist expression, as there are two Xist RNA domains in the cell, but only one domain with accumulated H3-3meK27. More importantly, the H3-3meK27 mark could not be detected when either a male somatic donor nucleus or a donor nucleus from a pluripotent female embryonic stem cell was used (supplementary Fig S6 online). Donor nuclei from both these sources do not possess an Xi and showed no H3-3meK27 accumulation at the two-cell stage of SCNT. By contrast, at this time, we detected the striking accumulation of both H3-3meK27 and Eed in donor nuclei from adult females in SCNT embryos.
With further development to the morula and blastocyst stages however, both RNA FISH analysis and allele-specific RT–PCR showed a shift back to monoallelic Xist RNA expression patterns (Fig 4B; supplementary Table S2 online). The fact that the Xist RNA coating of one of the two chromosomes eventually declines in some blastomeres suggests that even in SCNT embryos some form of counting or recognition of the number of X chromosomes must occur to ensure proper X-linked gene dosage for survival. A similar phenomenon has been described in both XpO embryos (Papaioannou & West, 1981) and in XpXp androgenotes (Okamoto et al, 2000). In the latter, both Xp chromosomes are initially coated in Xist RNA but one of these subsequently reverses at later stages. However, this loss of Xist RNA is not complete, as in some cells, biallelic Xist expression persists (Fig 4) to the blastocyst stage (data not shown). Presumably, such cells will die through nullisomy for the X chromosome if X inactivation is triggered.
Our study shows that reprogramming events after SCNT of donor nuclei have different effects on the active and inactive X chromosomes (Fig 5). The silent Xist locus on the Xa in somatic nuclei can be clearly activated after SCNT, which suggests that its mechanism of repression must differ from that of the Xist locus on the maternal Xm*, because the latter is not activated during pre-implantation development. A previous study suggests that a maternal imprint on Xm* is established during oocyte growth and serves to render the Xm* resistant to inactivation in pre-implantation embryos (Tada et al, 2000). The precise nature of the imprint on the maternal X that maintains repression of the Xist locus on Xm* is unknown (Takagi, 2003). A comparative analysis of Xa and Xm* may provide further insight into the mechanisms that regulate repression of the Xist locus.
Figure 5.
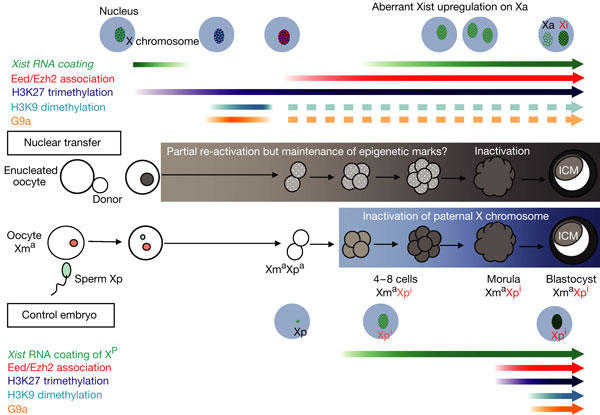
Comparative kinetics of epigenetic modifications of X chromosome in SCNT (top) and control embryos (bottom). There is rapid disappearance of Xist RNA coating from Xi in SCNT embryos commencing within 30 min after activation of the reconstructed embryo. Xist RNA re-appeared in these embryos from the four- to eight-cell stages, and colocalized with the H3-3meK27 and Eed/Ezh2 protein complex. Colocalization of G9a histone methyltransferase with H3-3meK27 and H3-2meK9 was also observed 30 min after activation, but disappeared at the two-cell stage. H3-3meK27 appeared precociously and initially in the absence of the Eed/Ezh2 complex at 8 h in SCNT embryos. Biallelic expression of Xist RNA was observed from the four- to eight-cell stages onwards in SCNT embryos, but eventually declined in blastocysts. The H3-3meK27 was associated with and persisted on only one of the two Xist domains associated with the original Xi. Compare the kinetics of the epigenetic events in SCNT with control embryos, which differ markedly. See text for detailed explanations.
