Abstract
Mitochondria are enveloped by two closely apposed boundary membranes with different properties and functions. It is known that they undergo fusion and fission, but it has remained unclear whether outer and inner membranes fuse simultaneously, coordinately or separately. We set up assays for the study of inner and outer membrane fusion in living human cells. Inner membrane fusion was more sensitive than outer membrane fusion to inhibition of glycolysis. Fusion of the inner membrane, but not of the outer membrane, was abolished by dissipation of the inner membrane potential with K+ (valinomycin) or H+ ionophores (cccp). In addition, outer and inner membrane fusion proceeded separately in the absence of any drug. The separate fusion of outer and inner membranes and the different requirements of these fusion reactions point to the existence of fusion machineries that can function separately.
Keywords: membrane fusion, mitochondrial fusion, mitochondrial fission, membrane potential, Drp1
Introduction
Mitochondria are enveloped by two closely apposed membranes with different properties and functions. The inner membrane delimits the matrix space and contains respiratory complexes for oxidative phosphorylation. The outer membrane mediates exchange between cytosol and intermembrane space (Frey & Mannella, 2000). Signalling cascades converging to mitochondria affect outer and inner membranes differentially, and various apoptotic stimuli that induce selective permeabilization of the outer membrane leave the permeability and function of the inner membrane transiently unaffected (Waterhouse et al, 2002). Mitochondria are dynamic organelles that fuse and divide. Fusion mediates molecular exchanges between mitochondria (Legros et al, 2004) and its disruption provokes severe defects (Chen et al, 2003).
Three essential fusion factors are known in yeast: two transmembrane proteins of the outer membrane (Fzo1 and Ugo1) and one intermembrane space protein associated with the inner membrane (Mgm1). The interactions between yeast Ugo1, Fzo1 and Mgm1 (Wong et al, 2003; Sesaki & Jensen, 2004) and the localization of Fzo1 to contact sites between the outer and inner membranes (Fritz et al, 2001) suggest that fusion of double membranes is mediated by a single protein complex. In contrast, the recent identification of fusion intermediates in vitro (Meeusen et al, 2004) suggests that the outer and inner membranes may fuse in successive reactions.
The situation is less clear in mammals. They have two homologues of Fzo1 (mitofusins Mfn1 and Mfn2; Rojo et al, 2002), one homologue of Mgm1 (OPA1; Olichon et al, 2003) and no homologue of Ugo1. The role of mitofusins in fusion has been clearly established (Chen et al, 2003), but the precise function of OPA1 remains unclear (Olichon et al, 2003; Cipolat et al, 2004; Griparic et al, 2004). The exchange of matrix proteins indicates fusion of mitochondrial double membranes (Legros et al, 2002), but it has remained unclear whether outer and inner membranes fuse in a single reaction or in successive steps. We set up assays for the study of outer and inner membrane fusion in living human cells and showed that the fusion reactions have different requirements and can proceed separately.
Results And Discussion
Deoxyglucose affects mitochondrial fusion reactions
To study the requirements of outer and inner membrane fusion, we fused cells containing differently labelled mitochondria in the presence of known inhibitors of energy production. Cells were first treated with deoxyglucose, an inhibitor of glycolysis, or oligomycin, an inhibitor of ATP synthase. Cells survived for days with either treatment, but a combination of both drugs led to rapid cell death (data not shown). Cellular ATP levels were almost unaffected by treatment with oligomycin, but decreased significantly in the presence of deoxyglucose (supplementary Table 1 online), as previously described for HeLa cells (Legros et al, 2002). This shows that glycolysis alone can maintain normal cellular ATP levels, but that oxidative phosphorylation cannot fully compensate the lack of cytosolic ATP synthesis.
