Abstract
Apolipoprotein E (ApoE), a constituent of the lipoproteins, may be relevant in herpes simplex virus type 1 (HSV-1) infection of the central nervous system (CNS), since HSV-1 binds to human serum ApoE lipoproteins. This study demonstrates the involvement of ApoE in the hematogenous route of HSV-1 to the CNS.
Apolipoprotein E (ApoE) is a constituent of very-low-density lipoprotein synthesized by the liver (29) and of members of a subclass of high-density lipoproteins involved in cholesterol transport among cells (18). ApoE mediates high-affinity binding of ApoE-containing lipoprotein particles to the low-density lipoprotein (LDL) receptor and is thus responsible for the cellular uptake of these particles (12). In humans, there are three alleles of the apoE gene (designated ɛ2, ɛ3, and ɛ4), encoding the respective isoforms of the protein (E2, E3, and E4) (19). ApoE has been implicated in regulation of the immune response (11), in nerve regeneration (17), and in muscle differentiation (20). ApoE may play an important modulatory function in the central nervous system (CNS), because while in the peripheral nervous system several other apolipoproteins are involved in lipid transport, in the CNS there is less redundancy of such molecules (19, 26). In the aged CNS, ApoE plays an important role in transporting esterified cholesterol to the neurons undergoing reinnervation, where it is taken up by the LDL receptor-related protein pathway and used as a precursor for the synthesis of new synaptic terminals (26). Numerous cell culture experiments have demonstrated receptor-mediated uptake of ApoE in neurons (4, 6, 24, 28). Furthermore, ApoE is necessary in the CNS to maintain cholesterol levels in the membrane (27) and plays a role in protecting against oxidative stress, excitotoxicity, and excessive calcium influx (31). Herpes simplex virus type 1 (HSV-1) is an important cause of severe human encephalitis (22), and it may reach the CNS by either the hematogenous or neural route. There is extensive evidence for the spread of HSV-1 within the nerves of the peripheral nervous system and the CNS (8, 9), and latent infection of the trigeminal ganglia by HSV-1 is virtually ubiquitous in humans (30). There are several routes of infection, but it is accepted that intraperitoneal inoculation, which mimics hematogenous infection by HSV-1 (14), is the normal route, and this type of infection has been particularly widely reported for young animals (14, 15, 16). Several authors have demonstrated the susceptibility of the adrenal gland to infection with HSV-1 and the crucial role of this gland in infection of the CNS after viremia with this virus has been established (9). Recent evidence raises the possibility that host genetic factors may influence the response to infection with HSV-1 (5, 13). The polymorphism of the apoE gene may be relevant to the development and course of herpes simplex virus infection for several reasons. First, it has been reported that sufferers from cold sores are more likely to carry the apoE ɛ4 allele than controls (3). Second, it has been suggested that a combination of subclinical infection of the brain with HSV-1 and carriage of apoE ɛ4 may act in concert to predispose to Alzheimer's disease (13). Third, there is accumulating evidence that apoE ɛ4 is associated with poor outcome after several forms of acute brain injury, including damage caused by trauma (7) and intracerebral hemorrhage (21). Furthermore, binding of HSV-1 to the various subclasses of serum lipoproteins has been described, including the interaction of purified glycoprotein B from HSV-1 with ApoE (10). Taken together, these reasons demonstrate the relationships between ApoE and HSV-1 infection. In the present study, we clearly showed that ApoE is essential for the colonization of the brain by HSV-1, an inherently interesting finding for the pathological scenario of viral infection.
