Abstract
Ca2+-regulated exocytosis of lysosomes was previously shown to be required for the repair of plasma membrane wounds. The small chemical vacuolin-1 alters the morphology of lysosomes without affecting the ability of cells to reseal their plasma membrane after injury. On the basis of a failure to detect Ca2+-triggered lysosomal exocytosis in vacuolin-1-treated cells, a recent study proposed that lysosomes are dispensable for resealing. Here, we show that vacuolin-1, despite altering lysosome morphology, does not inhibit the exocytosis of lysosomes induced by exposure to a Ca2+ ionophore, or by plasma membrane wounding. Thus, lysosomes cannot be excluded as agents of membrane repair in vacuolin-1-treated cells.
Keywords: calcium, exocytosis, lysosome, repair, vacuolin-1
Introduction
When the plasma membrane of animal cells is injured, Ca2+ influx activates a repair mechanism that involves exocytosis (McNeil & Steinhardt, 1997). Thus, the exocytic vesicle population mediating resealing must have the ability to respond to Ca2+ elevations by fusing with the plasma membrane. Conventional lysosomes were proposed as good candidates for this role (Andrews, 2000), as they are the only well characterized vesicle population that undergoes Ca2+-regulated secretion in most cell types (Rodriguez et al, 1997; Jaiswal et al, 2002). Further studies detected the ubiquitously expressed Ca2+ sensor synaptotagmin VII (Syt VII) on lysosomes (Martinez et al, 2000; Fukuda et al, 2004), and showed that the soluble Syt VII C2A domain is a useful tool for inhibiting lysosomal exocytosis (Martinez et al, 2000). This tool and also antibodies against Syt VII C2A or the cytoplasmic domain of the lysosomal glycoprotein Lamp-1 were used to show that blocking lysosomal exocytosis inhibits membrane repair (Reddy et al, 2001). Consistent with the role of Syt VII in regulating this process, defects in both lysosomal exocytosis and membrane resealing were observed in Syt VII-deficient mice (Chakrabarti et al, 2003; Roy et al, 2004).
However, a recent study proposed that lysosomal exocytosis triggered by Ca2+ is dispensable for membrane repair. This conclusion was drawn on the basis of effects of vacuolin-1, a small molecule recently identified in a microscopic screen of compounds that affect membrane traffic in fibroblasts (Cerny et al, 2004). Although the molecular target of vacuolin-1 is still unknown, it induces the formation of enlarged vacuoles derived from endosomes and lysosomes in several cell types. Cerny et al (2004) reported that lysosomal exocytosis induced by a Ca2+ ionophore or membrane wounding was blocked by vacuolin-1, whereas membrane resealing was unaffected. These results led us to further examine the effects of vacuolin-1, with the aim of better understanding the pathway of membrane repair that was apparently occurring in the absence of lysosomal exocytosis. Surprisingly, we found that lysosomes that were morphologically altered by vacuolin-1 fused normally with the plasma membrane under conditions that elevated intracellular Ca2+, including membrane injury.
Results And Discussion
The triazine-based molecule vacuolin-1 was obtained from ChemBridge Corporation (compound 5114069), which is the same source of the library of small chemicals that were originally screened by Cerny et al (2004). To determine whether the compound was active, HeLa cells were incubated with 1 μM vacuolin-1 for 1 h, followed by fixation and immunofluorescent staining with monoclonal antibodies (mAbs) against human Lamp-1. Markedly enlarged Lamp-1-positive vesicles were evident after exposure to the drug (Fig 1), confirming the previously described effect of vacuolin-1 on this human cell line (Cerny et al, 2004). Similar morphological changes on Lamp-1-positive compartments were observed in a rat cell line (NRK; not shown). Thus, our initial findings are consistent with the earlier suggestion that vacuolin-1 causes a pronounced vacuolation of the endocytic compartment in a large number of different cell types (Cerny et al, 2004).
Figure 1.
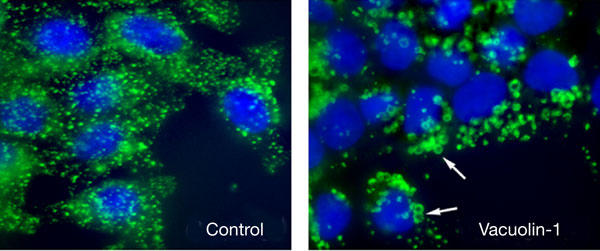
Vacuolin-1 alters the morphology of lysosomes. HeLa cells were plated on glass coverslips, grown to semiconfluency and treated or not with 5 μM vacuolin-1 for 1 h. Cells were then fixed, permeabilized and stained with anti-human Lamp-1 monoclonal antibodies followed by a secondary fluorescent antibody to detect lysosomes (green), and DAPI to stain cell nuclei (blue). The arrows point to enlarged Lamp-1-positive vesicles in vacuolin-1-treated cells.