To study the fusion of mitochondrial double membranes, we fused cells containing different fluorescent proteins targeted to the matrix (matrix red fluorescent protein (mtRFP) or matrix green fluorescent protein (mtGFP); Fig 1A). Cells were fixed 16 h after fusion, a period that is sufficient to achieve distribution of matrix proteins throughout mitochondria (Fig 1A, no drug). Mitochondrial fusion was inhibited in 70% of deoxyglucose-treated polykaryons: mixing of fluorescent proteins was restricted to small areas and large regions were devoid of one of the fluorescent proteins (Fig 1A, deoxyglucose). At this stage, it was not possible to infer whether deoxyglucose treatment had affected a step preceding fusion (such as mitochondrial mobility, tethering or docking) and/or the fusion of mitochondrial membranes. We therefore fused cells containing mtRFP with cells expressing a GFP molecule anchored to the outer membrane (GFPOM; see the supplementary information online). In the presence of deoxyglucose, all mtRFP-labelled mitochondria became positive for GFPOM in all polykaryons (Fig 1B), showing that outer membrane fusion and the preceding tethering/docking steps were not inhibited. In contrast, the absence of mtRFP from large regions in numerous polykaryons (Fig 1B) showed that inner membrane fusion was partially inhibited by deoxyglucose. The differential sensitivity of outer and inner membrane fusion to deoxyglucose showed that the fusion reactions can proceed separately and have different requirements. The inhibition of inner membrane fusion could be due to the changes in mitochondrial structure provoked by stimulated respiration (see later) or to the decrease of cellular ATP levels. In vitro studies have shown that, in the absence of cytosol, the fusion of docked yeast mitochondria requires GTP (not ATP) for completion (Meeusen et al, 2004). It is thus possible that deoxyglucose lowers the concentration of GTP and/or the GTP/GDP ratio.
Figure 1.
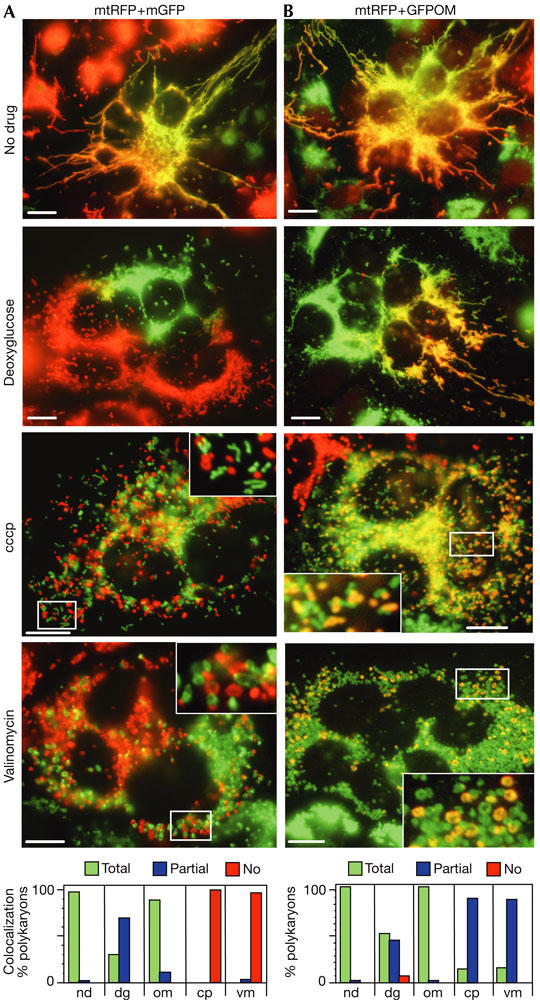
Outer and inner membrane fusion is differentially affected by drugs. Cells containing matrix red fluorescent protein (mtRFP) were fused with cells containing matrix green fluorescent protein (mtGFP; A) or GFPOM (a GFP molecule anchored to the outer membrane; B) and maintained under control conditions (nd) or in the presence of deoxyglucose (dg), oligomycin (om), cccp (cp) or valinomycin (vm). After 16 h, polykaryons were fixed and analysed for fluorescent protein distribution. Scale bars, 10 μm. The quantitative analysis of colocalization indicates the percentage of polykaryons (n⩾100 for each condition) with total, partial or no colocalization of mtRFP and mtGFP (A) or mtRFP and GFPOM (B). Matrix mtRFP and mtGFP as well as outer membrane GFPOM distributed throughout mitochondria under control conditions. Deoxyglucose partially prevented the diffusion of matrix mtRFP and mtGFP but not that of outer membrane GFPOM. Valinomycin and cccp abolished the exchange of mtRFP and mtGFP but not that of GFPOM. Intermixing of mtGFP, mtRFP and GFPOM was not significantly affected by oligomycin.
