Abstract
Apoptosis is implicated in the life cycle of the malaria parasite in mosquitoes. The genome project for the primary malaria vector Anopheles gambiae showed a significant expansion of the inhibitor of apoptosis protein (IAP) and caspase gene families in comparison with Drosophila. However, because of extensive sequence divergence, no orthologue was identified for the reaper/grim-like IAP antagonist genes that have a pivotal role in cell death regulation in Drosophila. Using a customized searching strategy, we identified michelob_x(mx), a gene not predicted by the genome project, as the missing IAP antagonist in the An. gambiae and other mosquito genomes. Mx has a highly conserved amino-terminal IAP-binding motif. Expression of Mx induces rapid cell death in insect cell lines and is a potent tissue ablator in vivo. Its proapoptotic activity is totally dependent on the IAP-binding motif. Like reaper in Drosophila, mx is transcriptionally induced by ultraviolet irradiation to mediate cell death.
Keywords: reaper, grim, apoptosis, Anopheles gambiae, Aedes aegypti
Introduction
Controlled cell death by apoptosis has an important role in insect host defence against parasites and other pathogens. In mosquitoes, apoptosis of midgut epithelial cells is observed after cell invasion by the Plasmodium ookinete, an essential step in the transmission life cycle of the malaria parasite (Abraham & Jacobs-Lorena, 2004). The destruction of the cell during apoptosis is initiated by the activation of caspases, which is controlled by several inter-related pathways (Danial & Korsmeyer, 2004). Mainly through studies on Drosophila melanogaster, we know that inhibitor of apoptosis proteins (IAPs) and IAP antagonists have a principal role in regulating cell death in insects. IAPs inhibit the activity of caspase. This inhibition is removed by the IAP antagonist after its binding to IAP (Palaga & Osborne, 2002).
In D. melanogaster, deletion mutants that involve the three IAP antagonists reaper, hid and grim, essentially all programmed cell death is blocked and the cell death response to cytotoxic stimuli is strongly impeded (White et al, 1994). Reaper, Hid, Grim (RHG) proteins share little overall sequence similarity except for a short, 7-amino-acid IAP-binding motif (IBM) at their amino-terminus. IBM binds to the surface groove formed in the baculoviral IAP repeats (BIR) domains of IAP, and thus releases caspases from inhibition by IAP (Wu et al, 2001). The functional mechanism of IBM is highly conserved. The binding of the IBM from human IAP antagonist Smac to Xiap is structurally similar, if not identical, to the binding of insect IBM to Diap1 (Wu et al, 2001).
Although most IAPs and caspases are ubiquitously expressed, Reaper/Grim-like IAP antagonists have restricted expression. During normal development, they are expressed in cells destined to die. These proapoptotic genes are also transcriptionally activated to mediate cell death in response to cytotoxic stimuli such as ionizing irradiation. As the IBM is at their extreme N terminus, the nascent proteins have their IBM exposed because of the removal of methionine in eukaryotic cells. This is in contrast with the other class of IAP antagonists, such as Smac and caspase 9, that require post-translational cleavage to expose their IBM. Although both classes of IAP antagonists exist in Drosophila, the Reaper/Grim-like IAP antagonists have not been identified outside the Drosophila genus, with the exception of a Reaper orthologue in the blowfly (Chen et al, 2004). This is apparently because of the rapid divergence of these proteins, as well as the small size of the signature IBM. The absence of reaper/grim-like genes in mosquitoes left a significant lacuna in our comprehension of cell death regulation in these disease-transmitting insects. Using a customized search strategy, we identified potential reaper/grim-like genes in mosquito genomes. Structural and functional comparison of Mx in mosquitoes versus Reaper/Grim in fruitflies showed interesting insights into the function and evolution of this family of proteins.
