Abstract
Attachment of Norwalk (NV), Snow Mountain (SMV), and Hawaii (HV) virus-like particles (VLPs) to specific ABH histo-blood group antigens was investigated by using human saliva and synthetic biotinylated carbohydrates. The three distinct Norwalk-like viruses (NLVs) have various capacities for binding ABH histo-blood group antigens, suggesting that different mechanisms for NLV attachment likely exist. Importantly, antisera from NV-infected human volunteers, as well as from mice inoculated with packaged Venezuelan equine encephalitis virus replicons expressing NV VLPs, blocked the ability of NV VLPs to bind synthetic H type 1, Leb, and H type 3, suggesting a potential mechanism for antibody-mediated neutralization of NV.
Norwalk-like viruses (NLVs) are members of the family Caliciviridae and are a leading cause of acute gastroenteritis worldwide. Studies into the basic biology of NLVs have been limited to recombinant molecular approaches and human challenge studies, primarily because no tissue culture model is available and because an animal model for NLV infection has only recently been reported (21). The genome of Norwalk virus (NV), the prototype NLV, is a single-stranded, positive-polarity RNA of approximately 7.5 kb in length and is organized into three open reading frames (ORFs). The ORF2-encoded major capsid protein self-assembles into Norwalk virus-like particles (VLPs) when expressed from recombinant baculoviruses or Venezuelan equine encephalitis virus (VEE) replicons in insect or mammalian cells, respectively (3, 9, 11).
Marionneau et al. have recently reported that baculovirus-expressed NV VLPs likely attach to either H types 1 or 3 on gastroduodenal epithelial cells of secretor-positive individuals (16). We describe a simple biochemical method to assess specific binding of three distinct VEE-expressed NLV VLPs to various ABH histo-blood group antigens from the type 1 and 3 biosynthesis pathways. We also evaluated and compared the receptor-blockade capacity of antisera from NV-infected human volunteers and from mice inoculated with two different candidate vaccines.
Cloning, expression, and production of genogroup II NLV VLPs.
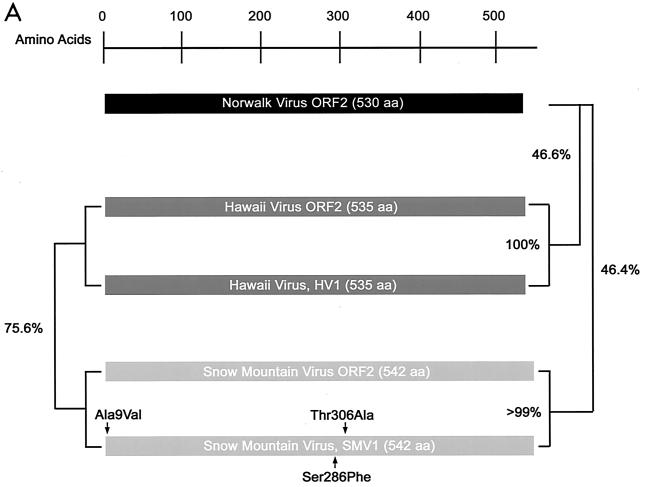
To assess the ability of different NLVs to bind ABH histo-blood group antigens, Snow Mountain virus (SMV) and Hawaii virus (HV) ORF2 capsid genes were cloned from the stools of SMV- or HV-infected human volunteers (Fig. 1A). HV1 is identical in amino acid sequence to the published HV ORF2 (14), and the SMV1 consensus clone contains three amino acid alterations (13).
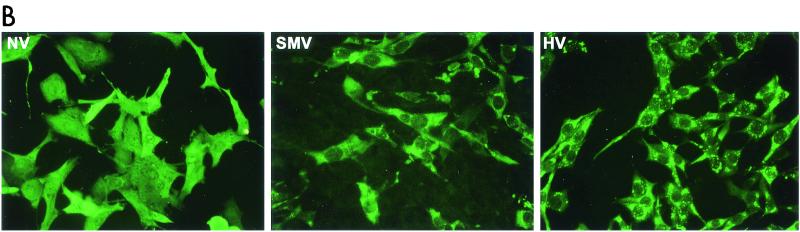
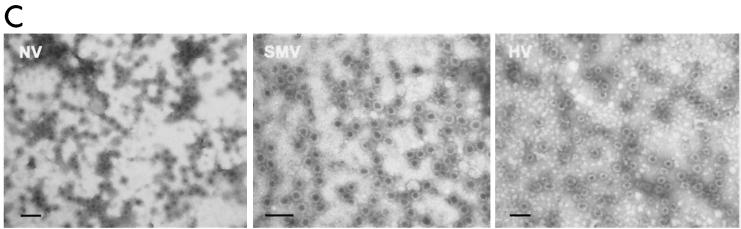
FIG. 1.