When mitochondrial ATP synthesis was inhibited with oligomycin, most of the polykaryons had fluorescent proteins distributed throughout the mitochondrial compartment (Fig 1, colocalization), suggesting that oxidative phosphorylation is dispensable for mitochondrial fusion. It confirms previous studies of mitochondrial fusion in human cells and in yeast cells devoid of mitochondrial DNA (and thus of a functional respiratory chain; Okamoto et al, 1998; Legros et al, 2002, 2004). In contrast, yeast mitochondria fuse in vitro only if they have been isolated from respiring cells (Meeusen et al, 2004). The differences between in vivo and in vitro fusion may be due to the absence in vitro of yet unknown factors or to changes of mitochondrial properties accompanying cell homogenization and subcellular fractionation.
Ionophores selectively inhibit inner membrane fusion
We have previously shown that dissipation of the mitochondrial membrane potential (ΔΨm) with the protonophore cccp abolishes mitochondrial fusion (Legros et al, 2002). To investigate whether mitochondrial fusion was dependent on the proton gradient or on ΔΨm, we compared the effects of cccp (a protonophore) and valinomycin (a potassiumspecific ionophore). Both drugs mediate the influx of cations until dissipation of ΔΨm and uncouple respiration from ATP synthesis. Accordingly, each ionophore had a minor effect on cellular ATP levels, as observed with oligomycin (supplementary Table 1 online). To study the effect of ionophores on mitochondrial double membrane fusion, cells expressing different matrix fluorescent proteins (mtGFP or mtRFP) were fused in the presence of cccp or valinomycin. After 16 h, the mitochondria of all polykaryons remained singly labelled, indicating complete inhibition of mitochondrial fusion (Fig 1A). The finding that both ionophores were inhibitory indicates that ΔΨm, rather than the proton or potassium gradients, is required for fusion, as in yeast (Meeusen et al, 2004).
To investigate whether ionophores inhibited fusion of the outer membrane, inner membrane or both, we fused cells containing mtRFP with cells containing GFPOM. After 16 h in the presence of ionophore, all mtRFP-containing mitochondria had become GFPOM positive (Fig 1B), suggesting that the outer membranes had fused in the absence of ΔΨm. The persistence of GFPOM-positive, mtRFP-negative mitochondria (Fig 1B) showed that inner membrane fusion was inhibited. Selective inhibition of inner membrane fusion after dissipation of ΔΨm confirms that outer and inner membrane fusion can proceed separately and have different requirements. These results contradict in vitro studies in which cccp, not valinomycin, abolished outer membrane fusion of yeast mitochondria (Meeusen et al, 2004). This divergence may show differences between yeast and human mitochondria or point to different requirements of in vivo and in vitro fusion.
Drp1-mediated outer membrane fission