To evaluate the role of ApoE in the hematogenous route of the virus, we induced infections of HSV-1 in apoE knockout and hemizygous mice, compared the results with those for wild-type mice, analyzed the relevance of ApoE in this process, and quantified the HSV-1 in several organs, using a highly powerful, sensitive technique, real-time quantitative PCR. Vero cells were grown in Dulbecco's modified Eagle medium supplemented with 5% fetal calf serum and antibiotics. HSV-1 was propagated and titrated by plaque assay in confluent monolayers of Vero cells (3). HSV-1 from the KOS strain (kindly supplied by L. Carrasco) was used in our experiments. All mice used in this study were 14-week-old females of the C57BL/6 strain. Between 10 and 15 mice, depending on the availability of animals, were used per group and time point. apoE knockout mice (C57BL/6J-ApoEtm1Unc) were generated under the protocol described by Piedrahita and coworkers (25) and obtained from the Jackson Laboratory (Bar Harbor, Maine). To use the same genetic background, all mice were of the C57BL/6 strain. The hemizygous females were produced by mating wild-type females with knockout males. All animals were apparently healthy. Experiments were carried out in accordance with the guidelines of the Animals (Scientific Procedures) Act of 1986. All animals had a quarantine period, and strict precautions were taken against contamination during inoculation and dissection. Mice were inoculated intraperitoneally with 106 PFU of a virus suspension. Wild-type mice were inoculated so that their ApoE levels could be compared to those of the knockout and hemizygous mice. Control mice (mock infected) received an equivalent volume of phosphate-buffered saline. Wild-type and knockout mice were culled 0.8, 2, 3, 4, and 5.7 days after inoculation, while hemizygotes were killed at two selected time points (days 4 and 5.7), when the viral load differences between wild-type and knockout mice were relevant. Organs were dissected, resulting in separation of the whole brain of all animals of the study into three regions (midbrain, ventricles, and cerebral cortex), and frozen. The DNA was extracted using conventional methods (NucleoSpin; catalog number K3053-2; Clontech, Palo Alto, Calif.). Cross-contamination of samples and false-positive PCR results were carefully avoided by frequent changing of gloves, use of exclusive pipettes, and strict spatial separation of the three main PCR steps. Real-time PCR was performed, using a LightCycler rapid thermal cycler (Roche Diagnostics Ltd., Lewes, United Kingdom), with 1 μM primers and 2 mM MgCl2. β-Actin primers (5′-AACCCTAAGGCCAACCGTGAAAAGATGACC-3′ and 5′-CCAGGGAGGAAGAGGATGCGGC-3′) were used as a positive control for the PCR (379-bp PCR product). Specific primers for a sequence in the gene for the viral DNA polymerase (pol) (5′-GGTGAACGTCTTTTCGCACT-3′ and 5′-GTGTTGTGCCGCGGTCTCA C-3′) were used (120-bp amplicon). PCR conditions were 95°C for 10 min, 45 cycles of 95°C for 30 s and 55°C (for β-actin) or 60°C (for pol) for 30 s, and 72°C for 40 s. An appropriate concentration range of virus was used for optimization of the standard curve of the real-time PCR, and the viral load determination was carried out using PFU as units. The PCR calibration for the β-actin housekeeping gene was performed using nanograms as units. Each experiment was performed in triplicate, and in each case, melting curve analysis, agarose gel electrophoresis, and restriction analysis confirmed the specificity of the amplification products. Briefly, the gene fragments analyzed were restricted with the AvaI endonuclease for the viral DNA pol (producing two fragments of 23 and 97 bp in size) and with the NlaIV enzyme for the β-actin (producing two fragments of 220 and 159 bp in size).