Next, we examined the effect of vacuolin-1 on the ability of HeLa and NRK cells to secrete lysosomal contents after exposure to ionomycin (Rodriguez et al, 1997). In marked contrast to the results reported by Cerny et al (2004), ionomycin-induced β-hexosaminidase secretion was detected with or without pretreatment for 1 h with 5 μM vacuolin-1. The levels of ionomycin-dependent β-hexosaminidase released, detected in the same incubation media to which ionophore was added, were similar for cells untreated or treated with vacuolin-1, reaching 12–14% in HeLa and 20–24% in NRK cells (Fig 2A,B). An examination of the experimental conditions used by Cerny et al suggested a potential explanation for this discrepancy. That study reported that the media containing ionomycin used for stimulating exocytosis was exchanged before assessment of the amount of β-hexosaminidase secreted (Cerny et al, 2004). Ca2+ ionophores trigger exocytosis within a few seconds of being added to cells (Jaiswal et al, 2002, 2004); thus, media exchange was likely to have resulted in the loss of a large fraction of the Ca2+-triggered secreted enzyme. To investigate this possibility, we repeated the ionomycin-induced exocytosis experiment under the same conditions as those described by Cerny et al (2004), exchanging the media after ionomycin stimulation. As predicted, the bulk of the secreted enzyme was found in the initial buffer containing ionomycin (Fig 2C). Markedly lower amounts were detected in the brief 1 min wash (Fig 2D). Levels of released β-hexosaminidase remained low after a further 10 min incubation in fresh medium (Fig 2E), which corresponds to the conditions reported in figure 3 of Cerny et al (2004). Immunofluorescence with anti-Lamp-1 antibodies before ionomycin stimulation confirmed that vacuolin-1 induced the expected lysosomal enlargement in the HeLa cells used in this experiment (Fig 2F). These results show that there is no differential β-hexosaminidase release kinetics in HeLa cells treated or not with vacuolin-1, which could explain the results reported by Cerny et al (2004). On the contrary, the small amounts of β-hexosaminidase released in the second incubation buffer were consistently marginally higher in vacuolin-1-treated cells. Thus, ionomycin-induced lysosomal exocytosis is not inhibited in vacuolin-1-treated cells, under all conditions tested (Cerny et al, 2004).
Figure 2.
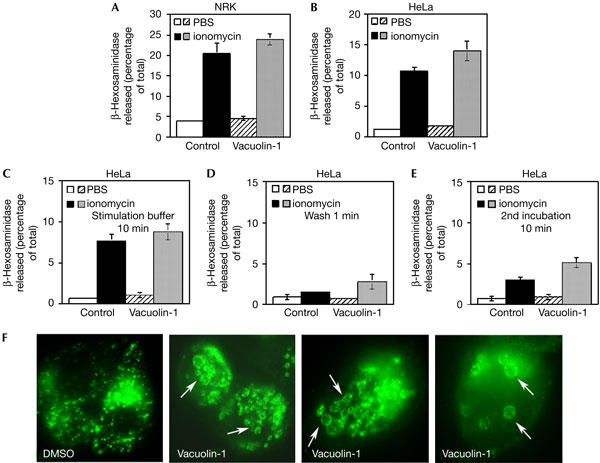
Vacuolin-1 does not inhibit the secretion of β-hexosaminidase induced by ionomycin. NRK (A) or HeLa (B) cells that were treated or not with 5 μM vacuolin-1 for 1 h were exposed or not to 5 μM ionomycin for 10 min, followed by collection of the supernatant and assay for β-hexosaminidase activity. The data are expressed as a percentage of the total cellular content of the enzyme, and correspond to the average of triplicates ±s.d. (C–E) Monolayers of HeLa cells were treated or not with 5 μM vacuolin-1 for 1 h and stimulated or not with 5 μM ionomycin for 10 min. β-Hexosaminidase activity was assayed in the supernatant collected after 10 min (C), in the 1 min wash (D) and in the subsequent 10 min incubation in fresh media (E). The data are expressed as a percentage of the total cellular content of the enzyme, and correspond to the average of triplicates ±s.d. (F) Immunofluorescence with anti-Lamp-1 antibodies of the cells used for the experiment shown in (C–E), before addition of ionomycin. The arrows point to the enlarged Lamp-1-positive vesicles induced by vacuolin-1. DMSO, dimethylsulphoxide.