In cccp-treated cells, mitochondria appeared as punctate structures that remained small and were largely scattered (Fig 1; supplementary Fig S1A online). Inhibition of fission with a dominant-negative mutant of dynamin-related protein 1 (Drp1 K38A) abolished cccp-induced fragmentation (supplementary Fig S1B online), as previously described (Legros et al, 2002). This confirms that fusion, not fission, was inhibited with cccp and that Drp1-mediated fission provokes mitochondrial fragmentation. The transfer of GFPOM and the punctate morphology of mitochondria in cccp-treated polykaryons (Fig 1) suggest that outer membrane fusion was followed by outer membrane fission. To investigate this, we developed a novel fusion assay in which one cell population (donor RMGO cells) is doubly labelled with matrix mtRFP and outer membrane GFPOM (Fig 2A). The second cell population (acceptor CN cells) has unlabelled mitochondria and is identified by CFPnuc, a cyan fluorescent protein targeted to the nucleus visible in the GFP channel (Fig 2A). Acceptor and donor cells were co-plated, fused and fixed after 16 h in the absence or presence of drugs. Polykaryons resulting from the fusion of donor and acceptor cells were identified by the coexistence of GFPOM, mtRFP and CFPnuc. Under control conditions, both mtRFP and GFPOM distributed throughout the network of filamentous mitochondria (Fig 2B). In the presence of deoxyglucose, when inner membrane fusion is inhibited (Fig 1), numerous polykaryons contained GFPOM-positive, mtRFP-negative mitochondria (Fig 2C). In the presence of cccp, the coexistence of doubly labelled donor mitochondria and acceptor mitochondria labelled with GFPOM only (Fig 3A) showed that only the outer membrane had undergone fusion, which was followed by outer membrane fission. To study fission, donor and acceptor cells were transfected with dominant-negative Drp1 K38A before cell fusion. The coexistence of filamentous and punctate mitochondria (Fig 3B) showed that Drp1 K38A had partially inhibited fission. Residual fission probably resulted from the fusion of some untransfected cells devoid of Drp1 K38A. Nevertheless, the observation of filamentous singly labelled acceptor mitochondria connected to doubly labelled donor mitochondria (Fig 3B, arrowheads) indicated that Drp1 K38A had inhibited outer membrane fission in mitochondria that had undergone outer membrane fusion. This shows that, in cccp-treated cells, outer membrane fusion is followed by Drp1-mediated outer membrane fission, which leads to the punctate mitochondrial morphology.
Figure 2.
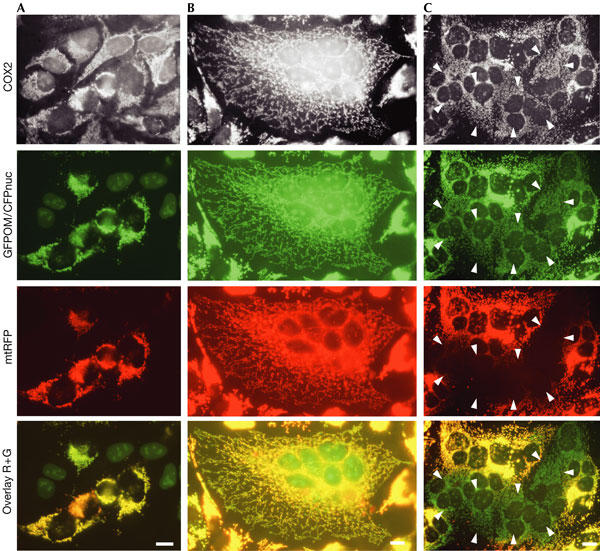
Assay for the parallel observation of outer and inner membrane fusion. Donor RMGO cells expressing two fluorescent proteins targeted to mitochondria (matrix red fluorescent protein (mtRFP) and a green fluorescent protein molecule anchored to the outer membrane (GFPOM)) and acceptor CN cells expressing CFPnuc (a cyan fluorescent protein targeted to the nucleus) were co-plated (A) and fused for 16 h under control conditions (B) or in the presence of deoxyglucose (C). After fixation, all mitochondria were visualized with antibodies against COX2. Mitochondria of RMGO cells are doubly labelled with mtRFP and GFPOM and the nucleus of CN cells is labelled with CFPnuc (A). After fusion of RMGO and CN cells, nuclei are labelled with CFPnuc and the entire mitochondrial compartment is labelled with GFPOM and mtRFP (B). In the presence of deoxyglucose, polykaryons depict mitochondria positive for GFPOM and negative for mtRFP (C, arrowheads). Scale bars, 10 μm.
Figure 3.