Expression and self-assembly of NLV capsid proteins. HV and SMV genomic RNA was isolated from the stools of SMV- or HV-infected human volunteers. The capsid genes were isolated by reverse transcription-PCR and were subcloned into the pVR21 VEE replicon vector as previously described for NV1 (3) with specific primer pairs 5′-AGTCTAGTCCGCCAAGATGAAGATGGCGTCGAATGAC-3′ and 5′-NNNTTAATTAATTATTGCACTCTTCTGCGCC-3′ (HV-5′ and HV-3′, respectively) for HV or HV-5′ and 5′-NNNNNNNGGCGCGCCTTACTGAACCCTTCTACGC-3′ (SMV-3′) for SMV. (A) Amino acid (aa) alignment of NV, HV, and SMV ORF2 regions. NV is a genogroup I isolate, whereas SMV and HV are genogroup II NLVs from distinct genogroup clusters (GII.2 and GII.1, respectively). Percentage of amino acid identities are shown, and arrows indicate amino acid variations from published sequences (12-14). The NV ORF2 capsid clone NV1 has been previously described and is identical to the published NV ORF2 amino acid sequence (9, 12). (B) BHK cells were infected with packaged VRPs encoding NV, SMV, or HV capsid proteins. Expression of NLV capsid proteins was determined by IFA with human antiserum directed to either NV, SMV, or HV. After determination of the VRP titers by IFA as described previously (3, 9), BHK cells were infected with VRPs encoding either NV, SMV, or HV capsid proteins at a multiplicity of infection of 2. At 36 h postinfection BHK cells were lysed by freeze-thaw and the extracts were purified through sucrose gradients and were analyzed by negative-stain electron microscopic analysis (C). Scale bar, 100 nm.
HV1 and SMV1 capsid sequences were inserted into the pVR21 VEE replicon vector, and packaged HV1- and SMV1-carrying VEE replicon particles (VRPs) were produced as previously described for NV1 (3). To determine if the VRPs express NLV capsid proteins, baby hamster kidney (BHK) cell cultures were infected with VRP-NV1, VRP-SMV1, or VRP-HV1. Immunofluorescence analysis (IFA) with antiserum from human volunteers challenged with either NV, SMV, or HV demonstrated that all three capsid constructs were expressed from the VEE replicons (Fig. 1B). Putative VLPs were harvested from VRP-infected BHK cell extracts and were purified by ultracentrifugation through 20 to 50% continuous sucrose gradients. As previously shown with NV (3, 9), negative-stain electron microscopic analysis clearly revealed that the SMV and HV capsid proteins self-assembled into VLPs (Fig. 1C).
Attachment of NLV VLPs to ABH histo-blood group antigens.
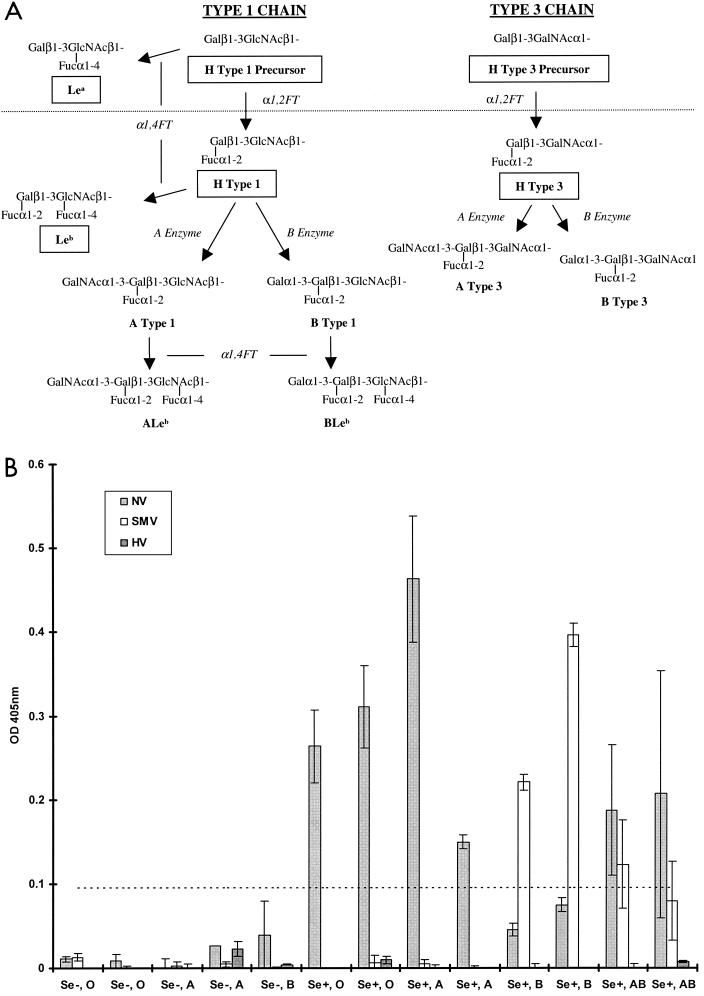
The ABH and Lewis histo-blood group antigens are carbohydrate epitopes present throughout many tissues of the human body (reviewed in reference 15). The type 1 and 3 chain ABH histo-blood group antigens are present on mucosal epithelial cell surfaces and in salivary secretions, with variations in the carbohydrate milieu in different individuals based on their secretor status and blood type (Fig. 2A). Recent observations suggest that NV likely attaches to either H types 1 or 3 present on gastroduodenal epithelial cells (16). To build upon these observations and to determine if other NLVs attach to ABH histo-blood group antigens, we examined whether NV, SMV, or HV VLPs attach to secreted ABH histo-blood group antigens in saliva. Human saliva samples were obtained and typed for the presence of ABH antigens in relation to blood type and secretor status by using antibodies to Lea, Leb, A antigen, and B antigen (Ortho Clinical Diagnostics, Raritan, N.J.). All samples were from Lewis-positive individuals. Saliva samples were boiled for 5 min prior to binding analysis to denature any potential anti-NLV antibody. Plates were coated with boiled saliva at a 1:500 dilution in carbonate buffer (pH 9.6) for 4 h at ambient temperature and were blocked overnight in 5% milk in Tris-buffered saline (TBS). NV, SMV, or HV VLPs (0.1 μg) were then added to saliva-coated wells in 1% milk-Tween-TBS and were incubated for 1 h at 37°C. VLP binding was detected by using convalescent antiserum from NV-, SMV-, or HV-infected human volunteers and goat anti-human immunoglobulin G (IgG) alkaline phosphatase conjugate. Plates were developed with p-nitrophenyl phosphate substrate (pNPP) (Sigma-FAST tablets; Sigma, St. Louis, Mo.), and the optical densities at 405 nm (OD405) were determined.