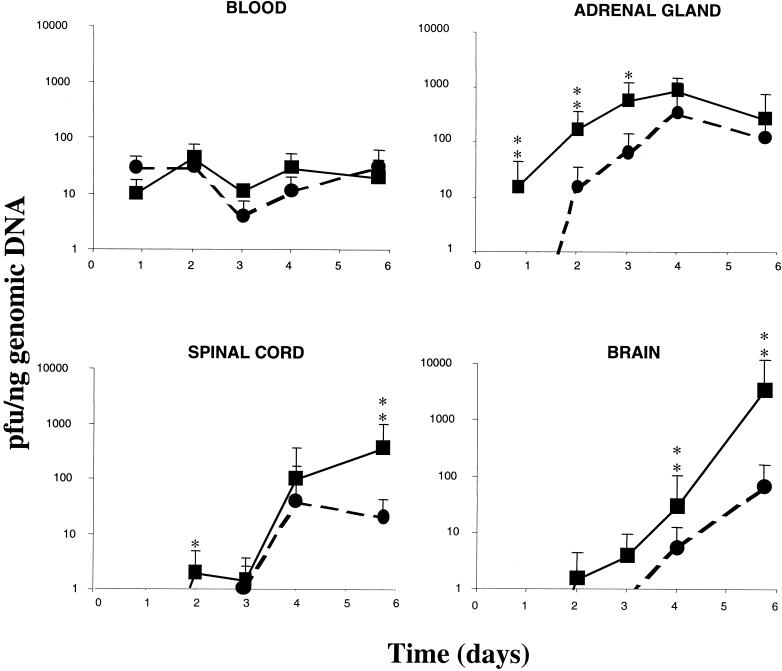
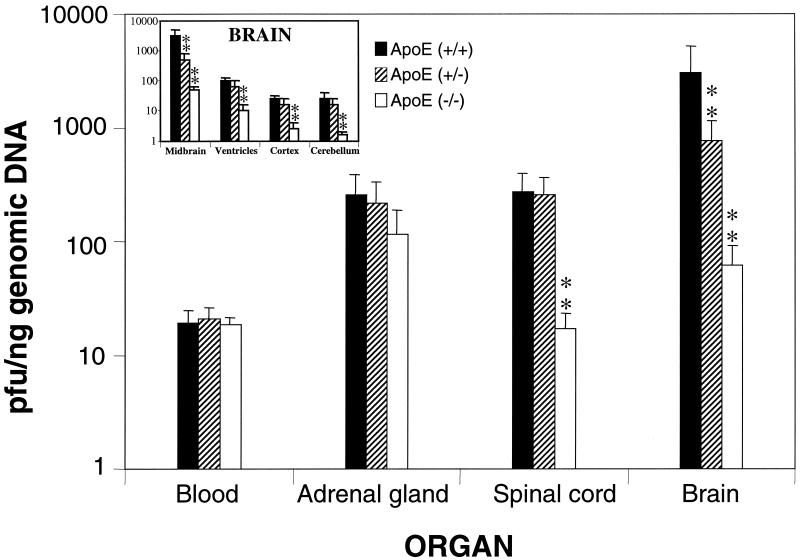
After inoculation, only a small number of mice (13.8%) presented clinical signs of disease and there were no significant differences linked to the apoE dose in the number of sick mice, indicating that the apoE knockout and hemizygous mice were not more susceptible to the disease than the wild-type animals. All mice remained asymptomatic until day 4. After this time, a small number of mice (7 out of 14 wild-type mice, 6 out of 12 apoE hemizygous mice, and 0 out of 10 apoE knockout mice) were bilaterally affected to some degree, at first showing symptoms ranging from slight weakness to loss of movement, later showing evident signs of ataxia, and finally exhibiting complete hind paralysis and loss of postural control. None of the mock-infected animals showed clinical abnormalities. The analysis of the viral load revealed a constant level of viremia over the time course of the experiment, independent of the identity of the mouse group, with different ApoE doses (Fig. 1). In fact, the statistical analysis revealed that there were no significant differences in the viral load in blood between knockout and wild-type mice at each time point. Virus levels in the adrenal gland increased from the point of first detection (day 0.8) until day 4, when the infection reached the spinal cord. On days 3 and 4, the virus was detectable in the brain, and the virus level increased until day 5.7. In the brain, HSV-1 was first detected at significant levels in the midbrain on day 4. On day 5.7, the virus reached the brain ventricles at significant levels, but it was also detected in the cortex and the cerebellum. The order of invasiveness was the same in both types of mice, with the midbrain being the first site of virus arrival after the spinal cord but with an evident time delay and lower viral load in the knockout mice compared to the wild-type mice (Fig. 1). Analysis of other organs (pancreas, heart, bone marrow, spleen, and liver) was not informative. The study of the apoE hemizygotes revealed that these mice had an intermediate viral load between those of the wild-type and knockout mice in the adrenal gland, spinal cord, and brain (Fig. 2). When the encephalon was separated into four areas, the midbrain was consistently the cerebral region with the highest viral load, while in the ventricles, cortex, and cerebellum, the viral load of hemizygotes was closer to that of the wild-type mice (Fig. 2; see inset).
FIG. 1.
Time courses of HSV-1 infection in the several analyzed organs. Solid lines represent the wild-type mouse group; dotted lines represent the apoE knockout mouse group. Values are expressed as means ± standard errors of the mean (∗, P < 0.01; ∗∗, P < 0.001 [Fischer's exact test]).