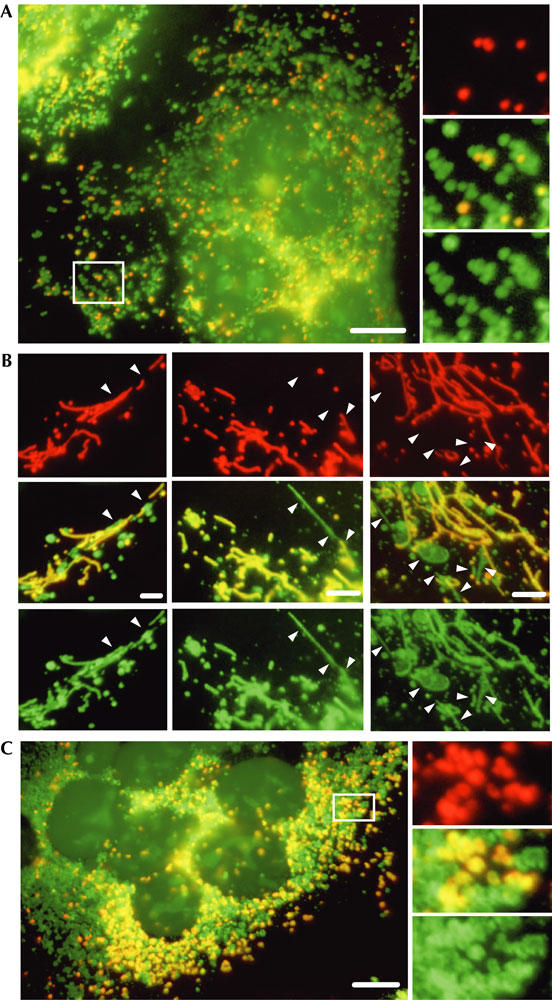
Outer membrane fusion is followed by Drp1-mediated outer membrane fission. Donor RMGO cells expressing two fluorescent proteins targeted to mitochondria (matrix red fluorescent protein (mtRFP) and a green fluorescent protein molecule anchored to the outer membrane (GFPOM)) and acceptor CN cells expressing CFPnuc (a cyan fluorescent protein targeted to the nucleus) were fused and maintained for 16 h in the presence of cccp (A,B) or valinomycin (C). Cells in (B) were transfected with dominant-negative Drp1 K38A before fusion. The selective inhibition of inner membrane fusion leads to the coexistence of doubly labelled donor mitochondria and acceptor mitochondria that are singly labelled with GFPOM (A–C). Under cccp treatment, donor and acceptor mitochondria are largely scattered, indicating that outer membrane fusion was followed by fission (A). After inhibition of fission with Drp1 K38A, some cccp-treated mitochondria remain filamentous and some GFPOM-positive acceptor mitochondria (arrowheads) remain in contact with doubly labelled donor mitochondria (B). Under valinomycin treatment, numerous donor and acceptor mitochondria remain in close proximity (C). Scale bars, 10 μm (A,C) and 3 μm (B).
Despite its similar effect on outer and inner membrane fusion, valinomycin induced significantly different changes in mitochondrial distribution and morphology: the mitochondria of valinomycin-treated cells were significantly enlarged and coalesced perinuclearly (Fig 1; supplementary Fig S1A online). Enlargement is probably due to the entry of water that follows potassium influx and swells mitochondria (Bernardi, 1999), but the mechanisms of perinuclear coalescence are unknown. Interestingly, the expression of Drp1 K38A did not hamper the appearance and coalescence of swollen mitochondria, especially after long incubations (supplementary Fig S1B online). After fusion of donor RMGO and acceptor CN cells in the presence of valinomycin, doubly labelled donor mitochondria coexisted with acceptor mitochondria that were positive for GFPOM only (Fig 3C), confirming fusion of only the outer membrane. A large number of donor and acceptor mitochondria were not scattered but remained in close proximity (Fig 3C), as observed previously (Fig 1; supplementary Fig S1 online). The transfection of dominant-negative Drp1 K38A before fusion of valinomycin-treated cells had no noticeable effect on the distribution of mitochondria and on the selective transfer of GFPOM (data not shown), suggesting that outer membrane fission did not follow outer membrane fusion.