The results discussed above suggested that ionomycin-triggered lysosomal exocytosis proceeds normally, in spite of the morphological changes induced by vacuolin-1. To reinforce this finding, we examined the translocation of Lamp-1 from lysosomes to the plasma membrane. In this assay, antibodies against the luminal domain of Lamp-1 are used to detect Lamp-1 on the surface of live cells (Reddy et al, 2001; Huynh et al, 2004). These assays were performed under conditions previously shown in our laboratory to reveal only surface-exposed and not intracellular Lamp-1 (Reddy et al, 2001; Huynh et al, 2004). In both HeLa and NRK cells, the punctate Lamp-1 surface staining observed after exposure to ionomycin was not altered in cells that were treated with vacuolin-1 (Fig 3A). Quantification of surface-exposed Lamp-1 by fluorescence-activated cell sorting (FACS) confirmed that treatment with 5 μM vacuolin-1 does not inhibit ionomycin-induced lysosomal exocytosis in both cell types (Fig 3B).
Figure 3.
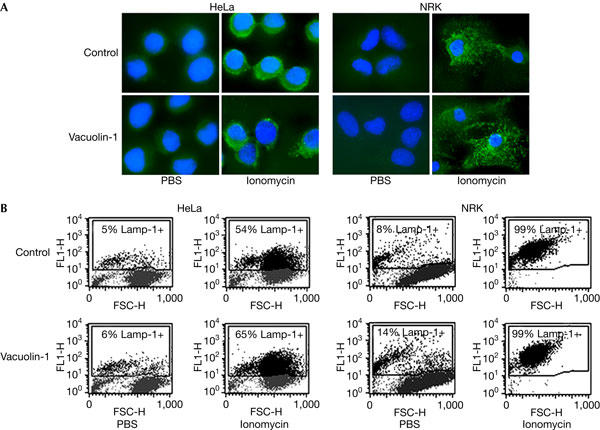
Vacuolin-1 does not inhibit the surface exposure of Lamp-1 induced by ionomycin. (A) HeLa and NRK cells that were plated on glass coverslips and treated or not with 5 μM vacuolin-1 for 1 h were exposed or not to 5 μM ionomycin for 10 min, transferred to ice and surface labelled with monoclonal antibodies against the luminal domain of Lamp-1, followed by fixation and staining with fluorescent secondary antibodies (green), and 4,6-diamidino-2-phenylindole to label cell nuclei (blue). Punctate Lamp-1 surface staining was observed after ionomycin exposure, in cells treated or nor with vacuolin-1. (B) HeLa and NRK cells that were treated or not with 5 μM vacuolin-1 for 1 h were trypsinized, exposed to 5 μM ionomycin for 10 min, surface stained for Lamp-1 and examined by fluorescence-activated cell sorting. FL1-H, fluorescence intensity; FSC-H, forward scatter.
Lysosomal exocytosis and surface exposure of Lamp-1 are induced by plasma membrane wounding, as a result of Ca2+ influx (Reddy et al, 2001). Cerny et al (2004) reported that surface exposure of Lamp-1 was blocked in vacuolin-1-treated cells that were wounded by scratching, so we investigated this issue by performing similar assays in HeLa cells. Scratching of HeLa cell monolayers induced translocation of the luminal domain of Lamp-1 to the cell surface, and this was observed in cells that were treated or not with 5 μM vacuolin-1 (Fig 4A). To quantify this response, we performed a previously characterized FACS-based wounding assay (Huynh et al, 2004). In this assay, carefully controlled wounding conditions are applied to the whole cell population by electroporation, and the lysosomal secretory response (surface exposure of Lamp-1) is measured by FACS. As shown in Fig 4B, treatment of NRK cells with 1 or 5 μM vacuolin-1 had no inhibitory effect on the surface exposure of Lamp-1 triggered by electroporation. Alterations in the sidescatter pattern of the cells confirmed that vacuolin-1 treatment increased cellular granularity (Fig 4B, left column).
Figure 4.