FIG. 2.
Quantification of the viral load in relation to ApoE dosage on day 5.7. The inset presents the results for the four dissected areas of the brain analyzed in this study. Values are expressed as means ± standard errors of the mean (∗∗, P < 0.001 [Fischer's exact test]).
Our results reveal the extreme susceptibility of the adrenal gland to infection with HSV-1 and the crucial role of this gland in hematogenous infection of the nervous system. Although the CNS is infected by viral spread through the nerves, the main pathway of HSV-1 to the organs in neonates (1) and in immunosuppressed humans (23) is the bloodstream. It is accepted that intraperitoneal inoculation is the normal route of infection, mimicking hematogenous infection by HSV-1 (14). In fact, our data show that the virus is immediately detectable in blood after inoculation in the peritoneum, due to the fast access from the peritoneal cavity to the bloodstream. Previous studies involving adrenalectomy in mice have demonstrated that this organ is the main source of virus entering the spinal cord (9). In its developmental and functional aspects, the medulla of the adrenal gland can be considered to be a modified ganglion of the sympathetic part of the autonomous nervous system, innervated by sympathetic and parasympathetic nerves. In this sense, tracing of the transneuronal path of the virus from the adrenal gland has revealed the existence of anatomical and functional connections between the adrenal gland and the hypothalamic suprachiasmatic nucleus (2). Our exploration of this pathway showed, in good accordance with the results in reference 2, that HSV-1 replicates in the adrenal gland, reaches the spinal cord, and migrates to the brain. HSV-1 DNA is detected in the midbrain, indicating that this is the first site reached by the virus, which later appears in the brain ventricles and finally spreads to the cortex and the cerebellum. The observations in the present study indicate that ApoE is involved in HSV-1 infection of the mouse brain. ApoE has been implicated in regulation of the immune response (11), and for this reason, we might expect that apoE knockout mice would be more susceptible to disease than the wild-type mice. In our study, however, HSV-1 detection and clinical signs detected in the mice showed that, in contrast to expectations, the disease starts when the virus reaches the spinal cord and that ApoE plays an important role in this process. We may therefore expect the ApoE-deficient mice to be more resistant to the virus that the wild-type mice, as the virus has more difficulties in accessing the CNS in the former case. Furthermore, the proportion of infected knockout mice with clinical signs of disease is equal to or less than that of wild-type mice, indicating that the potential immunosuppression of the ApoE-deficient mice is not relevant to this approach.
In summary, in this study we clearly showed that the ApoE dose is directly linked to the invasiveness of HSV-1 in the brain. Here, the amount of virus in hemizygotes was between that in wild-type and in knockout mice, indicating that the level of ApoE present and HSV-1 invasiveness in the brain are related. Outside of the CNS, there were few differences seen between the level of HSV-1 colonization and ApoE dose, which may reflect the great redundancy of apolipoproteins in blood and in organs irrigated by the bloodstream. The colonization of the brain by HSV-1, and the potential for the virus to survive in a latent form, could be implicated in a great number of chronic neurodegenerative disorders and could constitute the initial step for a reactivation and subsequent degeneration of infected neurons in which HSV-1 lodges. Moreover, the deep involvement of the apoE gene in the process by which HSV-1 infects the brain could be more relevant, because the type of ApoE present in mice bears some resemblance to ApoE4 in humans. ApoE3 has a cysteine at position 112 and arginine at position 158, whereas ApoE2 has cysteines and ApoE4 has arginines at both positions (32). Mouse ApoE, like ApoE4, contains two arginines at both positions. In conclusion, the present study supports the hypothesis of involvement of ApoE in the hematogenous route of HSV-1 to the CNS.
Acknowledgments
This research was supported by the Fundación Ramón Areces and the Comunidad de Madrid.
We thank F. Mayor for his continuous encouragement and help and L. Carrasco for providing the HSV-1 strain.