Mitochondrial ultrastructure in drug-treated cells
Electron microscopy showed that mitochondria have an orthodox structure (light matrix space and narrow cristae) in control cells (Fig 4A). In cells treated with deoxyglucose, mitochondria were a normal size but contained an electron-dense matrix and some swollen cristae (Fig 4B). These features, characteristic of the ‘condensed conformation' that mitochondria adopt after stimulation of mitochondrial respiration (Hackenbrock et al, 1971), indicate that oxidative phosphorylation is stimulated in deoxyglucose-treated cells. As predicted by fluorescence microscopy, the mitochondria of cccp-treated cells were small, whereas those of valinomycin-treated cells were significantly larger than those in control cells (Fig 4C,D). In valinomycin-treated cells, large swollen mitochondria with a clear matrix space and few cristae coexisted with fewer mitochondria having a more orthodox structure (Fig 4D). Interestingly, numerous mitochondria contained separate matrix compartments that were enveloped by a single outer membrane, but had different electron densities and differently structured cristae (Fig 4D, arrowheads; supplementary Fig S2 online). This indicates that the mitochondria enveloped by a single outer membrane contained matrices separated by an unfused inner membrane and that mitochondrial outer membrane fission is inhibited in valinomycin-treated cells. It cannot be excluded that a potassium gradient across the inner membrane is required for outer membrane fission. However, it is tempting to speculate that the increase in size provoked by swelling, rather than the absence of a potassium gradient, inhibits outer membrane fission.
Figure 4.
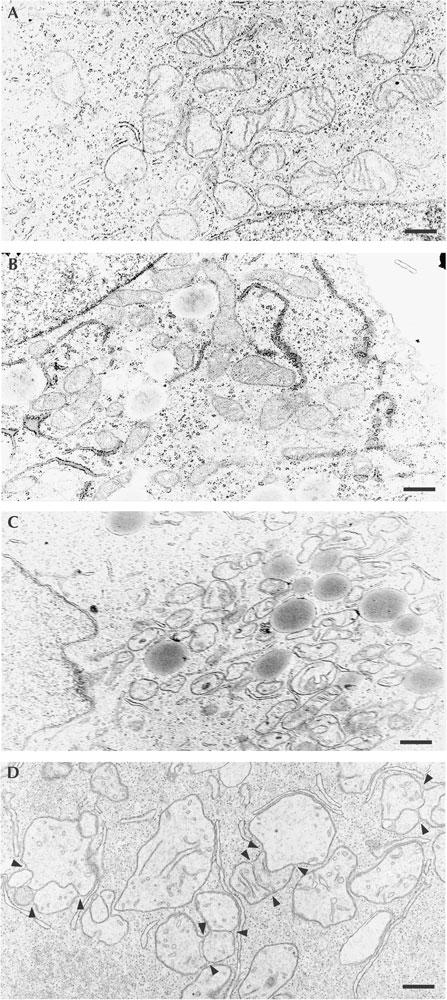
Mitochondrial ultrastructure in drug-treated cells. Cells maintained under control conditions (A) or treated for 16 h with deoxyglucose (B), cccp (C) or valinomycin (D) were processed for electron microscopy. Mitochondria depict an ‘orthodox conformation' under control conditions (A) and adopt a ‘condensed conformation' (electron-dense matrix and swollen cristae) after incubation with deoxyglucose (B). The mitochondria of cccp-treated cells are small (C) and those of valinomycin-treated cells are significantly enlarged (D). Valinomycin-treated cells show mitochondria enveloped by a continuous outer membrane but containing matrix spaces with different electron densities and cristae morphologies, suggesting that these matrix spaces are separated by unfused inner membrane (arrowheads). Scale bars, 400 nm.