Results And Discussion
The Anopheles gambiae genome project identified 12 caspases and 7 IAPs, representing a significant increase compared with Drosophila, which has 7 caspases and 4 IAPs (Christophides et al, 2002). Four of the mosquito IAPs seem to be orthologues of Diap1, the Drosophila IAP that binds to RHG proteins and has a central role in regulating caspase activation and cell death (Palaga & Osborne, 2002). The significant increase of caspases and death-regulating IAP genes may reflect the functional requirement of fine-tuning cell death in response to parasites and viruses commonly encountered as a consequence of blood feeding on infected vertebrates. However, the key regulators of this pathway, the IAP antagonists such as Reaper and Grim in Drosophila, were not identified because of ‘rapid sequence diversification' (Christophides et al, 2002). To circumvent this problem, we first identified orthologues of RHG proteins in distantly related Drosophila species such as Drosophila virilis and Drosophila mojavensis. Using the orthologue sequences, we were able to build a hidden Markov model (HMM)-based profile for the IBM motif (Bailey & Elkan, 1994). A motif search program (Zhou et al, 1999) was then customized to search for potential open reading frames (ORFs) in the mosquito genome that have an IBM, immediately following the methionine. Several putative matches were identified among genomic or expressed sequence tags (ESTs) of An. gambiae and were found to be conserved in Aedes mosquitoes as well. One of them, michelob_x(mx), was chosen for functional characterization because of the presence of ESTs in Aedes aegypti complementary DNA libraries made from animals fed with virus-contaminated blood.
Using the sequence information for primer design, genomic fragments of around 900 base pairs that contained mx were amplified from the genomic DNA of Aedes albopictus and An. gambiae. Although both the amplified fragments contained an intron, transfecting either fragment into Drosophila S2 cells was sufficient to induce rapid cell death, which could be blocked by diap1. The intron-less Ae. albopictus mx cDNA was then obtained through reverse transcription–PCR (RT–PCR), using RNA extracted from C6/36 cells treated with 254 nm ultraviolet (UV) irradiation, and used for functional characterization of Mx. In addition, mx cDNA clones for Ae. aegypti were identified by searching The Institute for Genomic Research (TIGR) Ae. aegypti Gene Index (http://www.tigr.org). The expression of mx in An. gambiae was also verified by identifying a corresponding EST (BM653729) from the A.Gam.ad.cDNA1 library as well as RT–PCR testing of RNA samples extracted from adult animals.
Examination of the sequences indicated that Mx proteins from the three mosquito genomes have an invariable N-terminal IBM that is almost identical to that of the Drosophila Grim protein (Fig 1). As expected, sequence alignment outside the IBM region showed little overall similarity. However, two structural features beyond local amino-acid identity are worth mentioning. First, there is a polyglutamine stretch of 10–20 residues immediately following the IBM (Fig 1). This feature was also observed for Grim, and is conserved among Mx proteins from different mosquito species. The length of this polyglutamine region varies in different species and its function remains unclear. Second, the carboxy-terminal region of Mx is rich in arginines and lysines, a feature that is shared by Reaper but not Grim.
Figure 1.
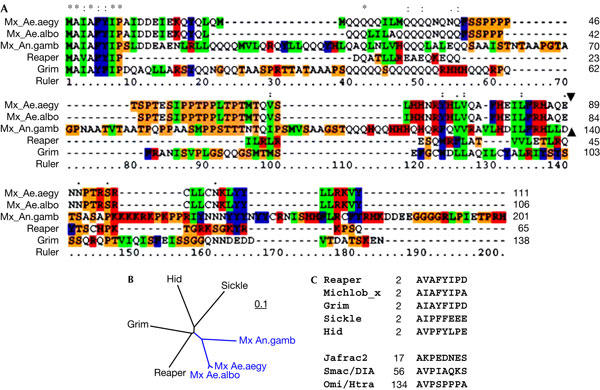
Comparison of Mx sequences with Reaper and Grim. (A) Alignment of Mx sequences from Anopheles gambiae (An.gamb), Aedes albopictus (Ae.albo) and Aedes aegypti (Ae.aegy), and Grim (NP_524137) and Reaper (NP_524138) sequences from the fruitfly. The relative position of the single intron (indicated with solid triangle) in mx genes is conserved between Anopheles and Aedes. There are no introns in grim or reaper. The colour scheme reflects percentage identity among the five aligned sequences (red, 100%; blue, >75%; and green, >50%). (B) Protein distance tree showing that Mx is slightly closer to Reaper and Grim than to Hid and Sickle. The scale bar reflects the rate of nucleotide change per position. (C) Comparison of the inhibitor of apoptosis protein (IAP)-binding motifs in fly (Reaper, Grim, Hid (NP_524136), Sickle (NP_524139) and Jafrac2 (Q9V3Q4)), mosquito (Michlob_x) and human (Smac/IDIA (NP_063940), OmiI/HtrA (O43464)) IAP antagonists. Numbers indicate the position of the first Ala in the host proteins.