FIG. 2.
Binding of NLV VLPs to saliva components from individuals of different blood types and secretor status. (A) General schematic of the type 1 and 3 chain ABH histo-blood group antigen production pathways adapted from that described in reference 15. Synthetic carbohydrates used in this paper are boxed. Enzymatic activities are in italics (FT, fucosyltransferase). The type 1 and 3 chains are the primary histo-blood group carbohydrates present in saliva and on epithelial cell surfaces of the gastric mucosa, presumed to be the site of NLV attachment (16, 20). H antigens are the first carbohydrates synthesized from precursor disaccharides by the enzymatic activity of the α1,2 fucosyltransferase encoded by either the fut1 or fut2 gene. About 20% of the European population encodes a mutant form of the fut2 gene resulting in the nonsecretor phenotype. Nonsecretors do not express a functional FUT2 enzyme and will thus not produce carbohydrates downstream of the H precursors (dashed line) on gastric mucosal surfaces or in saliva. In secretors, the A or B enzyme expressed by blood type A or B individuals, respectively (or both in type AB individuals), can further modify the H antigens along the particular type chain. Individuals of blood type O do not produce A or B enzymes and therefore do not express A or B antigens or downstream carbohydrates on mucosal surfaces. The Lea and Leb antigens are synthesized by the FUT3 Lewis enzymes (α1-3/4 fucosyltransferases) from either the H type 1 precursor or H type 1, respectively. In saliva and in the gastric mucosa, Lewis-positive secretors will generally produce Leb and relatively less Lea, whereas nonsecretors will only produce Lea. (B) Binding of NLV VLPs to saliva from individuals of various secretor status and blood type. VLPs were added to saliva-coated microwells, and VLP binding was detected with appropriate human anti-NLV antisera, anti-IgG alkaline phosphatase conjugated secondary antibody, and pNPP substrate. The OD405 quantifies net VLP binding to saliva after background subtraction, and the dashed line represents an arbitrary negative cutoff value equivalent to twice the average background OD. Mean values from duplicate wells are shown, and error bars represent the standard deviation. The x axis displays the individuals' secretor phenotype and blood type. Note that saliva samples could only be obtained from one Se− blood type B and no Se− blood type AB individuals.
NV VLPs bound efficiently to saliva components from secretor-positive (Se+), blood type O, A, and AB individuals but not to saliva from secretor-negative (Se−) individuals (Fig. 2B). The fact that NV VLPs bound poorly to saliva containing ABH antigens in blood group B individuals might also account for the observation that blood group B individuals are more resistant to NV challenge (10). SMV VLPs bound to saliva from Se+ blood type B and AB individuals, suggesting that SMV and NV attach to different ABH histo-blood group carbohydrates. Dose-dependent binding of SMV VLPs based on B antigen expression was also evident (data not shown), suggesting that SMV utilizes B type 1, B type 3, or another downstream carbohydrate for attachment. HV VLPs did not bind to saliva components regardless of secretor phenotype or blood type, suggesting yet a third possible mechanism for NLV attachment. These data suggest that blood group and secretor status may be susceptibility alleles for some, but not all, NLV infections in humans.
To clearly identify which particular ABH histo-blood group antigens are involved in NLV attachment, a microwell-based assay was designed to biochemically detect and quantify levels of synthetic, biotinylated ABH histo-blood group carbohydrate binding to NLV VLPs. High-binding microwells were coated with NLV VLPs or sucrose-purified transmissible gastroenteritis virus virions. The coated wells were incubated with serially diluted, synthetic biotinylated ABH histo-blood group carbohydrates as described in the legend to Fig. 3. Carbohydrate binding was detected using a streptavidin-alkaline phosphatase conjugate and substrate.