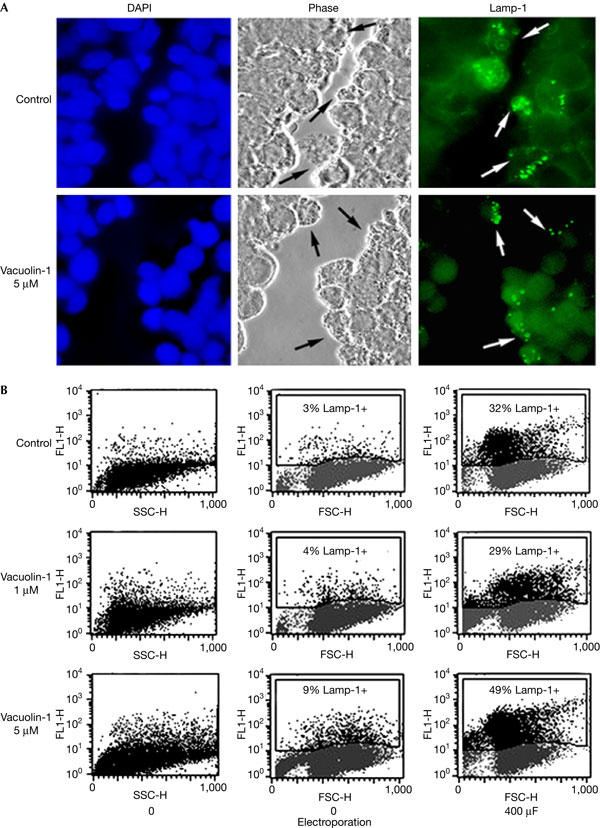
Vacuolin-1 does not inhibit the surface exposure of Lamp-1 induced by plasma membrane wounding. (A) Confluent monolayers of HeLa cells grown on glass coverslips were treated or not with 5 μM vacuolin for 1 h, scratched several times in a grid fashion with a scalpel, transferred to ice and surface labelled with anti-Lamp-1 monoclonal antibodies. After fixation, cells were incubated with a fluorescent secondary antibody (green), and 4,6-diamidino-2-phenylindole to stain cell nuclei (blue). The arrows point to wounded cells that exposed the luminal domain of Lamp-1 on the cell surface. (B) NRK cells, treated or not with vacuolin-1 for 1 h, were wounded in suspension by electroporation, surface stained for Lamp-1 and examined by fluorescence-activated cell sorting. No inhibition of injury-induced lysosomal exocytosis was observed after vacuolin-1 treatment. FL1-H, fluorescence intensity; FSC-H, forward scatter; SSC-H, side scatter.
Again, the explanation for the difference between our results and those reported by Cerny et al is unclear. The protocol described in their study for scrape wounding and Lamp-1 detection on the cell surface seems to have involved a large interval, of several minutes, between wounding and the incubation with anti-Lamp-1 antibodies (Cerny et al, 2004). The failure of Cerny et al to detect surface-exposed Lamp-1 in vacuolin-1-treated cells (which, according to our data, occurs normally and immediately after cell injury) may reflect differences in the rate of re-internalization of lysosomal membrane proteins. Once the molecular target of vacuolin-1 is identified, it will be of interest to investigate its potential role in membrane recycling mechanisms. Our present results indicate that the target of vacuolin-1 is not likely to be LYST, the protein carrying the mutation that causes abnormally enlarged lysosomes in cells from Chediak–Higashi patients (Shiflett et al, 2002). Unlike observations made after lysosomal enlargement by vacuolin-1, electroporation-induced lysosomal exocytosis is impaired in human Chediak–Higashi and mouse beige fibroblasts (Huynh et al, 2004).
Taken together, our observations indicate that lysosomal exocytosis cannot be excluded as a mechanism for the resealing of injured vacuolin-1-treated cells. Our results do not exclude the possibility that further Ca2+-regulated compartments, distinct from lysosomes but present in all cell types, also participate in membrane repair. An answer to this question will require the development of specific tools to block the exocytosis of these putative compartments, such as the AHNAK-containing intracellular vesicles proposed to exocytose in response to Ca2+ in some cell types (Borgonovo et al, 2002).
Methods
Cells, antibodies and vacuolin-1. HeLa and NRK cells were grown in DMEM containing 10% FBS. mAbs against human (H4A3) and rat (LY1C6) Lamp-1 were obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA, USA) and Dr Ira Mellman (Yale University), respectively. Vacuolin-1, identified as compound 5114069, was obtained from ChemBridge (San Diego, CA, USA), solubilized in dimethylsulphoxide (DMSO) at 2 mM and added to cells at 1 or 5 μM. Identical volumes of DMSO alone were added to control cultures.