For the Xi, after a rapid loss of Xist RNA coating, there is precocious appearance of the H3-3meK27 mark without association with the Polycomb Eed/Ezh2 complex, which may indicate that some epigenetic marks on Xi are not erased in the oocyte. Xist RNA on the Xi is, however, detected subsequently in SCNT embryos, but later than at the two-cell stage, which is the case on Xp in control embryos. This study suggests a possible mechanism for previous observations on preferential inactivation of the Xi in the extraembryonic tissues in cloned embryos (Eggan et al, 2000; Nolen et al, 2005), which could be due to the persistence of chromatin marks such as H3-3meK27 on the Xi, presumably because they are not erased by the oocyte. In control embryos, the epigenetic mark on Xp is likely to be at the level of the Xist gene itself, as we could not detect H3-3meK27 methylation and other chromatin marks before the 16-cell stages (Okamoto et al, 2004). The epigenetic marks present on the Xi and Xa in the somatic nucleus are thus different from those on Xp and Xm, although both Xi and Xp undergo preferential inactivation in pre-implantation embryos but involve a different sequence of epigenetic events.
We propose that some of the epigenetic marks on the Xi endure through the initial stages of genomic reprogramming, and result in an unusual series of epigenetic changes in SCNT embryos. It is probable that the persistent epigenetic marks on the Xi and Xa are subsequently fully erased in the epiblast of the blastocysts, judging from recent evidence showing re-activation of Xp in the epiblast (Mak et al, 2004; Okamoto et al, 2004). This is consistent with random X inactivation in the fetuses of both normal and cloned embryos. Epigenetic reprogramming during SCNT is thus a stepwise process that is initiated in the oocyte but continues in the epiblast, and may involve diverse mechanisms.
Methods
See the supplementary information online for details.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Figures
Supplementary Tables
Acknowledgments
We thank A. Otte for the generous gift of Eed antibody. This work was supported by a grant from the Wellcome Trust to M.A.S. Work in the laboratory of E.H. is funded by the Centre National de la Recherche Scientifique (ATIPE), the French Ministry for Research (ACI programme) and the Curie Institute (PIC programme). I.O. is supported by the CNRS. M.A.S., T.J. and E.H. are also supported under the European network of excellence ‘Epigenome' programme.
References
- Brockdorff N, Ashworth A, Kay GF, Cooper P, Smith S, McCabe VM, Norris DP, Penny GD, Patel D, Rastan S (1991) Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nature 351: 329–331 [DOI] [PubMed] [Google Scholar]
- Brown CJ, Ballabio A, Rupert JL, Lafreniere RG, Grompe M, Tonlorenzi R, Willard HF (1991) A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 349: 38–44 [DOI] [PubMed] [Google Scholar]
- de Napoles M et al. (2004) Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev Cell 7: 663–676 [DOI] [PubMed] [Google Scholar]
- Eggan K, Akutsu H, Hochedlinger K, Rideout W III, Yanagimachi R, Jaenisch R (2000) X-chromosome inactivation in cloned mouse embryos. Science 290: 1578–1581 [DOI] [PubMed] [Google Scholar]
- Erhardt S, Su IH, Schneider R, Barton S, Bannister AJ, Perez-Burgos L, Jenuwein T, Kouzarides T, Tarakhovsky A, Surani MA (2003) Consequences of the depletion of zygotic and embryonic enhancer of zeste 2 during preimplantation mouse development. Development 130: 4235–4248 [DOI] [PubMed] [Google Scholar]
- Heard E (2004) Recent advances in X-chromosome inactivation. Curr Opin Cell Biol 16: 247–255 [DOI] [PubMed] [Google Scholar]