FIG. 3.
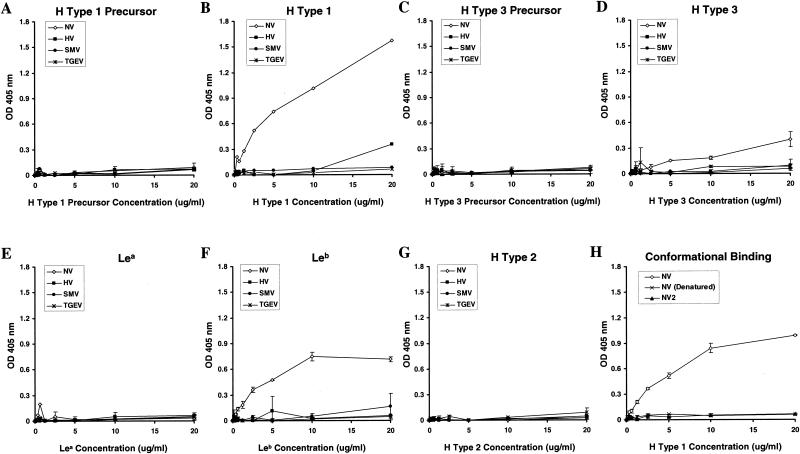
(A to G) Binding of synthetic ABH histo-blood group antigens with NLV VLPs. Purified NLV VLPs (100 μl at 2 μg/ml in TBS) were added to high-binding enzyme immunoassay plates (Costar, Corning, N.Y.). Sucrose-purified virions of the coronavirus transmissible gastroenteritis virus (TGEV) were used as an unrelated virus control. Plates were incubated for 4 h at ambient temperature and were blocked overnight at 4°C with 5% milk in phosphate-buffered saline (5% BLOTTO). Synthetic ABH and Lewis histo-blood group carbohydrates were purchased from Glycotech (Rockville, Md.). Each synthetic carbohydrate is biotinylated with a multivalent polyacrylamide linker between the biotin and carbohydrate moieties. The lyophylized carbohydrates were resuspended in sterile phosphate-buffered saline at 1 mg/ml, diluted to 20 μg/ml in 5% BLOTTO, and serially diluted twofold in the microtiter wells, yielding 100 μl/well. The plates were incubated for 3.5 to 4 h at 37°C followed by five washes with phosphate-buffered saline-Tween (0.05%). A 1:250 dilution (100 μl/well; in 5% BLOTTO) of a streptavidin-alkaline phosphatase conjugate solution (1 mg/ml; Sigma) was then added, and the plates were incubated at 37°C for 1 h. After a final wash step the plates were developed with 150 μl of pNPP substrate/well and the OD405 was determined. Wells incubated with blocking solution instead of synthetic carbohydrates were used as plate blanks. (H) Conformation-dependent binding of H type 1 with NV VLPs. High-binding microwells were coated with (2 μg/ml) NV VLPs (NV), heat denatured NV VLPs [NV (Denatured)], or sucrose-purified NV2 protein (NV2), which does not assemble into intact VLPs (9). Serially diluted, synthetic, biotinylated H type 1 was added to the microwells, and the ability of the carbohydrate to bind to the NV capsid proteins was determined. Average ODs for all panels are shown, and error bars represent the standard deviation of duplicate wells.
Neither NV, SMV, nor HV VLPs bound synthetic, biotinylated H type 1 precursor (Fig. 3A), which lacks the FUT2-linked α1-2 fucose in H type 1. However, under identical conditions NV VLPs, but not SMV or HV VLPs, clearly bound H type 1 carbohydrate in a dose-dependent manner (Fig. 3B), supporting the notion that different NLVs utilize different pathways for docking and entry. NV VLPs also bound H type 3, although not as well as H type 1 (Fig. 3D). This interaction is specific, as NV VLPs did not bind the H type 3 precursor (Fig. 3C). SMV and HV VLPs did not detectably bind H type 3 or its precursor (Fig. 3C and D). Neither NV, SMV, nor HV VLPs detectably bound Lea (Fig. 3E). However, NV VLPs clearly bound synthetic Leb carbohydrate (Fig. 3F). Interestingly, this is the first suggestion that Leb may function as a target for NV attachment. The A, B, and FUT3 enzymes compete for the common H type 1 substrate. Individuals of blood type O do not maintain active A or B enzyme and will likely retain more H type 1 substrate for subsequent FUT3 Lewis enzymatic activity. Therefore, the interaction of NV VLPs with Leb, but not Lea, agrees with the findings that saliva from secretor-positive blood type O individuals binds NV VLPs and that individuals of blood type O are most susceptible to NV infection (10). Finally, we evaluated the ability of the VLPs to attach to H type 2, which is present on red blood cells but is not produced at the surfaces of the gastric mucosa or in salivary secretions. Consistent with the tissue distribution of H type 2, NV, SMV, and HV VLPs did not bind synthetic H type 2 carbohydrate (Fig. 3G).