β-Hexosaminidase secretion assay. Cells that were treated or not with vacuolin-1 for 1 h were trypsinized and resuspended in PBS containing Ca2+ and Mg2+ with or without 5 μM ionomycin (Sigma, St Louis, MO, USA). After 10 min, the cells were pelleted and the supernatant was assayed for β-hexosaminidase activity, as described previously (Rodriguez et al, 1997). The total activity contained in the cell pellet was determined after solubilization with 1% Triton X-100. Ionomycin-induced exocytosis experiments were performed according to Cerny et al (2004). HeLa cell monolayers that were grown in six-well plates and were exposed or not to 5 μM vacuolin-1 for 1 h were transferred to Hepes-buffered Eagle's minimum essential medium (HMEM) at 37°C and stimulated with 5 μM ionomycin for 10 min. The media were then exchanged, three rapid washes were performed, and the amount of β-hexosaminidase released during 10 min in fresh HMEM was determined (along with the β-hexosaminidase content in first-wash buffer, and the total amount present in the cells after solubilization with 1% Triton X-100).
Immunofluorescence, electroporation and FACS analysis. Detection of total Lamp-1 on fixed and permeabilized cells or of surface-exposed Lamp-1 in intact cells was performed as described previously (Martinez et al, 2000; Reddy et al, 2001). Wounding by electroporation and FACS detection of surface-exposed Lamp-1 were performed as described previously (Huynh et al, 2004). Electroporation was performed at 200 V, with 400 μF capacitance.
Acknowledgments
We thank M. Strongin from ChemBridge Corporation for helpful information on compound 5114060 (vacuolin-1). This work was supported by National Institutes of Health grant RO1 GM04625 to N.W.A.
References
- Andrews NW (2000) Regulated secretion of conventional lysosomes. Trends Cell Biol 10: 316–321 [DOI] [PubMed] [Google Scholar]
- Borgonovo B, Cocucci E, Racchetti G, Podini P, Bachi A, Meldolesi J (2002) Regulated exocytosis: a novel, widely expressed system. Nat Cell Biol 4: 955–962 [DOI] [PubMed] [Google Scholar]
- Cerny J, Feng Y, Yu A, Miyake K, Borgonovo B, Klumperman J, Meldolesi J, McNeil PL, Kirchhausen T (2004) The small chemical vacuolin-1 inhibits Ca(2+)-dependent lysosomal exocytosis but not cell resealing. EMBO Rep 5: 883–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Kobayashi KS, Flavell RA, Marks CB, Miyake K, Liston DR, Fowler KT, Gorelick FS, Andrews NW (2003) Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. J Cell Biol 162: 543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Kanno E, Satoh M, Saegusa C, Yamamoto A (2004) Synaptotagmin VII is targeted to dense-core vesicles and regulates their Ca2+-dependent exocytosis in PC12 cells. J Biol Chem 279: 52677–52684 [DOI] [PubMed] [Google Scholar]
- Huynh C, Roth D, Ward DM, Kaplan J, Andrews NW (2004) Defective lysosomal exocytosis and plasma membrane repair in Chediak–Higashi/beige cells. Proc Natl Acad Sci USA 101: 16795–16800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal JK, Andrews NW, Simon SM (2002) Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J Cell Biol 159: 625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal JK, Chakrabarti S, Andrews NW, Simon SM (2004) Synaptotagmin VII restricts fusion pore expansion during lysosomal exocytosis. PLoS Biol 2: 1224–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K, Andrews NW (2000) Synaptotagmin VII regulates Ca(2+)-dependent exocytosis of lysosomes in fibroblasts. J Cell Biol 148: 1141–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil PL, Steinhardt RA (1997) Loss, restoration and maintenance of plasma membrane integrity. J Cell Biol 137: 1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A, Caler E, Andrews N (2001) Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell 106: 157–169 [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Webster P, Ortego J, Andrews NW (1997) Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J Cell Biol 137: 93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy D, Liston DR, Idone VJ, Di A, Nelson DJ, Pujol C, Bliska JB, Chakrabarti S, Andrews NW (2004) A process for controlling intracellular bacterial infections induced by membrane injury. Science 304: 1515–1518 [DOI] [PubMed] [Google Scholar]
- Shiflett SL, Kaplan J, Ward DM (2002) Chediak–Higashi syndrome: a rare disorder of lysosomes and lysosome related organelles. Pigment Cell Res 15: 251–257 [DOI] [PubMed] [Google Scholar]