Our results suggest that it is inappropriate to designate Mx as the orthologue of Reaper or Grim because it lacks a Grim helix 3 (GH3) domain. That is, sequence alignment as well as motif analysis using MEME (Multiple EM for Motif Elicitation) failed to identify a discernible GH3 motif in the mx sequence. The GH3 domain, shared by Drosophila IAP antagonists Grim, Reaper and Sickle, can induce cell death in the absence of the IBM motif. The proapoptotic activity of GH3 is mediated either through induction of IAP degradation (Olson et al, 2003) or by the release of cytochrome c from mitochondria (Claveria et al, 2002). It has been found that the region connecting IBM and GH3 in Reaper has similarity with a family of nonstructural viral proteins and can inhibit cellular protein translation (Colon-Ramos et al, 2003). This region does not seem to be present in Mx.
When mx was expressed in Drosophila S2 cells or in the mosquito C6/36 cells (Ae. albopictus), it induced cell death within 20 h of transfection, similar to what has been observed for Drosophila IAP antagonists. However, unlike Reaper and Grim, which retain proapoptotic ability even when their N-terminal IBM is removed, the proapoptotic activity of Mx is totally dependent on its N-terminal IAP-binding motif (Fig 2A). When this motif, composed of amino acids 2–8, was removed, the killing ability of Mx was abolished (Fig 2A,B). This is not due to protein stability, as there were much higher levels of mutant Mx(−IBM) protein than wild-type Mx (Fig 2B). As the remaining proapoptotic activity of Reaper and Grim, both without IBM, is mainly mediated by their GH3 domains, the fact that Mx minus IBM has no proapoptotic activity verifies the functional absence of a GH3 motif.
Figure 2.
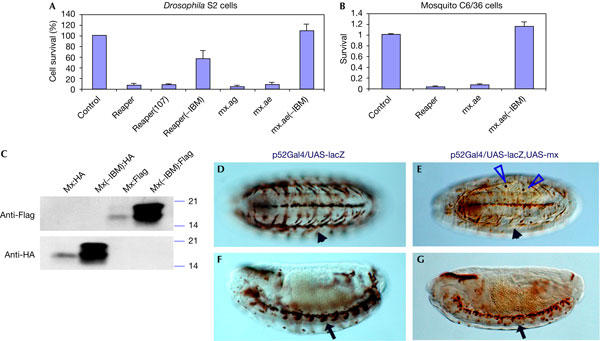
The IAP (inhibitor of apoptosis protein)-binding motif (IBM) is required for the proapoptotic activity of Mx. (A) Expression of Mx in S2 cells induces cell apoptosis similar to Reaper, or Reaper(107), which has two helix-disturbing substitutions in its GH3 (Grim helix 3) domain (Leu35Pro and Ala36Pro). Although Reaper (−IBM) retained partial proapoptotic activity, removal of the IBM (amino acids 2–8) from Mx totally abolished its proapoptotic activity. Error bars represent the standard error of 4–5 measurements. mx.ag, mx cloned from Anopheles gambiae; mx.ae, mx cloned from Aedes albopictus; mx from Aedes aegypti behaved the same as the two presented here (data not shown). (B) Expression of mx induced cell death in mosquito C6/36 cells, which is dependent on the IBM domain. Mx from Ae. albopictus was used. (C) Western blot analysis of cell lysates from transfected H1299 cells. At 48 h after transfection, there is significant accumulation of mutant protein Mx(−IBM) in cells transfected with pRK5-Mx(−IBM):HA (haemagglutinin) or pRK5-Mx(−IBM):Flag. In contrast, there is little accumulation of the wild-type protein in cells transfected with pRK5-Mx:HA or pRK5-Mx:Flag. Equal amounts of whole-cell lysates were loaded on all lanes. (D–G) Expression of mx in transgenic fly strain induces cell/tissue ablation. P52Gal4 is an enhancer trap insertion in the GP150 gene (Melnattur et al, 2002). It guides the expression of UAS-lacZ in ventral musculature (arrowhead in (D), ventral view, stage 15) as well as in the central nervous system midline glia and neurons (arrow in (F), sagittal view, stage 15). Expression of mx (E,G) caused the elimination of muscle cells; the arrowhead in (E) (ventral view, stage 15) points to the empty space that should be occupied by the β-galactosidase (β-Gal)-expressing muscle cells (compare with D). Corresponding with the disappearance of β-Gal-positive muscle cells is the emergence of round β-Gal-positive apoptotic inclusion bodies (blue triangles in E) inside migrating microphages. Similarly, expression of mx also eliminated midline neurons (arrow in G, compare with F).