To verify the carbohydrate binding results, the binding assays were repeated in microwells coated with 10 μg of each VLP stock/ml, which confirmed that the NV VLPs bound only H type 1, H type 3, and Leb (data not shown). Also, although the initial binding data suggest that HV might bind H type 1 at relatively high carbohydrate concentrations (Fig. 3B), neither HV nor SMV VLPs coated at 10 μg/ml could detectably bind any of the synthetic carbohydrates tested (data not shown).
To determine if the interaction of NV capsid proteins with H type 1 depends on the ability of the proteins to self-assemble into VLPs, the binding capacity of NV2 protein and heat-denatured VLPs was evaluated. The NV2 capsid construct contains three amino acid mutations from the wild type, which collectively ablate the ability of the protein to self-assemble into VLPs (3). Neither NV2 nor denatured NV1 VLPs could detectably bind H type 1 (Fig. 3H). Also, with both the binding assay described above and an NV VLP competition-based assay we were unable to detect any interaction of H type 1 with a series of 15-mer overlapping peptides that span the entire NV capsid protein (data not shown), confirming that H type 1 binding is dependent on VLP assembly.
Blockade of NV:ABH histo-blood group antigen binding by antisera from infected human volunteers.
One basic mechanism by which viruses are neutralized is the antibody-mediated prevention of viral attachment to host cells (4, 5, 18, 22). Therefore, the development of an assay system that measures antibody-mediated neutralization of virus binding to its receptor would be valuable for NLV immunity and vaccine studies. We utilized the carbohydrate binding assay to determine if infection with NV stimulates antibody responses that block attachment of NV to its putative receptor(s).
Human volunteers were dosed with 107 to 108 PCR-detectable units of the NV 8fIIb challenge inoculum. Infection in the challenged volunteers based on seroconversion or PCR detection of virus in stool has been determined and will be described in greater detail elsewhere (C. Moe, unpublished data). Ten randomly chosen serum sample pairs from day 0 and days 21 to 23 postinfection (prechallenge and convalescent, respectively) were obtained from volunteers determined to be infected. NV VLP-specific serum IgG titers were quantified by enzyme-linked immunosorbent assay (ELISA) (9), and all volunteers had preexisting anti-NV antibody. Serum samples were serially diluted and preincubated in NV VLP-coated microwells prior to addition of the appropriate synthetic carbohydrate and completion of the binding assay as described above.
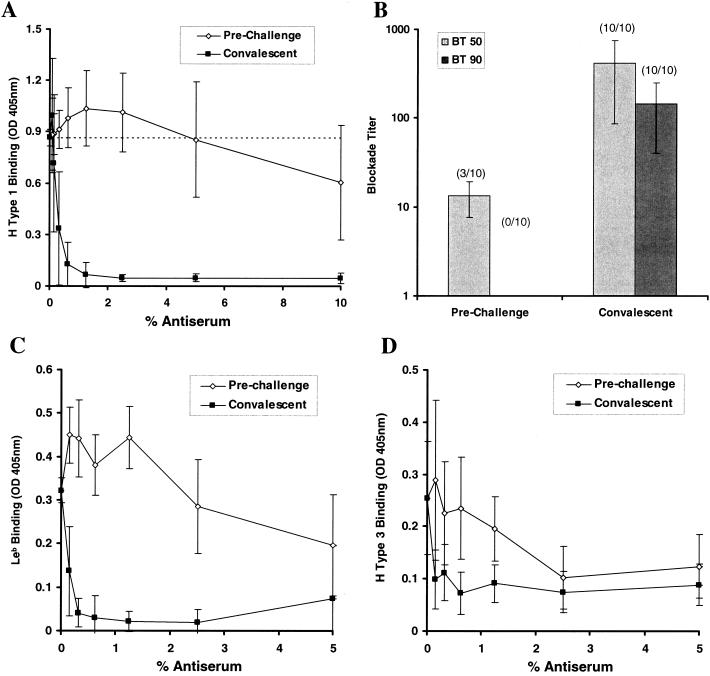
Convalescent human antisera, but not preinfection antisera, efficiently blocked binding of H type 1 to NV VLPs (Fig. 4A). The dilution (blockade titer) at which antisera could block 50 and 90% of the signal produced from H type 1:NV VLP binding (BT 50 and BT 90, respectively) was determined for each sample (Fig. 4B). Of the 10 serum pairs, 3 preinfection samples had detectable BT 50 titers, which might suggest prior exposure to NV or a closely related NLV. In contrast, all convalescent antisera samples had BT 50 and BT 90 activity at sera concentrations of 0.33% ± 0.17% and 1.13% ± 0.81%, respectively. The >30-fold difference in BT 50 titers between the 3 preinfection and 10 convalescent-phase serum samples is significant (P < 0.005; Student's t test). The blockade capacity (BT 50) of the convalescent antiserum correlates with the NV-specific IgG titer in the serum samples as determined by ELISA (R2 > 0.9; linear trend analysis), whereas the blockade titers of the preinfection serum samples do not correlate with their respective anti-NV IgG titers (R2 < 0.5) (data not shown). Convalescent antiserum from the infected volunteers also inhibited the binding of NV VLPs with Leb and H type 3 (Fig. 4C and D).