- Heard E, Rougeulle C, Arnaud D, Avner P, Allis CD, Spector DL (2001) Methylation of histone H3 at Lys-9 is an early mark on the X chromosome during X inactivation. Cell 107: 727–738 [DOI] [PubMed] [Google Scholar]
- Huynh KD, Lee JT (2003) Inheritance of a pre-inactivated paternal X chromosome in early mouse embryos. Nature 426: 857–862 [DOI] [PubMed] [Google Scholar]
- Kay GF, Penny GD, Patel D, Ashworth A, Brockdorff N, Rastan S (1993) Expression of Xist during mouse development suggests a role in the initiation of X chromosome inactivation. Cell 72: 171–182 [DOI] [PubMed] [Google Scholar]
- Kohlmaier A, Savarese F, Lachner M, Martens J, Jenuwein T, Wutz A (2004) A chromosomal memory triggered by Xist regulates histone methylation in X inactivation. PLoS Biol 2: E171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon MF (1961) Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature 190: 372–373 [DOI] [PubMed] [Google Scholar]
- Mak W, Nesterova TB, de Napoles M, Appanah R, Yamanaka S, Otte AP, Brockdorff N (2004) Reactivation of the paternal X chromosome in early mouse embryos. Science 303: 666–669 [DOI] [PubMed] [Google Scholar]
- Nolen LD, Gao S, Han Z, Mann MR, Gie Chung Y, Otte AP, Bartolomei MS, Latham KE (2005) X chromosome reactivation and regulation in cloned embryos. Dev Biol 279: 525–540 [DOI] [PubMed] [Google Scholar]
- Ogura A, Inoue K, Takano K, Wakayama T, Yanagimachi R (2000) Birth of mice after nuclear transfer by electrofusion using tail tip cells. Mol Reprod Dev 57: 55–59 [DOI] [PubMed] [Google Scholar]
- Okamoto I, Tan S, Takagi N (2000) X-chromosome inactivation in XX androgenetic mouse embryos surviving implantation. Development 127: 4137–4145 [DOI] [PubMed] [Google Scholar]
- Okamoto I, Otte AP, Allis CD, Reinberg D, Heard E (2004) Epigenetic dynamics of imprinted X inactivation during early mouse development. Science 303: 644–649 [DOI] [PubMed] [Google Scholar]
- Panning B, Dausman J, Jaenisch R (1997) X chromosome inactivation is mediated by Xist RNA stabilization. Cell 90: 907–916 [DOI] [PubMed] [Google Scholar]
- Papaioannou VE, West JD (1981) Relationship between the parental origin of the X chromosomes, embryonic cell lineage and X chromosome expression in mice. Genet Res 37: 183–197 [DOI] [PubMed] [Google Scholar]
- Peters AH et al. (2003) Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol Cell 12: 1577–1589 [DOI] [PubMed] [Google Scholar]
- Plath K et al. (2003) Role of histone H3 lysine 27 methylation in X inactivation. Science 300: 131–135 [DOI] [PubMed] [Google Scholar]
- Rougeulle C, Chaumeil J, Sarma K, Allis CD, Reinberg D, Avner P, Heard E (2004) Differential histone H3 Lys-9 and Lys-27 methylation profiles on the X chromosome. Mol Cell Biol 24: 5475–5484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheardown SA et al. (1997) Stabilization of Xist RNA mediates initiation of X chromosome inactivation. Cell 91: 99–107 [DOI] [PubMed] [Google Scholar]
- Silva J, Mak W, Zvetkova I, Appanah R, Nesterova TB, Webster Z, Peters AH, Jenuwein T, Otte AP, Brockdorff N (2003) Establishment of histone h3 methylation on the inactive X chromosome requires transient recruitment of Eed–Enx1 polycomb group complexes. Dev Cell 4: 481–495 [DOI] [PubMed] [Google Scholar]
- Tada T, Obata Y, Tada M, Goto Y, Nakatsuji N, Tan S, Kono T, Takagi N (2000) Imprint switching for non-random X-chromosome inactivation during mouse oocyte growth. Development 127: 3101–3105 [DOI] [PubMed] [Google Scholar]
- Takagi N (2003) Imprinted X-chromosome inactivation: enlightenment from embryos in vivo. Semin Cell Dev Biol 14: 319–329 [DOI] [PubMed] [Google Scholar]
- Takagi N, Sasaki M (1975) Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature 256: 640–642 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures
Supplementary Tables