The only Drosophila IAP antagonist that does not have a functional GH3 motif is head involution defective (Hid). Like Mx, Hid minus IBM has no cell-killing function (Vucic et al, 1998). However, we do not believe that Mx is the orthologue of Hid, for two reasons. First, the IBM of Mx (AIAF) is the signature for Reaper/Grim-like IAP antagonists (Zhou, 2005). Second, our search identified another potential IAP antagonist (Michelob_y) in the mosquito genomes (L.Z., unpublished data) that has sequence features more reminiscent of Hid and Sickle, although functional analyses have yet to be performed.
To test the proapoptotic activity of Mx in vivo, we generated a transgenic fly strain carrying P{UAS-mx}. The P52Gal4 insertion directs Gal4 expression in a set of discrete cells, including neurons and glias in the central nervous system midline as well as some ventral epidermal cells (Melnattur et al, 2002). This Gal4 strain has been used successfully in characterizing functional interaction as well as the functional difference of various Drosophila IAP antagonists (Zhou et al, 1997; Wing et al, 1998). When the P{UAS-mx} strain was crossed with the P52Gal4:UAS-lacZ strain, the progenies carrying both p{UAS-mx} and P52Gal4:UAS-lacZ were embryonic lethal. Anti-β-galactosidase (β-Gal) staining showed that many epithelial cells, as well as the ventral unpaired median neurons expressing P52Gal4, were eliminated by mx (Fig 2D–G). Expression of reaper or hid alone in this context induces little cell death, and only when both are expressed together can significant tissue ablation be achieved (Zhou et al, 1997). In this regard, mx is similar to grim, in that expression by itself is able to cause tissue ablation in vivo (Wing et al, 1998). It should be noted that the ability of individual IAP antagonists to induce cell death differs markedly, depending on tissue types and developmental stages. For instance, expression of hid alone in the eye disc during the third-instar larval stage was able to induce significant cell death (Grether et al, 1995).
As in Drosophila IAP antagonists, proapoptotic activity of Mx is dependent on the cellular level of IAPs (Fig 3A). Cell death induced by Mx is blocked when the DNA ratio for mx and diap1 is 1:2. By contrast, a ratio of 1:5 failed to block completely the induction of cell death by Grim or Reaper, presumably owing to the fact that GH3 can induce cell death through IAP degradation (Colon-Ramos et al, 2003) or through IAP-independent mechanisms (Holley et al, 2002; Yoo et al, 2002). In this sense, Mx functions similar to the GH3-less Reaper(107), which has two helix-disturbing substitutions in the GH3 domain. Cell death induced by Mx is also efficiently blocked by expressing p35, a caspase inhibitor from a baculovirus.
Figure 3.

Mx-induced cell death is blocked by Diap1 and P35. (A) Unlike Reaper- or Grim-induced cell death, which cannot be totally blocked by Diap1, cell death induced by Mx is readily blocked by Diap1, the principal death-inhibiting IAP (inhibitor of apoptosis protein) from the fruitfly, and by p35, a viral caspase inhibitor. In this regard, it is similar to Reaper(107), which has two helix-disrupting substitutions in the GH3 domain. The colour of the bars reflects the ratio of the two testing constructs in a total of 0.9 μg per sample. Error bars represent the standard error of repeated measurements (n=3). (B) Direct interaction between Diap1 and Mx is detected by co-immunoprecipitation (IP). This interaction is abolished when the IBM (amino acids 2–8) is removed from Mx. NCI-H1299 cells were transfected with 1 μg of pRK5-HA (haemagglutinin):Diap along with 3 μg of pRK5-Mx:Flag, or pRK5-Mx(IBM):Flag, or empty vector. At 48 h after transfection, cells were lysed. Equal amounts of whole-cell lysates were immunoprecipitated with an anti-HA antibody (12CA5). The immunoprecipitate was resolved by SDS–polyacrylamide gel electrophoresis and transferred to the membrane, followed by immunoblotting (IB) with anti-Flag.