FIG. 4.
Human antisera blockade of NV VLP:ABH histo-blood group attachment. Prechallenge and convalescent-phase serum samples were obtained from 10 human volunteers infected with a live NV challenge inoculum. Sera were serially diluted and added to NV-coated microwells prior to addition of synthetic, biotinylated carbohydrates and completion of the binding assay. (A) The OD405 was determined to measure H type 1:NV VLP binding in the presence of various concentrations of the preincubated, prechallenge, and convalescent human antisera. Results indicate the mean OD405 from 10 human serum sample pairs, and error bars represent standard deviation. The dashed line represents 100% H type 1 binding (no preincubated serum). (B) The serum dilution (blockade titer) at which 50 and 90% H type 1 is blocked from binding to the NV VLPs (BT 50 and BT 90, respectively) was determined on the basis of OD values. Results indicate the mean blockade titer of the serum samples, with detectable BT 50 and BT 90 values at the concentrations tested, and the number of samples with detectable BT 50 or BT 90 titers is indicated by parentheses. Error bars indicate blockade titer standard deviation from only those samples with detectable BT 50 and BT 90 values. (C and D) Human antisera blockade of Leb:NV VLP and H type 3:NV VLP binding. Serum samples were preincubated with NV VLP-coated wells prior to addition of the biotinylated carbohydrates and completion of the binding assay. Results indicate mean OD values for 10 serum pairs, and error bars represent standard deviation.
Blockade of NV:ABH histo-blood group antigen binding by antisera from experimentally vaccinated mice.
Multiple-candidate VLP-based NLV vaccine strategies have been proposed to date. Oral inoculation of baculovirus-expressed NV VLPs stimulates systemic and mucosal antibody responses in mice and boosts antibody responses in human volunteers previously exposed to NV (1, 2, 7). It was recently demonstrated that inoculation of mice with packaged VEE replicon particles expressing Norwalk VLPs stimulates robust systemic, mucosal, and heterotypic antibody responses (9). However, the lack of a convenient tissue culture or animal model for NLV infection does not allow the use of conventional animal challenge or tissue culture neutralization assays to evaluate the relative effectiveness of any candidate NLV vaccine.
The synthetic carbohydrate binding assay was utilized to identify potentially neutralizing antibody responses in mice inoculated with the two candidate vaccines. Two groups of four 6-week-old male BALB/c mice were either inoculated with two doses of the VRP-NV1 replicon construct or were orally dosed with NV VLPs on days 0 and 23. Preimmune and day 35 blood samples were collected by tail bleed, and serum anti-NV IgG titers were determined by ELISA (9). Of the four mice orally inoculated with NV VLPs, only one mouse had detectable anti-NV IgG in the day 35 antisera (9). However, all four mice inoculated with VRP-NV1 produced robust titers of anti-NV serum IgG (data not shown), confirming our previous report (9).
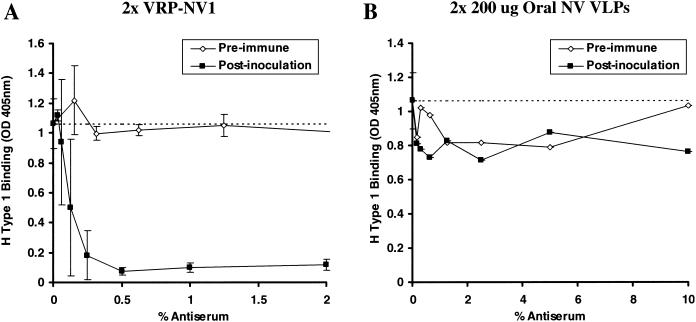
Day 35 antisera from all mice inoculated with VRP-NV1 blocked nearly 100% of H type 1:NV VLP binding with only 0.5% (1:200 dilution) preincubated serum (Fig. 5A). Based on anti-NV IgG titers, the postinoculation mouse antisera was >5-fold more effective at blocking H type 1:NV VLP binding than the human antisera (data not shown). Preimmune sera from the same mice at concentrations as high as 10% did not detectably block H type 1 binding (Fig. 5A and data not shown). Antiserum from the single mouse that responded to oral inoculation of NV VLPs did not appear to block binding of H type 1 to the VLP-coated wells any better than preimmune serum from the same mouse (Fig. 5B). Day 35 antisera from mice inoculated with VRP-NV1 also similarly blocked Leb:NV and H type 3:NV binding (data not shown).
FIG. 5.