REFERENCES
- 1.Arvin, A. M., A. S. Yeager, F. W. Bruhn, and M. Grossman. 1982. Neonatal herpes simplex infection in the absence of mucocutaneous lesions. J. Pedriatr. 100:715-721. [DOI] [PubMed] [Google Scholar]
- 2.Buijs, R. M., J. Wortel, J. J. Van Heerikhuize, M. G. Feenstra, G. J. Ter Horst, H. J. Romijn, and A. Kalsbeek. 1999. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur. J. Neurosci. 5: 1535-1544. [DOI] [PubMed] [Google Scholar]
- 3.Carrascosa, A. L., J. F. Santaren, and E. Vinuela. 1982. Production and titration of African swine fever virus in porcine alveolar macrophages. J. Virol. Methods 3:303-310. [DOI] [PubMed] [Google Scholar]
- 4.DeMattos, R. B., L. K. Curtiss, and D. L. Williams. 1998. A minimally lipidated form of cell-derived apolipoprotein E exhibits isoform-specific stimulation of neurite outgrowth in the absence of exogenous lipids or lipoproteins. J. Biol. Chem. 273:4206-4212. [DOI] [PubMed] [Google Scholar]
- 5.Dobson, C. B., and R. F. Itzhaki. 1999. Herpes simplex virus type 1 and Alzheimer's disease. Neurobiol. Aging 20:457-465. [DOI] [PubMed] [Google Scholar]
- 6.Fagan, A. M. G., Bu, Y. Sun, A. Daugherty, and D. M. Holtzman. 1996. Apolipoprotein E-containing high density lipoprotein promotes neurite outgrowth and is a ligand for the low density lipoprotein receptor-related protein. J. Biol. Chem. 271:30121-30125. [DOI] [PubMed] [Google Scholar]
- 7.Friedman, G., P. Froom, L. Sazbon, I. Grinblatt, M. Shochina, J. Tsenter, S. Babaey, B. Yehuda, and Z. Groswasser. 1999. Apolipoprotein E-ε4 genotype predicts a poor outcome in survivors of traumatic brain injury. Neurology 52:244-248. [DOI] [PubMed] [Google Scholar]
- 8.Hill, T. J. 1985. Herpes simplex virus latency, p. 175-240. In B. Roizman (ed.), The herpesvirus, vol. 3. Plenum Press, New York, N.Y.
- 9.Hill, T. J., D. L. Yirrell, and W. A. Blyth. 1986. Infection of the adrenal gland as a route to the central nervous system after viraemia with herpes simplex virus in the mouse. J. Gen. Virol. 67:309-320. [DOI] [PubMed] [Google Scholar]
- 10.Huemer, H. P., H. J. Menzel, D. Potratz, B. Brake, D. Falke, G. Utermann, and M. P. Dierich. 1988. Herpes simplex virus binds to human serum lipoprotein. Intervirology 29:68-76. [DOI] [PubMed] [Google Scholar]
- 11.Hui, D. Y., J. A. Harmony, T. L. Innerarity, and R. W. Mahley. 1980. Immunoregulatory plasma lipoproteins. Role of apolipoprotein E and apolipoprotein B. J. Biol. Chem. 255:11775-11781. [PubMed] [Google Scholar]
- 12.Hui, D. Y., T. L. Innerarity, and R. W. Mahley. 1981. Lipoprotein binding to canine hepatic membranes. Metabolically distinct apo-E and apo-B,E receptors. J. Biol. Chem. 256:5646-5655. [PubMed] [Google Scholar]
- 13.Itzhaki, R. F., W. R. Lin, D. Shang, G. K. Wilcock, B. Faragher, and G. A. Jamieson. 1997. Herpes simplex virus type 1 in brain and risk of Alzheimer's disease. Lancet 349:241-244. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, R. T. 1964. Pathogenesis of herpes simplex virus encephalitis. I. Virus pathway to the nervous system of suckling mice demonstrated by fluorescent antibody staining. J. Exp. Med. 119:343-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kern, E. R., J. C. Overall, and L. A. Glasgow. 1973. Herpesvirus hominis infection in newborn mice. I. An experimental model and therapy with iododeoxyuridine. J. Infect. Dis. 128: 290-299. [DOI] [PubMed] [Google Scholar]