A direct interaction between Mx and IAP is verified by co-immunoprecipitation assay. Although Mx interacts directly with Diap1 when both are expressed in mammalian cells, there is no interaction when the IBM is removed (Fig 3B), indicating that the interaction between Mx and Diap1 is mediated through the IBM. Expression of Mx in mammalian cells (H1299 and MCF7) induces cell death within 24 h of transfection. Collectively, this evidence indicates that Mx induces cell death by removing the inhibitory effect of IAP on caspase activation, and probably acts solely through this mechanism. The absence of a GH3 domain in Mx may indicate that the joining of both GH3 and IBM in Drosophila IAP antagonists occurred after the separation of Drosophila and mosquito lineages about 250 million years ago (Rohdendorf, 1974). This is a likely scenario given that a protein that has only a GH3 domain but does not have an IBM was recently identified in Drosophila, which has functional interaction with the GH3-less IAP antagonist Hid (L. Zhou, in preparation). Identifying orthologues of Reaper/Mx from other insects or vertebrates should provide further insight into this hypothesis. Alternatively, it is possible that GH3 was lost during the evolution of Mx in mosquitoes, after mosquito/Drosophila divergence.
An important characteristic of reaper-like IAP antagonist genes is that they are regulated at the transcriptional level. During development, reaper, grim and sickle are expressed specifically in cells just before their genetically programmed death. Expression of reaper, sickle and hid is also induced after cytotoxic stimuli such as X-ray or UV treatment. To test whether mx is also transcriptionally regulated, we treated the mosquito C6/36 cell line with 10 mJ/cm2 UV and monitored the expression of mx by quantitative PCR. A significant increase in mx was observed 30 min after the treatment, and after 1 h the level of mx in irradiated cells was more than fourfold that in control cells (Fig 4). This induction of mx by UV was followed by massive apoptosis that initiated about 2 h after treatment. Introducing doublestranded RNA (dsRNA) for mx through transient transfection before UV irradiation significantly suppressed UV-induced cell death. This suppression of cell death is significant at a low dosage (1 mJ/cm2 UV). This is not surprising, as studies in Drosophila have shown that several cell-death regulatory genes are induced by irradiation to mediate cell killing. The relatively low transfection efficiency (5–10%) prevented us from measuring the extent to which mx expression is suppressed by RNA interference. However, even if dsRNA caused a complete block of mx expression, cell death can still proceed if the level of other proapoptotic genes is high enough. This may explain why, at higher dosages (>5 mJ/cm2 UV), dsRNA for mx is not able to rescue the cells (Fig 4B). This evidence suggests that, similar to what we know about cell-death regulation in Drosophila, cell death in mosquitoes is also controlled by several regulatory genes. Identifying the other components of the cell-death regulatory network will be necessary for a comprehensive understanding of cell-death regulation in mosquitoes.
Figure 4.