Mouse antisera blockade of H type 1:NV VLP binding. Groups of four mice were experimentally vaccinated with two doses of either subcutaneously inoculated VRP-NV1 or orally inoculated NV VLPs. VRPs were administered at 107 infectious units via footpad injection, and 200 μg of sucrose-purified NV VLPs was administered by oral gavage by using methods previously described (9). Baseline and day 35 (12 days postboost) antisera were serially diluted and preincubated in NV VLP coated microwells, followed by addition of synthetic, biotinylated H type 1. H type 1 binding was measured as described in the legend to Fig. 4 and in the text. Dashed lines represent 100% H type 1 binding (no preincubated serum). (A) Mean ODs from wells preincubated with the various serum concentrations from four mice inoculated with VRP-NV1. Error bars represent standard deviation. Note that data for preimmune sera from only three of the four VRP-NV1-vaccinated mice is shown because of insufficient sample volume from one mouse. (B) OD values from microwells preincubated with serially diluted sera from one mouse that responded to the orally inoculated NV VLPs. The three other mice did not respond to the experimental vaccination (9). Note the x-axis scale differences in panels A and B.
We have developed a simple biochemical assay that measures NV VLP binding to H type 1 and other ABH histo-blood group antigens. These data build upon earlier observations (10, 16) and collectively suggest that Se+ individuals are more likely to become infected with NV than Se− individuals. Also, based on saliva and carbohydrate binding assays with SMV and HV VLPs, we've identified at least two potential additional mechanisms for NLV attachment, with one mechanism (HV) possibly not related to secretor phenotype or the ABH histo-blood group antigens. This report warrants additional studies to fully elucidate the extent and significance of ABH attachment of NLVs and whether the carbohydrates act as primary receptors or merely enhance NLV infectivity or attachment to a common cellular receptor.
The experiments described in this manuscript may provide a useful surrogate for examining and monitoring NLV neutralizing antibody as a result of viral infection or vaccination. NV-challenged human volunteers that became infected mounted robust serum IgG responses that efficiently blocked binding of NV VLPs with H type 1, H type 3, and Leb. Also, immunization with VEE replicons expressing NV VLPs, but not oral VLP inoculation, stimulated robust titers of attachment-blocking serum antibody in mice. It is unclear at this point if the anti-NV IgG titers in the orally inoculated mice are simply insufficient to detect NV VLP:H type 1 blockade or if the antibody does not recognize the appropriate H type 1 binding epitopes. Nevertheless, immunization with VEE replicons expressing the NV VLPs stimulated considerably higher titers of attachment-blocking antibody than oral inoculation of the NV VLPs. We anticipate that the coadministration of a mucosal adjuvant and/or intranasal inoculation of NV VLPs would improve the production and detection of ABH:NV VLP blocking antibody (2, 7, 19).
Studies into NV immune mechanisms are complicated by the fact that anti-NV serum IgG cross-reacts with various other NLV capsid proteins (8), and the NLV infection history of the challenged volunteers is unknown. Therefore, it is not known whether the preexisting anti-NV IgG in the challenged volunteers emerged from a previous infection with NV or merely represents cross-reactive, nonneutralizing antibody that has remained from a previous, alternative NLV infection. The methods described in this study could provide a means for detecting and measuring type-specific NLV neutralizing antibody to clarify our understanding of a patient's NLV exposure history. We hypothesize that the three individuals with prechallenge anti-NV IgG that partially blocked H type 1 binding may have been recently exposed to NV or a genetically similar NLV, whereas the anti-NV IgG in the other seven individuals recognized cross-reactive epitopes and is indicative of prior exposure to other NLV(s) but not NV.
Importantly, the mucosal immune response likely plays a critical role in NLV immunity (6, 17, 19). Therefore, it is important to determine whether the carbohydrate-blocking antiserum is indicative of a protective antibody response in the human volunteers. Examinations of receptor-blocking antibody in mucosal secretions and determining the potential function of both serum and mucosal receptor-blocking antibody are needed to fully understand their significance in NLV immunity.
Acknowledgments
We thank Nancy Davis, Mark Heise, and Robert E. Johnston for helpful discussions during writing of this paper and for providing the pVR21 VEE replicon vector. We also thank Martha Collier for producing packaged VEE replicons.
This work was supported by research grants from the National Institutes of Health (RSB-AI23946 and RSB-GM63228), the U.S. Environmental Protection Agency (R-82936501), and the North Carolina Biotechnology Center.