- 16.Lascano, E. F., and M. I. Berria. 1980. Histological study of the progression of herpes simplex virus in mice. Arch. Virol. 64:67-79. [DOI] [PubMed] [Google Scholar]
- 17.LeBlanc, A. C., and J. F. Poduslo. 1990. Regulation of apolipoprotein E gene expression after injury of the rat sciatic nerve. J. Neurosci. Res. 25: 162-171. [DOI] [PubMed] [Google Scholar]
- 18.Mahley, R. W. 1986. The molecular basis of atherosclerosis: concepts derived from studies of lipoprotein metabolism and cell biology. Clin. Investig. Med. 9:304-308. [PubMed] [Google Scholar]
- 19.Mahley, R. W. 1988. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240:622-630. [DOI] [PubMed] [Google Scholar]
- 20.Majack, R. A., C. K. Castle, L. V. Goodman, K. H. Weisgraber, R. W. Mahley, E. M. Shooter, and P. J. Gebicke-Haerter. 1988. Expression of apolipoprotein E by cultured vascular smooth muscle cells is controlled by growth state. J. Cell Biol. 107:1207-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarron, M. O., K. W. Muir, C. J. Weir, A. G. Dyker, I. Bone, J. A. R. Nicoll, and K. R. Lees. 1998. The apolipoprotein E ɛ4 allele and outcome in cerebrovascular disease. Stroke 29:1882-1887. [DOI] [PubMed] [Google Scholar]
- 22.Meyer, H. M., R. T. Johnson, I. P. Crawford, H. E. Dascomb, and N. G. Rogers. 1960. Central nervous system syndromes of viral etiology. A study of 713 cases. Am. J. Med. 29:334. [DOI] [PubMed] [Google Scholar]
- 23.Montgomerie, J. Z., D. M. Becroft, M. C. Croxson, P. B. Doak, and J. D. North. 1969. Herpes-simplex-virus infection after renal transplantation. Lancet 2:867-871. [DOI] [PubMed] [Google Scholar]
- 24.Nathan, B. P., K. C. Chang, S. Bellosta, E. Brisch, N. Ge, R. W. Mahley, and R. E. Pitas. 1995. The inhibitory effect of apolipoprotein E4 on neurite outgrowth is associated with microtubule depolymerization. J. Biol. Chem. 270:19791-19799. [DOI] [PubMed] [Google Scholar]
- 25.Piedrahita, J. A., S. H. Zhang, J. R. Hagaman, P. M. Oliver, and N. Maeda. 1992. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc. Natl. Acad. Sci. USA 89:4471-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poirier, J., A. Minnich, and J. Davignon. 1995. Apolipoprotein E, synaptic plasticity and Alzheimer's disease. Ann. Med. 27:663-670. [DOI] [PubMed] [Google Scholar]
- 27.Posse de Chaves, E. I., A. E. Rusinol, D. E. Vance, R. B. Camperot, and J. E. Vance. 1997. Role of lipoproteins in the delivery of lipids to axons during axonal regeneration. J. Biol. Chem. 272:30766-30773. [DOI] [PubMed] [Google Scholar]
- 28.Puttfarcken, P. S., A. M. Manelli, M. T. Falduto, G. S. Getz, and M. J. LaDu. 1997. Effect of apolipoprotein E on neurite outgrowth and beta-amyloid-induced toxicity in developing rat primary hippocampal cultures. J. Neurochem. 68:760-769. [DOI] [PubMed] [Google Scholar]
- 29.Shore, V. G., and B. Shore. 1973. Heterogeneity of human plasma very low density lipoproteins. Separation of species differing in protein components. Biochemistry 12:502-507. [DOI] [PubMed] [Google Scholar]
- 30.Steiner, I., and P. G. Kennedy. 1995. Herpes simplex virus latent infection in the nervous system. J. Neurovirol. 1:19-29. [DOI] [PubMed] [Google Scholar]
- 31.Wanj, X., and E. Gruenstein. 1997. Rapid elevation of neuronal cytoplasmic calcium by apolipoprotein E peptide. J. Cell Physiol. 173:73-83. [DOI] [PubMed] [Google Scholar]
- 32.Weisgraber, K. H. 1994. Apolipoprotein E: structure-function relationships. Adv. Protein Chem. 45:249-302. [DOI] [PubMed] [Google Scholar]