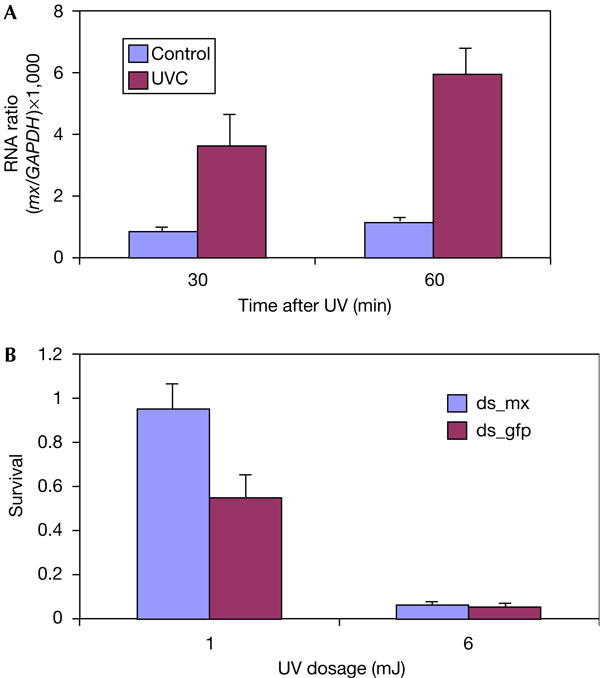
mx is transcriptionally upregulated by ultraviolet light to mediate cell death. (A) mx RNA is rapidly induced after ultraviolet (UV) treatment of C6/36 mosquito cells. Units are relative to the messenger RNA level of the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase). Error bars represent standard errors (n=3). (B) Transfection of double-stranded mx RNA (ds_mx) suppresses UV-induced cell death in C6/36 cells. Double-stranded green fluorescent protein (GFP) RNA (ds_gfp) was used as a control. Transfection of dsRNA and DNA was followed by 2 h recovery before UV treatment. Error bars represent standard errors (n=4 for 1 mJ and n=3 for 6 mJ).
In summary, using a customized motif search strategy, we have successfully identified and characterized the first IAP antagonist, mx, in mosquito genomes. Although the Mx IBM region is nearly identical to that of the Drosophila Grim protein, it lacks a GH3 domain, indicating evolutionary diversification among the mosquito and Drosophila lineages. Furthermore, removal of the mx IBM completely abolishes its apoptotic activity. Like reaper, mx is also transcriptionally regulated to induce cell death. At present, a role for Mx in regulating cell death during virus and parasite infection in mosquitoes is unknown. A further analysis of the functional role of mx should provide more insight into pathogen–host interaction and cell-death regulation in mosquitoes. It is worth noting that because mx was not predicted by the An. gambiae genome project, there is no probe set for this gene in the popular ‘Plasmodium/Anopheles Genome Array' manufactured by Affymetrix. Gene expression profiling using this DNA array would have totally missed information on important cell-death regulatory genes such as mx. This serves as a reminder that the efficacy of genomic analysis can only be as good as our understanding of the genome.
Speculation
It is possible that mx, like reaper in Drosophila, has a pivotal role in regulating cell death during development and in response to environmental stress and infection.
Methods
Data-mining strategy. Models and scoring matrices for the IBM motifs were built with the MEME program (Bailey & Elkan, 1994), and tested empirically by searching the Swiss-Pro annotated protein data set (see Supplementary information III online). Genomic sequences for Anopheles and Aedes were obtained from Ensembl and TIGR, respectively. A motif search program implemented in C was customized to search for the IBM motifs in the genomic as well as EST sequences from mosquito genomes (Zhou et al, 1999).
Cloning mx. Genomic DNA flanking the predicted ORF of mx was first amplified from Ae. albopictus and An. gambiae genomic DNA and tested for cell-killing ability in S2 cells. An intron-less cDNA was then obtained by RT–PCR using RNA extracted from UV-irradiated C6/36 mosquito cells. Other mx cDNA clones were isolated from the Ae. aegypti cDNA libraries.
RNA interference assay in C6/36 cells. Transcription, annealing and purification of dsRNA were carried out using the MEGAscript RNAi kit from Ambion (Austin, TX, USA). dsRNAs were transfected to C6/36 before UV irradiation.
(For a more detailed description of methods, as well as additional sections on in vivo and in vitro cell death assay, UV treatment of C6/36 cells, co-immunoprecipitation, and western blot analysis, please refer to supplementary information I online.)
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We are grateful to Dr J.R. Nambu and Dr A.l. Handler for their comments on the manuscript, to Dr Y. Zhang for her help in generating the transgenic fly strain, to Dr N. Xavier and Dr A.A. James for kindly providing us An. gambiae genomic DNA and RNA samples and to Ms M. Wall for proofreading the manuscript. This work is supported by National Institutes of Health grants CA95542 (L.Z.), CA88815 (L.X.) and AI50936 (D.W.S.).