REFERENCES
- 1.Ball, J. M., D. Y. Graham, A. R. Opekun, M. A. Gilger, R. A. Guerrero, and M. K. Estes. 1999. Recombinant Norwalk virus-like particles given orally to volunteers: phase I study. Gastroenterology 117:40-48. [DOI] [PubMed] [Google Scholar]
- 2.Ball, J. M., M. E. Hardy, R. L. Atmar, M. E. Conner, and M. K. Estes. 1998. Oral immunization with recombinant Norwalk virus-like particles induces a systemic and mucosal immune response in mice. J. Virol. 72:1345-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baric, R. S., B. Yount, L. Lindesmith, P. R. Harrington, S. R. Greene, F. C. Tseng, N. Davis, R. E. Johnston, D. G. Klapper, and C. L. Moe. 2002. Expression and self-assembly of Norwalk virus capsid protein from Venezuelan equine encephalitis virus replicons. J. Virol. 76:3023-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bass, D. M., and U. Upadhyayula. 1997. Characterization of human serotype 1 astrovirus-neutralizing epitopes. J. Virol. 71:8666-8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards, M. J., and N. J. Dimmock. 2001. A haemagglutinin (HA1)-specific FAb neutralizes influenza A virus by inhibiting fusion activity. J. Gen. Virol. 82:1387-1395. [DOI] [PubMed] [Google Scholar]
- 6.Estes, M. K., J. M. Ball, R. A. Guerrero, A. R. Opekun, M. A. Gilger, S. S. Pacheco, and D. Y. Graham. 2000. Norwalk virus vaccines: challenges and progress. J. Infect. Dis. 181(Suppl. 2):S367-S373. [DOI] [PubMed] [Google Scholar]
- 7.Guerrero, R. A., J. M. Ball, S. S. Krater, S. E. Pacheco, J. D. Clements, and M. K. Estes. 2001. Recombinant Norwalk virus-like particles administered intranasally to mice induce systemic and mucosal (fecal and vaginal) immune responses. J. Virol. 75:9713-9722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hale, A. D., D. C. Lewis, X. Jiang, and D. W. Brown. 1998. Homotypic and heterotypic IgG and IgM antibody responses in adults infected with small round structured viruses. J. Med. Virol. 54:305-312. [PubMed] [Google Scholar]
- 9.Harrington, P. R., B. Yount, R. E. Johnston, N. Davis, C. Moe, and R. S. Baric. 2002. Systemic, mucosal, and heterotypic immune induction in mice inoculated with Venezuelan equine encephalitis replicons expressing Norwalk virus-like particles. J. Virol. 76:730-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutson, A. M., R. L. Atmar, D. Y. Graham, and M. K. Estes. 2002. Norwalk virus infection and disease is associated with ABO histo-blood group type. J. Infect. Dis. 185:1335-1337. [DOI] [PubMed] [Google Scholar]
- 11.Jiang, X., M. Wang, D. Y. Graham, and M. K. Estes. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 66:6527-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang, X., M. Wang, K. Wang, and M. K. Estes. 1993. Sequence and genomic organization of Norwalk virus. Virology 195:51-61. [DOI] [PubMed] [Google Scholar]
- 13.King, A. D., and K. Y. Green. 1997. Sequence analysis of the gene encoding the capsid protein of the Snow Mountain human calicivirus. Virus Genes 15:5-7. [DOI] [PubMed] [Google Scholar]
- 14.Lew, J. F., A. Z. Kapikian, J. Valdesuso, and K. Y. Green. 1994. Molecular characterization of Hawaii virus and other Norwalk-like viruses: evidence for genetic polymorphism among human caliciviruses. J. Infect. Dis. 170:535-542. [DOI] [PubMed] [Google Scholar]
- 15.Marionneau, S., A. Cailleau-Thomas, J. Rocher, B. Le Moullac-Vaidye, N. Ruvoen, M. Clement, and J. Le Pendu. 2001. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie 83:565-573. [DOI] [PubMed] [Google Scholar]
- 16.Marionneau, S., N. Ruvoen, B. Le Moullac-Vaidye, M. Clement, A. Cailleau-Thomas, G. Ruiz-Palacois, P. Huang, X. Jiang, and J. Le Pendu. 2002. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology 122:1967-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsui, S. M., and H. B. Greenberg. 2000. Immunity to calicivirus infection. J. Infect. Dis. 181(Suppl. 2):S331-S335. [DOI] [PubMed] [Google Scholar]
- 18.Ober, B. T., B. Teufel, K. H. Wiesmuller, G. Jung, E. Pfaff, A. Saalmuller, and H. J. Rziha. 2000. The porcine humoral immune response against pseudorabies virus specifically targets attachment sites on glycoprotein gC. J. Virol. 74:1752-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogra, P. L., H. Faden, and R. C. Welliver. 2001. Vaccination strategies for mucosal immune responses. Clin. Microbiol. Rev. 14:430-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravn, V., and E. Dabelsteen. 2000. Tissue distribution of histo-blood group antigens. APMIS 108:1-28. [DOI] [PubMed] [Google Scholar]
- 21.Subekti, D. S., P. Tjaniadi, M. Lesmana, J. McArdle, D. Iskandriati, I. N. Budiarsa, P. Walujo, I. H. Suparto, I. Winoto, J. R. Campbell, K. R. Porter, D. Sajuthi, A. A. Ansari, and B. A. Oyofo. 2002. Experimental infection of Macaca nemestrina with a Toronto Norwalk-like virus of epidemic viral gastroenteritis. J. Med. Virol. 66:400-406. [DOI] [PubMed] [Google Scholar]
- 22.Whitton, J. L., and M. B. A. Oldstone. 1996. Immune response to viruses, p. 345-374. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 1. Lippincott-Raven, Philadelphia, Pa.