Outer and inner membranes can fuse separately
The capacity to uncouple outer and inner membrane fusion with drugs (this work) or after in vitro reconstitution (Meeusen et al, 2004) suggests that both reactions may also be uncoupled in living cells. To compare the fusion of outer and inner membranes in the absence of drugs, we used an assay based on the fusion of doubly labelled donor mitochondria with nonlabelled acceptor mitochondria (RMGO and CN cells; Fig 2). Donor and acceptor cells were co-plated, fused and fixed after 4 h, a time period that is insufficient for diffusion of matrix fluorescent proteins throughout mitochondria. Fixed cells were decorated with antibodies against mitochondrial proteins to visualize the entire mitochondrial compartment. If outer and inner membrane fusion proceeded simultaneously, acceptor mitochondria should be equally labelled with both GFPOM and mtRFP. However, if outer and inner membrane fusion proceeded separately, some mitochondria should be labelled with only GFPOM, at least transiently. At 4 h after fusion, most of the polykaryons contained mitochondria that were doubly labelled with GFPOM and mtRFP (Fig 5A, quantification), indicating that fusion of outer and inner membranes had occurred simultaneously. However, the presence of a significant proportion of polykaryons (Fig 5, quantification) containing GFPOM-positive, mtRFP-negative mitochondria (Fig 5, arrowheads and boxed area) suggested that the transfer of GFPOM (by outer membrane fusion) was not accompanied by that of mtRFP (by inner membrane fusion). To investigate the possible influence of mitochondrial respiration on the coupling of outer and inner membrane fusion, we fused cells devoid of mitochondrial DNA that lack a functional respiratory chain and rely on glycolysis for energy supply. The separate fusion of outer and inner membranes (Fig 5B) was observed in a similar proportion of polykaryons (Fig 5B, quantification), showing that separate fusion of outer and inner membranes occurs with similar frequency in respiring and nonrespiring mitochondria. We performed control experiments to confirm that the segregation of GFPOM and mtRFP was due to the separate fusion of outer and inner membranes. After fusion between the RMGO cells, all mitochondria were labelled with GFPOM and mtRFP (data not shown), thus excluding unspecific segregation of GFPOM and mtRFP by PEG treatment and/or cell fusion. After fusion of doubly labelled donor cells expressing mtRFP and mtGFP (instead of GFPOM) with acceptor CN cells, acceptor mitochondria were all labelled with both matrix fluorescent proteins (supplementary Fig S3 online), excluding that segregation may result from the various properties (such as size, solubility, oligomeric state and fluorescence intensity) of GFP and RFP (DsRed). Altogether, these results show that, in living cells and in the absence of any drug, outer and inner membrane fusion can proceed separately.
Figure 5.
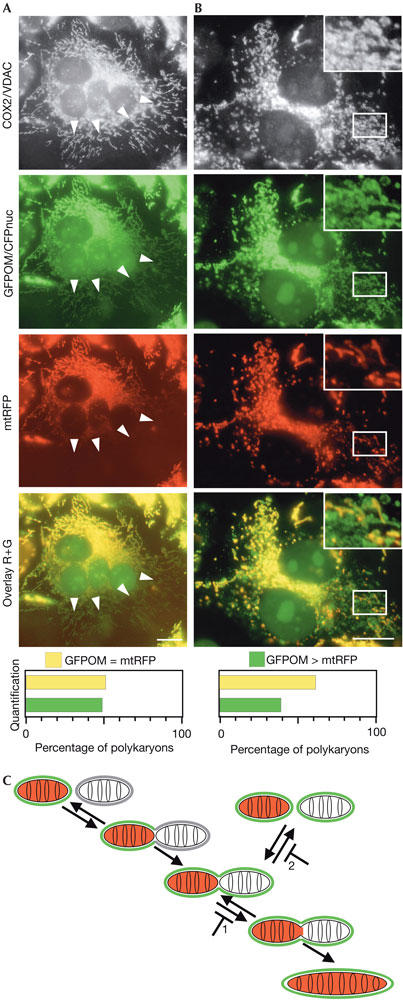
Outer and inner membranes can fuse separately. RMGO cells expressing two fluorescent proteins targeted to mitochondria (matrix red fluorescent protein (mtRFP) and a green fluorescent protein molecule anchored to the outer membrane (GFPOM)) and CN cells expressing a cyan fluorescent protein targeted to the nucleus (CFPnuc) were co-plated and fused for 4 h. RMGO and CN cells were either normal (A) or devoid of mitochondrial DNA (B). After fixation, all mitochondria were visualized with antibodies against COX2 (A) or VDAC (B). A significant fraction of all polykaryons (n⩾100) contain mitochondria positive for GFPOM but negative for mtRFP (arrowheads in (A), inset in (B)). (C) Model of mitochondrial fusion and fission reactions. Under normal conditions, the fusion of outer and inner membranes mediates the successive transfer of GFPOM and mtRFP; mitochondria singly labelled with GFPOM exist only transiently. Inhibition of inner membrane fusion with cccp (1) does not affect outer membrane fusion or fission and leads to fragmentation and scattering of mitochondria. Inhibition of inner membrane fusion and outer membrane fission with valinomycin (1+2) does not affect outer membrane fusion and leads to the accumulation of mitochondria with unfused inner membranes. Scale bars, 10 μm.