References
- Abraham EG, Jacobs-Lorena M (2004) Mosquito midgut barriers to malaria parasite development. Insect Biochem Mol Biol 34: 667–671 [DOI] [PubMed] [Google Scholar]
- Bailey TL, Elkan C (1994) Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol 2: 28–36 [PubMed] [Google Scholar]
- Chen P, Ho SI, Shi Z, Abrams JM (2004) Bifunctional killing activity encoded by conserved reaper proteins. Cell Death Differ 11: 704–713 [DOI] [PubMed] [Google Scholar]
- Christophides GK et al. (2002) Immunity-related genes and gene families in Anopheles gambiae. Science 298: 159–165 [DOI] [PubMed] [Google Scholar]
- Claveria C, Caminero E, Martinez AC, Campuzano S, Torres M (2002) GH3, a novel proapoptotic domain in Drosophila Grim, promotes a mitochondrial death pathway. EMBO J 21: 3327–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon-Ramos DA et al. (2003) Inhibition of translation and induction of apoptosis by Bunyaviral nonstructural proteins bearing sequence similarity to Reaper. Mol Biol Cell 14: 4162–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116: 205–219 [DOI] [PubMed] [Google Scholar]
- Grether ME, Abrams JM, Agapite J, White K, Steller H (1995) The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev 9: 1694–1708 [DOI] [PubMed] [Google Scholar]
- Holley CL, Olson MR, Colon-Ramos DA, Kornbluth S (2002) Reaper eliminates IAP proteins through stimulated IAP degradation and generalized translational inhibition. Nat Cell Biol 4: 439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnattur K, Rawson E, Nambu JR (2002) P[52A-GAL4] is an insertion in the Drosophila GP150 gene. Genesis 34: 29–33 [DOI] [PubMed] [Google Scholar]
- Olson MR, Holley CL, Gan EC, Colon-Ramos DA, Kaplan B, Kornbluth S (2003) A GH3-like domain in reaper is required for mitochondrial localization and induction of IAP degradation. J Biol Chem 278: 44758–44768 [DOI] [PubMed] [Google Scholar]
- Palaga T, Osborne B (2002) The 3D's of apoptosis: death, degradation and DIAPs. Nat Cell Biol 4: E149–E151 [DOI] [PubMed] [Google Scholar]
- Rohdendorf B (1974) The Historical Development of Diptera. Edmonton, Alberta, Canada: University of Alberta Press [Google Scholar]
- Vucic D, Kaiser WJ, Miller LK (1998) Inhibitor of apoptosis proteins physically interact with and block apoptosis induced by Drosophila proteins HID and GRIM. Mol Cell Biol 18: 3300–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H (1994) Genetic control of programmed cell death in Drosophila. Science 264: 677–683 [DOI] [PubMed] [Google Scholar]
- Wing JP, Zhou L, Schwartz LM, Nambu JR (1998) Distinct cell killing properties of the Drosophila reaper, head involution defective, and grim genes. Cell Death Differ 5: 930–939 [DOI] [PubMed] [Google Scholar]
- Wu JW, Cocina AE, Chai J, Hay BA, Shi Y (2001) Structural analysis of a functional DIAP1 fragment bound to grim and hid peptides. Mol Cell 8: 95–104 [DOI] [PubMed] [Google Scholar]
- Yoo SJ, Huh JR, Muro I, Yu H, Wang L, Wang SL, Feldman RM, Clem RJ, Muller HA, Hay BA (2002) Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat Cell Biol 4: 416–424 [DOI] [PubMed] [Google Scholar]
- Zhou L (2005) The ‘unique key' feature of the IAP-binding motifs in RHG proteins. Cell Death Differ 20 May 2005; doi:10.1038/sj.cdd.4401637 [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Schnitzler A, Agapite J, Schwartz LM, Steller H, Nambu JR (1997) Cooperative functions of the reaper and head involution defective genes in the programmed cell death of Drosophila central nervous system midline cells. Proc Natl Acad Sci USA 94: 5131–5136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Song Z, Tittel J, Steller H (1999) HAC-1, a Drosophila homolog of APAF-1 and CED-4 functions in developmental and radiation-induced apoptosis. Mol Cell 4: 745–755 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