Speculation
The different requirements and kinetics of outer and inner membrane fusion, as well as the possibility of uncoupling them, point to the existence of fusion machineries that can function separately (Fig 5C). This enables regulation of mitochondrial fusion at two levels. It is theoretically possible that regulated outer membrane fusion is followed by uncontrolled inner membrane fusion. However, it is also possible that inner membrane fusion is regulated separately and/or represents the main (or only?) target of regulation. Indeed, the dependence of inner membrane fusion on ΔΨm provides the means to link the dynamics of mitochondria to its respiratory activity. Further characterization of outer and inner membrane fusion reactions and of the molecules involved in these processes is required to validate any of these hypotheses.
Methods
Reagents and experimental procedures are described in the supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We thank P. Frachon for valuable assistance, and F. Chevessier and P. de Camilli for expression vectors encoding CFPnuc and GFPOM, respectively. We are grateful to A. Rouche and P. Bozin for assistance in electron microscopy. M.R. is an investigator of CNRS. This work was supported by INSERM and by grants from AFM and from Ministère Délégué à la Recherche (ACI BCMS).
References
- Bernardi P (1999) Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev 79: 1127–1155 [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160: 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolat S, de Brito OM, Dal Zilio B, Scorrano L (2004) OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA 101: 15927–15932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey TG, Mannella CA (2000) The internal structure of mitochondria. Trends Biochem Sci 25: 319–324 [DOI] [PubMed] [Google Scholar]
- Fritz S, Rapaport D, Klanner E, Neupert W, Westermann B (2001) Connection of the mitochondrial outer and inner membranes by Fzo1 is critical for organellar fusion. J Cell Biol 152: 683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griparic L, Van Der Wel NN, Orozco IJ, Peters PJ, Van Der Bliek AM (2004) Loss of the intermembrane space protein Mgm1/Opa1 induces swelling and localized constrictions along the lengths of mitochondria. J Biol Chem 279: 18792–18798 [DOI] [PubMed] [Google Scholar]
- Hackenbrock CR, Rehn TG, Weinbach EC, Lemasters JJ (1971) Oxidative phosphorylation and ultrastructural transformation in mitochondria in the intact ascites tumor cell. J Cell Biol 51: 123–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros F, Lombes A, Frachon P, Rojo M (2002) Mitochondrial fusion in human cells is efficient, requires the inner membrane potential and is mediated by mitofusins. Mol Biol Cell 13: 4343–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros F, Malka F, Frachon P, Lombès A, Rojo M (2004) Organization and dynamics of human mitochondrial DNA. J Cell Sci 117: 2653–2662 [DOI] [PubMed] [Google Scholar]
- Meeusen S, McCaffery JM, Nunnari J (2004) Mitochondrial fusion intermediates revealed in vitro. Science 305: 1747–1752 [DOI] [PubMed] [Google Scholar]
- Okamoto K, Perlman PS, Butow RA (1998) The sorting of mitochondrial DNA and mitochondrial proteins in zygotes: preferential transmission of mitochondrial DNA to the medial bud. J Cell Biol 142: 613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, Lenaers G (2003) Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem 278: 7743–7746 [DOI] [PubMed] [Google Scholar]
- Rojo M, Legros F, Chateau D, Lombes A (2002) Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci 115: 1663–1674 [DOI] [PubMed] [Google Scholar]
- Sesaki H, Jensen RE (2004) Ugo1p links the Fzo1p and Mgm1p GTPases for mitochondrial fusion. J Biol Chem 279: 28298–28303 [DOI] [PubMed] [Google Scholar]
- Waterhouse NJ, Ricci JE, Green DR (2002) And all of a sudden it's over: mitochondrial outer-membrane permeabilization in apoptosis. Biochimie 84: 113–121 [DOI] [PubMed] [Google Scholar]
- Wong ED, Wagner JA, Scott SV, Okreglak V, Holewinske TJ, Cassidystone A, Nunnari J (2003) The intramitochondrial dynamin-related GTPase, Mgm1p, is a component of a protein complex that mediates mitochondrial fusion. J Cell Biol 160: 303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


